Abstract
Background
Currently, the standard procedure used to evaluate hepatic steatosis is the liver biopsy. This is an invasive practice that presents inherent risks. Increasing evidence suggests that magnetic resonance imaging (MRI) and MR spectroscopy (MRS) may represent an accurate method to determine the hepatic lipid content. The aim of this study was to evaluate the effect of sleeve gastrectomy on liver steatosis, quantified by MRI and MRS.
Patients and Methods
A prospective observational study of patients undergoing laparoscopic sleeve gastrectomy was performed. All patients underwent a MRI and a MRS study 2 weeks before the intervention and 6 months after the surgery. Anthropometric, biochemical, and radiological parameters were analyzed.
Results
Twenty-three patients were included, 21 females and 2 males, with a mean age of 47.6 ± 10.6 years and mean pre-op BMI 47.6 ± 6.7 Kg/m2. Six months after surgery, mean BMI was 32.2 ± 5.1 Kg/m2, with a mean excess weight loss of 68.2 ± 18.6%. Mean preoperative hepatic volume was 1999.9 ± 436.2 ml and 6 months after surgery it decreased to 1568 ± 170.3 ml (p = 0.005). Mean preoperative percentage of lipid content was 14.2 ± 15.4% and 6 months after surgery, it decreased to 4.3 ± 3.2% (p = 0.007). A significant reduction of steatosis grade was observed, with disappearance of preoperative steatosis in 54.9% of the patients.
Conclusion
Six months after sleeve gastrectomy, a significant reduction of liver steatosis is observed, as demonstrated by reduction in the percentage of intrahepatocitary lipids and liver volume, determined by MRS and MRI. These imaging techniques can be considered as noninvasive, accurate methods for monitoring liver steatosis in morbidly obese patients undergoing bariatric surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is characterized by lipid deposit in the hepatocytes of the liver parenchyma, exceeding the 5% of the liver weight. NAFLD includes a spectrum of disorders ranging from simple steatosis, a relatively benign condition, to nonalcoholic steatohepatitis (NASH) and pericellular or perisinusoidal fibrosis. In some cases, these entities might lead to a cirrhosis stage, similar to the observed in alcoholic hepatitis. The mechanism of NAFLD is still unknown, but involves the development of insulin resistance, inflammatory cytokines, and oxidative stress [1, 2]. NAFLD is strongly associated with obesity, mainly with central one, and has become the most common liver disorder in Western countries [3]. The prevalence of NAFLD, in any degree, is estimated around 70% in obese subjects and up to 95% in morbidly obese patients [4]. Among patients undergoing bariatric surgery, liver steatosis is the most common degree of the disease, appearing NASH in about 25% of the cases and cirrhosis in 1–3% [5].
Bariatric surgery achieves an improvement or even complete resolution of NAFLD in morbidly obese patients [4, 6]. This fact has been demonstrated after Roux-en–Y gastric bypass (RYGBP), adjustable gastric banding and malabsorptive procedures (duodenal switch and biliopancreatic diversion) [7–9], but little is known about the effect of laparoscopic sleeve gastrectomy (LSG).
Currently, the standard procedure used to evaluate hepatic steatosis is the histopathological examination of cross-liver sections and the semi-quantitative estimation of the percentage of hepatocytes containing macrovesicular fat [10]. This is an invasive practice that presents inherent risks. Increasing evidence suggests that magnetic resonance imaging (MRI) and MR spectroscopy (MRS) may represent an accurate method to determine the hepatic lipid content, comparable to the liver biopsy to measure and follow up the fatty liver disease. Thus, they have been postulated as potential screening tools for patients at risk of liver steatosis, such as morbidly obese patients [11–15].
The aim of this study was to evaluate the effect of sleeve gastrectomy on liver steatosis, quantified by MRI and MRS.
Patients and Methods
From March 2013 to July 2014, a prospective observational study of patients undergoing laparoscopic sleeve gastrectomy as bariatric technique was performed.
Inclusion criteria were a body mass index (BMI) ≥ 40 Kg/m2 or BMI ≥35 Kg/m2 with comorbidities. Exclusion criteria included the presence of daily alcohol intake of more than 20 g per day, chronic hepatitis B or C virus infection, hepatotoxic drugs intake or other known liver diseases, presence of pacemaker or any metallic implant not compatible with MR, and claustrophobia.
Preoperative Evaluation
A multidisciplinary team, including surgeons, endocrinologists, endoscopists, anesthetics, psychiatrists, and psychologists, and specialized nurses, performed a combined medical, nutritional, and endocrinological work-up to evaluate potential surgical candidates. Preoperative assessment included abdominal ultrasound, upper gastrointestinal endoscopy, functional respiratory tests, and analytical evaluation of the nutritional status. Psychiatrists and psychologists assessed additional interviews to evaluate the implication of the patient in following a strict diet in the postoperative course. A dietician established a diet consisting of a total daily energy intake of 1200 Kcal, similar to the one they have to follow after the operation. A weight loss of at least a 5% of the patient’s overweight was considered an indispensable condition to be selected as candidate for laparoscopic sleeve gastrectomy. Patients with documented gastroesophageal reflux disease or patients not achieving the required weight loss (reflection of bad adhesion to the diet) were excluded and selected for undergoing a gastric bypass.
Surgical Technique
A longitudinal resection from 1.5-2 cm distance to the His angle to approximately 3–4 cm proximally to the pylorus was performed using a 40-French bougie inserted along the lesser curve. A staple line inversion was performed with a continuous oversewing of Polypropilene 2/0, before extracting the bougie.
Magnetic Resonance
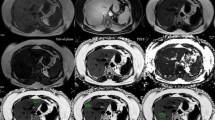
All patients underwent a MRI and a MRS study 2 weeks before the intervention and 6 months after the surgery. All studies were acquired in a Philips Intera 1.5 T (Philips Medical, The Nederlands) with a standard SENSE body coil provided by the manufacturer. The MRI protocol included axial T1-wi dual gradient-echo (TR/TE, 122 ms/5–2 ms; flip angle, 80°; slice thickness, 6 mm; matrix 256 × 256). In addition, axial breath hold three dimensional (3D) T1-weighted high resolution isotropic volume examination (THRIVE) (TR/TE, 4/2 ms; flip angle, 10°; reconstructed slice thickness, 2 mm; matrix, 256 × 256) was acquired for volumetric analysis.
Before spectroscopy, for a better voxel placement, fast T2 single shot TSE sequences (TR/TE, 611/100 ms; flip angle 90°, slice thickness/GAP, 9/0.9 mm) were acquired in coronal, sagital, and axial planes. MRS was acquired with the single-voxel technique, two voxels were located in different regions within the left and right hepatic lobes to provide a more generalized distribution of fat within the liver. The average voxel volume was of 30 × 30 × 30 mm and 20 × 20 × 20 mm for the voxels located in the right lobe and left hepatic lobe, respectively. The voxel was located avoiding blood vessels, the gall bladder, and fatty tissue. After volume-selective autoshimming, the spectra were obtained with a point-resolved spectroscopy (PRESS) technique (TR/TE/scans = 2000 ms/35 ms/64) without water suppression. Each spectrum consisted of 1024 complex data points in the time domain with a spectral width of 2000 Hz. Chemical shift was placed relative to the water signal intensity.
Spectra were analyzed offline in the time domain using the AMARES algorithm included in the MRUI software package as described previously. The relative lipid content was defined by following the formulae [15]:
For volumetric analysis, manual volumetric segmentations of the liver were performed by manual tracing of the liver on volumetric sequences in the axial plane avoiding the gall bladder and main hepatic vessels at a dedicated workstation (Advantage Workstation, Version 4.3; GE Healthcare Europe, Buc, France).
Steatosis by MRS was determined as absent when detected percentage of lipid content (PLC) was lower than 5%, mild when lower than 10%, moderate when 10–30%, and severe when >30%, as described in literature [12–16].
Variables
Anthropometric, biochemical and radiological parameters were obtained preoperatively and 6 months after surgery. Anthropometric measurements included preoperative weight and BMI and postoperative weight loss, BMI, and excess weight loss (EWL). Biochemical parameters include liver enzymes alanine aminotransferase (AST) and aspartate aminotransferase (ALT). Radiological parameters, referring to the MRI and MRS, included hepatic volume and PLC.
Statistical Analysis
All statistical analysis was performed using SPSS Version 19.0 (SPSS Inc., Chicago, IL). Results are expressed as mean ± standard deviation or number and percentages in non-Gaussian variables. Paired student t tests and Friedman’s test were used to compare data before and after surgery. A p value <0.05 was considered statistically significant.
The study was approved by the local Ethics Committee and all the patients signed a written informed consent before entering the study.
Results
Initially, 25 patients were included in the study, but 2 were excluded, because their obesity (abdominal circumference) prevented them entering into MRI scanner. Finally, 23 patients were included for the analysis, 21 females (91.3%) and 2 males (8.7%), with a mean age of 47.6 ± 10.6 years. Comorbidities are described in Table 1.
Anthropometric Measurements
Mean preoperative weight was 119.9 ± 14.7 Kg, mean preoperative height was 159.9 ± 6.4 cm and mean pre-op BMI 47.6 ± 6.7 Kg/m2. Six months after surgery, mean weight was 82.7 ± 11.4 Kg and mean BMI 32.2 ± 5.1 Kg/m2, with a mean excess weight loss of 68.2 ± 18.6%.
Biochemical Measurements
Preoperatively, increased AST levels (>40 U/I) were found in 13% of the patients and increased ALT (>40 U/I) in 26.1%, postoperatively, all the patients presented AST and ALT levels within the normal range.
A significant decrease of AST and ALT levels was observed between pre and postoperative determinations (Table 2).
MRI and MRS Measurements
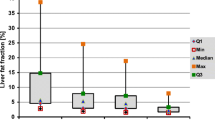
Mean preoperative hepatic volume was 1999.9 ± 436.2 ml, and 6 months after surgery, the liver volume significantly decreased to 1568 ± 170.3 ml (p = 0.005). Mean preoperative PLC was 14.2 ± 15.4%, and 6 months after surgery, these values decreased to 4.3 ± 3.2% (p = 0.007). Pre and postoperative steatosis grade, according to the previously mentioned classification, is described in Table 3. A significant reduction of steatosis grade was observed, with disappearance of preoperative steatosis in 54.9% of the patients. Six months after surgery, 91.3% of the patients did not present any grade of steatosis.
Correlations
The preoperative PLC positively correlated with preoperative AST (Pearson 0.742, p = 0.022) and ALT levels (Pearson 0.668, p = 0.049). Notwithstanding, the decrease of liver enzymes or the postoperative values did not show any correlation with PLC decrease or postoperative PLC values.
Discussion
The diagnosis of NAFLD is usually made after an incidental discovery of unexplained elevation of liver enzyme levels or when steatosis is observed on imaging. Liver biopsy remains the gold standard for the diagnosis of liver steatosis and steatohepatitis, however, the liver cell injury and intrahepatocellular accumulation of lipids can be also evaluated by noninvasive tests [1]. Despite there are several series in literature with an elevated number of patients undergoing a liver biopsy during bariatric surgery, without showing any morbidity related with the biopsy [17], for posterior monitoring of the liver status, it is necessary to perform a percutaneous liver biopsy, implying an invasive procedure. Moreover, histological examination of cross-liver sections and the determination of the percentage of hepatocytes containing macrovesicular fat, only provides a two-dimensional estimation of a particular biopsy and is subject to inter-individual visual evaluation depending on the pathologist’s training, which usually results in overestimation of the liver fat content [18, 19]. Therefore, it is essential to establish new noninvasive approaches to accurately determine the hepatic fat concentration, allowing the correct diagnosis and monitoring of steatosis.
MRI represents a potential noninvasive technique for assessing hepatic steatosis in three-dimensions [19–21]. MRS is a suitable method to quantify hepatic lipid content, as the values obtained by MRS are directly related to the molecular triglyceride content. One of the main advantages of using spectroscopy instead of biopsy is that it can be performed contemporaneously with MR imaging and the sampling volumes are larger than the obtained in liver biopsy avoiding misinterpretations if the fat liver distribution is heterogeneous [22, 23].
In our group, we observed a steatosis incidence calculated with MRS of 63.6%. This steatosis rate is similar to the one observed by some authors, though other reports have shown higher prevalence that could vary from 65 to 80% [24, 25]. The steatosis grade was probably lower among our patients, as all of them followed a strict preoperative diet, and a weight loss of at least a 5% of their overweight was a necessary condition to undergo the sleeve gastrectomy as bariatric procedure. It has been demonstrated that establishing strict dietary treatments with a total caloric intake of less than 1000 kcal/day between 2 and 8 weeks before surgery, in order to maximize weight loss during this period of time, result in a reduction of the surgical risk [26]. This weight loss is associated with a reduction of hepatomegalia secondary to liver steatosis. We have observed a positive correlation between hepatic lipid content assessed by MRS and hepatic volume, suggesting that the hepatic volume loss observed after surgery is related to the decrease in the hepatic lipid content, as it has been previously reported in patients undergoing bariatric surgery [27].
Alterations of AST and ALT are the most frequent conditions found in NAFLD in morbidly obese patients [2], similar to our results. Notwithstanding, Dixon et al. [28] reported that changes in aminotransferase concentrations seems not to predict changes in steatosis. In the same line, a recent study observed a significant reduction of steatosis after bariatric surgery, documented with liver biopsy, but without correlation with AST or ALT levels [29]. In our series, we observed a correlation between liver enzymes and PLC in the preoperative determinations, but the decrease of liver enzymes or the postoperative values did not show any correlation with PLC decrease or postoperative PLC values. Liver steatosis rate in morbidly obese patients ranges between 65 and 80%, but steatohepatitis appears in only 25% of the case. Hepatocyte fat infiltration might lead to a certain degree of inflammation and cytolysis, but this is especially relevant in severe steatosis grades. Thus, in the preoperative determinations of our series, with more than 25% of patients presenting severe steatosis, more steatohepatitis would be present and therefore, a correlation with liver enzymes is possible. Postoperatively, steatosis remains in few patients and just in mild grade with probably absence of steatohepatitis, preventing a positive correlation between both entities [1].
Improvement of liver steatosis after bariatric surgery has been widely demonstrated, but mainly after gastric bypass or malabsorptive procedures. Little is known about the effect of sleeve gastrectomy on liver steatosis in humans, but in animal models, sleeve gastrectomy has demonstrated a significant reduction of the steatosis grade. Liver steatosis regression has been associated with an improvement in the lipid profile, which is more likely to occur after malabsorptive techniques [30, 31]. A recently published study of our group demonstrated a significant reduction of triglyceride levels and an increase of HDL-cholesterol 12 months after undergoing a sleeve gastrectomy, achieving both parameters values within the normal range [32]. Therefore, an improvement of liver steatosis was observed, as expected. Our results are similar to those reported after Roux-en-Y gastric bypass (90–95% of resolution of the steatosis) [31].
Conclusion
Six months after sleeve gastrectomy, a significant reduction of liver steatosis is observed, as demonstrated by a statistically significant reduction in the percentage of intrahepatocitary lipids and liver volume, determined by MRS and MRI (respectively). These imaging techniques can be considered as noninvasive, accurate methods for monitoring liver steatosis in morbidly obese patients undergoing bariatric surgery. Mid- and long-terms results are needed to ensure that these are sustained results.
References
Wilkins T, Tadkod A, Hepburn I, et al. Nonalcoholic fatty liver disease: diagnosis and management. Am Fam Physician. 2013;88:35–42.
Alves de Carvalho MS, Coelho Cabral P, Kruze Grande de Arruda I, et al. Risk factors associated with hepatic steatosis; a study in patients in the Northeast Brazil. Nutr Hosp. 2012;27:1344–50.
Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–57.
Clark JM, Alkhuraishi AR, Solga SF, et al. Roux-en-Y gastric bypass improves liver histology in patients with non-alcoholic fatty liver disease. Obes Res. 2005;13:1180–6.
Pillai AA, RInella ME. Non-alcoholic fatty liver disease: is bariatric surgery the answer? Clin Liver Dis. 2009;13:689–710.
Weiner RA. Surgical treatment of non-alcoholic steatohepatitis and non-alcoholic fatty liver disease. Dig Dis. 2010;28:274–9.
Johansson HE, Haenni A, Zethelius B. Platelet counts and liver enzymes after bariatric surgery. J Obes. 2013; Article ID 567984.
Tai CM, Huang CK, Hwang JC, et al. Improvement of nonalcoholic fatty liver disease after bariatric surgery in morbidly obese Chinese patients. Obes Surg. 2012;22:1016–21.
Sakçak M, Avsar MF, Hamamci EO, et al. Comparison of early and late changes in immunoglobulins and acute phase reactants after laparoscopic adjustable gastric banding in patients with morbid obesity. Obes Surg. 2010;20:610–5.
Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–906.
Guiu B, Petit JM, Loffreoy R, et al. Quantification of liver fat content: comparison of triple-echo chemical shift gradient-echo imaging and in vivo proton MR spectroscopy. Radiology. 2009;250:95–102.
Moreno-Pérez O, Portilla J, Escoín C, et al. Impact of vitamin D insufficiency on insulin homeostasis and beta cell function in nondiabetic male HIV-infected patients. HIV Med. 2013;14:540–8.
Ma X, Holalkere N-S, Kambadakone RA, et al. Imaging-based quantification of hepatic fat: methods and clinical applications. Radiographics. 2009;29:1253–77.
Machann J, Thamer C, Schnoedt B, et al. Hepatic lipid accumulation in healthy subjects: a comparative study using spectral fat-selective MRI and volume-localized 1H-MR spectroscopy. Magn ResonMed. 2006;55:913–7.
van Werven JR, Marsman HA, Nederveen AJ, et al. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology. 2010;256:159–68.
Thomas EL, Hamilton G, Patel N, et al. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut. 2005;54:122–7.
Subichin M, Clanton J, Makuszewski M, et al. Liver disease in the morbidly obese: a review of 1000 consecutive patients undergoing weight loss surgery. Surg Obes Relat Dis. 2015;11:137–41.
Jimenez-Aguero R, Emparanza JI, Beguiristain A, et al. Novel equation to determine the hepatic triglyceride concentration in humans by MRI: diagnosis and monitoring of NAFLD in obese patients before and after bariatric surgery. BMC Medicine. 2014;12:137–51.
Younossi ZM, Gramlich T, Liu YC, et al. Nonalcoholic fatty liver disease: assessment of variability in pathologic interpretations. Mod Pathol. 1998;11:560–5.
Reeder SB, Cruite I, Hamilton G, et al. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–49.
Tang A, Tan J, Sun M, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422–31.
Chan DC, Watts GF, Ng TWK, et al. Measurement of liver fat by magnetic resonance imaging: relationships with body fat distribution, insulin sensitivity and plasma lipids in healthy men. Diabetes Obes Metab. 2006;8:698–702.
Ishii M, Yoshioka Y, Ishida W, et al. Liver fat content measured by magnetic resonance spectroscopy at 3.0 tesla independently correlates with plasminogen activator inhibitor-1 and body mass index in type 2 diabetic subjects. Tohoku J Exp Med. 2005;206:23–30.
Oliveira CPMS, Faintuch J, Rascovski A, et al. Lipid peroxidation in bariatric candidates with nonalcoholic fatty liver disease (NAFLD)—preliminary findings. Obes Surg. 2005;15:502–5.
Frantzides CT, Carlson MA, Moore RE, et al. Effect of body mass index on nonalcoholic fatty liver disease in patients undergoing minimally invasive bariatric surgery. J Gastrointest Surg. 2004;8:849–55.
Carbajo MA, Castro MJ, Kleinfinger S, et al. Effects of a balanced energy and high protein formula diet (Vegestart complet®) vs. low-calorie regular diet in morbid obese patients prior to bariatric surgery (laparoscopic single anastomosis gastric bypass): a prospective, double-blind randomized study. Nutr Hosp. 2010;25:939–48.
Lewis MC, Phillips ML, Slavotinek JP, et al. Change in liver size and fat content after treatment with Optifast very low calorie diet. Obes Surg. 2006;16:697–701.
Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006;10:1278–86.
Campos GM, Bambha K, Vittinghoff E, et al. A clinical scoring system for predicting nonalcoholic steatohepatitis in morbidly obese patients. Hepatology. 2008;47:1916–23.
Vargas V, Allende H, Lecube A, et al. Surgically induced weight loss by gastric bypass improves non alcoholic fatty liver disease in morbid obese patients. World J Hepatol. 2012;4:382–8.
Pontiroli AE, Benetti A, Folini L, et al. Other aspects of bariatric surgery: liver steatosis, ferritin and cholesterol metabolism. Nutr Hosp. 2013;28:104–8.
Ruiz-Tovar J, Oller I, Tomas A, et al. Midterm impact of sleeve gastrectomy, calibrated with a 50-Fr bougie, on weight loss, glucose hemostasis, lipid profiles and comorbidities in morbidly obese patients. Am Surg. 2012;78:969–74.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in the study.
Statement of Human Rights
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Rights and permissions
About this article
Cite this article
Alsina, M.E., Ruiz-Tovar, J. & Bernabeu, A. Evolution of Liver Steatosis Quantified by MR Imaging and MR Spectroscopy, in Morbidly Obese Patients Undergoing Sleeve Gastrectomy: Short-Term Outcomes. OBES SURG 27, 1724–1728 (2017). https://doi.org/10.1007/s11695-016-2473-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2473-9