Abstract
Objective
Adiposity has been inversely associated with vitamin D concentration across a range of body mass index values and cultural groups. As obesity has increased markedly worldwide, a greater number of patients with severe obesity have been treated with gastric restrictive and/or malabsorptive surgical procedures. The purpose of this review was to describe current knowledge about vitamin D and severe obesity, and the impact of obesity surgery on vitamin D status.
Research Methods and Procedures
A systematic review was conducted with search terms obesity, vitamin D, osteoporosis, bone disease, gastric bypass, and obesity surgery in various combinations. Publications were limited to those since 2000 to control for similarity in vitamin D assays and obesity prevalence levels.
Results
Mean 25-hydroxy vitamin D was <80 nmol/l in more than 1,900 patients preoperatively, and was not restored to the optimal concentration of >80 nmol/l postoperatively. Both secondary hyperparathyroidism and bone loss were common, particularly when the obesity surgery included a malabsorptive component. Standard postsurgical supplementation with vitamin D and calcium have not been adequate to suppress secondary hyperparathyroidism or to restore 25-hydroxy vitamin D status.
Discussion
The mechanisms behind vitamin D deficiency in severe obesity and evidence-based corrective actions have not been well-defined. Of particular concern are adolescents who qualify for and elect surgical treatment of their obesity, where subsequent metabolic bone disease may be long-standing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
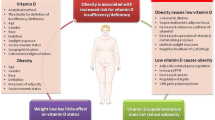
There is a recent consensus that prior definitions of adequate vitamin D status based on the “normal” population distribution of serum 25-hydroxy vitamin D concentration should be redefined because the vitamin concentration will vary with sunlight exposure, geographic location, and level of vitamin D intake [1]. By earlier definitions, serum 25-hydroxy vitamin D < 25 nmol/l was considered deficient and higher levels were considered adequate. These deficiency levels are clearly associated with osteomalacia but vitamin D sufficiency is not guaranteed with slightly higher levels [2]. Because insufficient vitamin D status results in poor calcium absorption, hypocalcemia and compensatory increases in secretion of parathyroid hormone (PTH), recent research has focused on determining the serum 25-hydroxy vitamin D concentrations needed to optimize calcium absorption and suppress PTH secretion, with the aim of supporting optimal bone metabolism [2]. These studies suggest that 25-hydroxy vitamin D concentration ≥80 nmol/l optimizes calcium absorption and suppresses PTH secretion, significantly beyond that seen with 25-hydroxy vitamin D of 50 nmol/l. Thus, for this paper, we accept the definition of true deficiency as <25 nmol/l because of the recognized association with osteomalacia in adults, levels 25–80 as associated with osteoporosis, and optimal vitamin status as ≥80 nmol/l [2]. In light of these changes in interpretation, the data regarding vitamin D status in severe obesity should be reconsidered.
For more than 30 years, vitamin D status has been consistently reported as depressed in patients with obesity [3]. In data representing more than 25,000 individuals from eight nonoverlapping studies with a considerable range of adult ages, races, and ethnic groups, obesity was significantly negatively associated with vitamin D status [4–11]. In postmenopausal women from across the world, obesity was associated with a 2.4-fold greater odds (95% CI = 1.83, 3.14, p < 0.001) of vitamin D insufficiency than nonobese women [9]. Vitamin D concentrations were suboptimal in 80% of obese individuals [5, 12] and associated with secondary hyperparathyroidism [6, 11]. Subjects with dark skin had lower vitamin D concentration than light-skinned subjects [7, 9, 10]. These data suggest that vitamin D deficiency is widespread in subjects at all levels of obesity.
Because the prevalence of obesity has increased dramatically in recent years [13], with a marked increase in severe obesity, any chronic nutritional problems associated with obesity will have great public health significance. While the prevalence of obesity defined as a body mass index (BMI) > 30 kg/m2 doubled between 1986 and 2000, the prevalence of BMI > 40 kg/m2 quadrupled and that of BMI > 50 kg/m2 increased by five times [14]. Because these latter two groups have qualified for obesity surgery since 1991 by NIH guidelines, there has also been an expansion of bariatric procedures. The number of surgeries increased from 13,354 in 1998 to 72,177 in 2002 [15], with low in-hospital complication rates but limited data on long-term outcomes such as vitamin D status and bone disease.
The surgical procedures used most commonly at present to treat severe obesity are of two general types. Most employ a restrictive component, where the size of the gastric pouch is reduced to enhance satiety with a reduced volume of food intake. Purely restrictive procedures are the vertical banded gastroplasty (VBG) and the newer adjustable gastric band (aGB). Resulting weight loss is greater when a procedure that results in malabsorption is added to the gastric restriction as with the gastric bypass (GBP) or the purely malabsorptive biliopancreatic diversion (BPD). Case reports of severe osteomalacia (vitamin D deficiency) at time points distant from VBG [16] and GBP [16–18] suggest that metabolic bone disease is a long-term complication of obesity surgery.
The purpose of this study was to describe current understanding of vitamin D status in patients with severe obesity treated with bariatric surgery, with emphasis on the impact of weight loss surgery on vitamin D and bone mineral status.
Methods
A systematic review of the published medical literature was undertaken, using the Pubmed database with the following search terms in various combinations: ”obesity,” “vitamin D,” “calciferol,” “obesity surgery,” “bone disease,” “osteoporosis,” and “bariatric surgery.” In addition, reference lists of papers obtained were reviewed for additional pertinent references. Since vitamin D assay methods have improved considerably in accuracy over the past 5 years [19, 20], and obesity rates have mushroomed worldwide in the same timeframe, included studies were limited to those published since 2000. Seventeen clinical descriptions of vitamin D status in obesity surgery patients but no epidemiologic studies or randomized trials were found.
The International Quality Assessment Scheme for Vitamin D metabolites was introduced to improve the quality of vitamin D assays [19]. Most assay methods using radioimmunoassay (RIA) or high performance liquid chromatography (HPLC) were within 7% of the all-laboratory trimmed mean value, with the exception of the Nichols Advantage assay that was later removed from the market. Thus, for this paper to ensure comparable data, only vitamin D concentrations from laboratories employing RIA or HPLC, or HPLC with tandem mass spectroscopy (MS) were used in the final descriptive figure.
Results
Vitamin D deficiency was common preoperatively. In 14 reports [20–34] representing data from 1,566 patients, only a single study [23] of 30 patients reported a mean 25-hydroxy vitamin D > 80 nmol/l. This study did not report the assay method for vitamin D, making their data difficult to evaluate. From 33 [34] to 80% [20, 32, 34, 35] of patients were judged vitamin D deficient by the authors. Two studies found BMI to be inversely related to 25-hydroxy vitamin D (R = −0.4, p = <0.01 [34]; R = −0.15, p = 0.012 [20]). Secondary hyperparathyroidism was also described in some patients preoperatively [20, 28].
Vitamin D status was not greatly improved postoperatively. Most studies did not find a significant change in vitamin D status [23, 25–28, 30]. Three studies found the 25-hydroxy vitamin D to be increased significantly but not to optimal levels [20, 24, 32], and parathyroid hormone (PTH) was still elevated in 40% of patients in one study [20] but improved in another study [32]. At 3 years after BPD, 50% of patients had low 25-hydroxy vitamin D, 26% were hypocalcemic and 63% had elevated PTH [36]. A study of patients up to 10 years after BPD found significantly decreased vitamin D concentration and increased secondary hyperparathyroidism over time [29]. No study assayed 25-hydroxy vitamin D by the gold standard HPLC method, but 8 studies used some form of RIA or radioimmunodiagnostics. Figure 1 depicts the reported 25-hydroxy vitamin D concentration from preoperative and postoperative (up to 36 months) after GBP [20, 25, 31, 32, 34].
While current practice includes recommendation for calcium and vitamin D supplements by many surgery groups [20, 26, 29, 31, 33, 36], the dose and/or formulation of these supplements reported thus far has not been adequate to suppress PTH or bone resorption. Only one study found slightly improved vitamin D status after aGB procedure with no supplements [24].
Discussion
Patients who qualify for obesity surgery present with vitamin D insufficiency with many patients frankly deficient and some with secondary hyperparathyroidism. Vitamin D status may then worsen after obesity surgery, even when supplemental calcium and vitamin D are prescribed.
By contrast to many obesity-related comorbid conditions, vitamin D deficiency and secondary hyperparathyroidism are not corrected by the significant weight loss seen after obesity surgery and may even be exacerbated by the accompanying malabsorption. When the limited intake of dietary calcium and vitamin D due to gastric restriction or food intolerance are paired with the malabsorption of GBP, worsening vitamin D deficiency and secondary hyperparathyroidism are not surprising. In fact, malabsorption of calcium from dietary and supplement sources may exacerbate hyperparathyroidism. These effects may be ameliorated by selecting restrictive procedures such as the aGB, where vitamin D concentration increased though not to optimal levels, but data to date only represent 2 years postsurgery in less than 70 patients [24, 30], and so must be considered preliminary.
The cause of the vitamin D deficiency state in obesity is not well-understood. It has been proposed that the low vitamin D status might be due to increased vitamin clearance from serum and enhanced storage of vitamin D by adipose tissue [37, 38], as demonstrated in models of vitamin D deficient nonobese rats after vitamin D repletion [3, 39–41], with vitamin replete rats [42] and at autopsy in humans [43]. In nonobese rats, vitamin D stored in adipose tissue was released during periods of fasting with 15% weight loss, but at a rate far below the release of triglyceride [40]. The authors suggest that this slow release of stored cholecalciferol may be a protection against toxicity of serum 25-hydroxycholecalciferol during times of excess vitamin D storage in adipose tissue, though their model uses pharmacologic doses of vitamin D (50 times usual intake) that may not be applicable to humans. Other possible causes include limited exposure to sunlight and reduced hepatic synthesis of 25-hydroxy vitamin D due to negative feedback from a liver that is functionally impaired due to steatosis [44]. Reduced bioavailability of vitamin D from both cutaneous and dietary sources is also suggested [37]. Another possible mechanism that deserves exploration is the possibility that total body clearance/elimination of vitamin D is increased during the inflammatory conditions of obesity.
Obesity is a proinflammatory condition characterized by the progressive infiltration of adipose tissue by macrophages [45]. Adipocytes secrete more than 50 adipokines including interleukin (IL)-6, visfatin, macrophage chemoattractant protein 1, plasminogen activator inhibitor-1, leptin, adiponectin, adipsin, and acylation stimulating protein. Other metabolic changes in adipose tissue cause release of proinflammatory cytokines such as tumor necrosis factor (TNF)-α and IL-6. The proinflammatory state described with obesity improves with weight loss.
Treatment with vitamin D improves proinflammatory conditions. In a prospective randomized controlled trial in patients with congestive heart failure, daily supplements of 2,000 IU (50 μg) vitamin D3 administered for 9 months resulted in improved serum vitamin D concentration and significant reductions in TNF-α and IL-6 [46]. While this study suggests that vitamin D supplementation may improve inflammatory conditions, similar studies have not yet been reported in patients with obesity.
There is to date no congruence on required vitamin D or calcium intake in patients undergoing obesity surgery. In the studies reviewed for this paper, vitamin D intake up to 20 μg (800 IU) was still associated with secondary hyperparathyroidism [27, 28]. One study suggested that their policy was to give 10,000 μg (400,000 IU) vitamin D2 intramuscularly bimonthly, though vitamin D serum concentration fell steadily after BPD [29] suggesting that patients may not have actually received the injections or that they were not clinically effective. Most studies did not report on adherence to vitamin supplement recommendations, so nonadherence could have been a factor in poor vitamin D status postoperatively. In these published reports of obese patients undergoing bariatric surgery, however, any vitamin D that may have been stored in adipose tissue that could have been released as the considerable weight loss occurred was still not able to suppress secondary hyperparathyroidism or to optimize serum 25-hydroxy vitamin D status to the level of 80 nmol/l. Because obese patients respond to cutaneous production and oral vitamin D less efficiently than optimal weight individuals [37], and because parenteral vitamin D is no longer available, these patients will be left with the challenge of oral vitamin dosing to surpass the limits of their malabsorption. Limited absorption of calcium and phosphorus may also be important after obesity surgery and require supplement use.
There are several limitations in this analysis. A very important one is the fact that none of the studies used the gold standard assay method of HPLC to analyze vitamin D concentration. Several used RIA, which is reportedly within 7% of the all laboratory trimmed mean. Perhaps more concerning, however, is the fact that a number of papers did not report the assay method used at all, and one of those reported levels that were much higher than all other investigators. While the assay methods reflect the realities of clinically available vitamin D assays across geographic regions, the variability in data attained makes the true extent of vitamin deficiency or insufficiency unclear, and yet most surgical groups noted high prevalence of vitamin D deficiency preoperatively.
A second limitation is the sparse available data on vitamin D status in patients with aGB, with none over time periods beyond 2 years. If the promising early data with stable vitamin D status are sustained through 5 to 10 years postoperatively, this procedure might be a good option for weight reduction with less bone loss in deficient or fragile individuals.
While the prevalence of severe obesity has increased most dramatically in Black women [13], and it has become clear that individuals with dark skin have lower vitamin D status [7, 9, 10], these surgical studies to date are limited in representation of Black women. In fact, only a single study reported vitamin D levels preoperatively in 69 Black Americans [20]. Because patterns of bone mineral deposition and loss as well as hyperparathyroidism may be different in Black than White Americans, further studies are needed to clarify the extent of vitamin D deficiency.
Many questions regarding vitamin D status and obesity remain to be clarified if improvement in the outcomes of obesity surgery is to be realized most fully (Table 1). While this review focused on obese adult patients, the answers to these questions will be even more important for the adolescents who have not yet reached maximal height but still qualify for and elect surgical treatment of their obesity and who are likely to live many years after their surgery.
References
Martini LA, Wood RJ. Vitamin D status and the metabolic syndrome. Nutr Rev 2006;64:479–86.
Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr 2004;80:1706–9S.
Rosenstreich SJ, Rich C, Volwiler W. Deposition in and release of vitamin D3 from body fat: evidence for a storage site in the rat. J Clin Invest 1971;50:679–87.
Arunabh S, Pollack S, Yet J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab 2003;88:157–61.
Hypponen E, Power C. Vitamin D status and glucose homeostasis in the 1958 British birth Cohort. Diabetes Care 2006;29:2244–6.
Kamycheva E, Sundsfjord J, Jorde R. FERUM parathyroid hormone level is associated with body mass index. The 5th Tromso Study. Eur J Endocrinol 2004;151:167–72.
Looker AC. Body fat and vitamin D status in black versus white women. J Clin Endocrinol Metab 2005;90:635–40.
Parikh SJ, Edelman M, Uwaifo GI, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab 2004;89:1196–9.
Rizzoli R, Eisman JA, Norquist J, Ljunggren O, Krishnarajah G, Lim S-K. Risk factors for vitamin D inadequacy among women with osteoporosis: an international epidemiological study. Int J Clin Pract 2006;60:1013–9.
Yanoff LB, Parikh SJ, Spitalnik A, et al. The prevalence of hypovitaminosis D and secondary hyperparathyroidism in obese Black Americans. Clin Endocrinol 2006;64:523–9.
Snijder MB, Van Dam RM, Visser M, et al. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab 2005;90:4119–23.
Bischof MG, Heinze G, Vierhapper H. Vitamin D status and its relation to age and body mass index. Horm Res 2006;66:211–5.
Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006;295:1549–55.
Sturm R. Increases in clinically severe obesity in the United States. Arch Intern Med 2003;163:2146–8.
Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA 2005;294:1909–17.
Goldner WS, O’Dorisio TM, Dillon JS, Mason EE. Severe metabolic bone disease as a long-term complication of obesity surgery. Obes Surg 2002;12:685–92.
Collazo-Clavell ML, Jimenez A, Hodgson SF, Sarr MG. Osteomalacia after Roux-en-Y gastric bypass. Endocr Pract 2004;10:287–8.
DePrisco D, Levine SN. Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci 2005;329:57–61.
Carter GD, Carter CR, Gunter E, et al. Measurement of vitamin D metabolites: An international perspective on methodology and clinical interpretation. J Steroid Biochem Mol Biol 2004;90:467–70.
Carlin AM, Rao DS, Yager KM, Genaw JA, Parikh NJ, Szymanski W. Effect of gastric bypass surgery on vitamin D nutritional status. Surg Obes Relat Dis 2006;2:638–42.
Carlin AM, Rao DS, Meslemani AM, et al. Prevalence of vitamin D depletion among morbidly obese patients seeking gastric bypass surgery. Surg Obes Relat Dis 2006;2:98–103.
Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab 2004;89:1061–5.
El-Kadre LJ, Rocha PRS, Tinoco AC, Tinoco RC. Calcium metabolism in pre- and post-menopausal morbidly obese women at baseline and after laparoscopic Roux-en-Y gastric bypass. Obes Surg 2004;14:1062–6.
Giusti V, Gasteyger C, Suter M, Heraief E, Gaillard RC, Burckhardt P. Gastric banding induces negative bone remodeling in the absence of secondary hyperparathyroidism: potential role of serum C telopeptides for follow-up. Int J Obes 2005;29:1429–35.
Goode LR, Brolin RE, Chowdhury HA, Shapses SA. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res 2003;12:40–47.
Hamouri N, Kim K, Anthone G, Crookes PF. The significance of elevated levels of parathyroid hormone in patients with morbid obesity before and after bariatric surgery. Arch Surg 2003;138:891–7.
Johnson JM, Maher JW, Samuel I, Heitsbusen D, Doherty C, Downs RW. Effects of gastric bypass procedures on bone mineral density, calcium, parathyroid hormone and vitamin D. J Gastrointest Surg 2005;9:1106–11.
Johnson JM, Maher JW, DeMaria EJ, Downs RW, Wolfe LG, Kellum KM. The long-term effects of gastric bypass on vitamin D metabolism. Ann Surg 2006;243:701–5.
Manco M, Calvani M, Nanni G, et al. Low 25-hydroxyvitamin D does not affect insulin sensitivity in obesity after bariatric surgery. Obes Res 2005;13:1692–700.
Pugnale N, Giusti V, Suter M, et al. Bone metabolism and risk of secondary hyperparathyroidism in 12 months after gastric banding in obese pre-menopausal women. Int J Obes 2003;27:110–16.
Riedt CS, Brolin RE, Sherrell RM, Field MP, Shapses SA. True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity 2006;14:1950–8.
Sanchez-Hernandez J, Ybarra J, Gich I, et al. Effects of bariatric surgery on vitamin D status and secondary hyperparathyroidism: a prospective study. Obes Res 2005;15:1389–95.
Slater GD, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg 2004;8:48–55.
Ybarra J, Sanchez-Hernandez J Gich I, et al. Unchanged hypovitaminosis D and secondary hyperparathyroidism in morbid obesity after bariatric surgery. Obes Res 2005;15:330–5.
Flancbaum L, Belsley S, Drake V, Colarusso T, Tayler E. Preoperative nutritional status of patients undergoing Roux-en-Y gastric bypass for morbid obesity. J Gastrointest Surg 2006;10:1033–7.
Newbury L, Dolan K, Hatzifotis M, Low N, Fielding G. Calcium and vitamin D depletion and elevated parathyroid hormone following biliopancreatic diversion. Obes Surg 2003;13:893–5.
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690–3.
Rizzoli R, Eisman JA, Norquist J, et al. Risk factors for vitamin D inadequacy among women with osteoporosis: an international epidemiological study. Int J Clin Pract 2006;60:1013–9.
Lorentzon R, Danielson A. The effects of different vitamin D states on intestinal absorption of vitamin D3 and its metabolites in rats. Acta Physiol Scand 1985;123:437–44.
Brouwer DAJ, Van Beek J, Ferwerda H, et al. Rat adipose tissue rapidly accumulates and slowly releases an orally-administered high vitamin D dose. Br J Nutr 1998;79:527–32.
Holman CA, Mawer EB, Smith DJ. Tissue distribution of cholecalciferol (Vitamin D3) in the rat. Proc Biochem Soc 1970;120:29–30P.
Lawson DEM, Sedrani SH, Douglas J. Interrelationships in rats of tissue pools of cholecalciferol and 25-hydroxcholecalciferol formed in UV light. J Biochem 1986;233:535–40.
Mawer EB, Backhouse J Holman, CA, Lumb GA, Stanbury SW. The distribution and storage of vitamin D and its metabolites in human tissues. Clin Sci 1972;43:413–31.
Bell NH, Epstein S, Greene A, Bhary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest 1985;76:370–3.
John BJ, Irukulla S, Abulafi AM, Kumar D, Mendall MA. Systematic review: adipose tissue, obesity and gastrointestinal diseases. Aliment Pharmacol Ther 2006;23:1511–23.
Schleithoff SS, Zitterman A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2006;83:754–9.
Author information
Authors and Affiliations
Corresponding author
Additional information
There is no conflict of interest to report for any of the authors.
Rights and permissions
About this article
Cite this article
Compher, C.W., Badellino, K.O. & Boullata, J.I. Vitamin D and the Bariatric Surgical Patient: A Review. OBES SURG 18, 220–224 (2008). https://doi.org/10.1007/s11695-007-9289-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-007-9289-6