Abstract
Physicochemical properties of giant salamander skin gelatin (GSSG) prepared at 45, 50, 55, 60, 65 °C were studied in comparison with those of tiger puffer skin gelatin (TPSG) and commercial bovine gelatin (CBG). It is showed that GSSG contained lower content of imino acid (179–181 residues/1000) than CBG (204 residues/1000) and TPSG (188 residues/1000). GSSG45 exhibited a protein pattern with α1- and β-chain, which attenuated along with the formation of fragments with molecule weight < 97.2 KD when extraction temperature increased. Amide I and II of GSSG were shifted to higher wavenumber while amide A and B to lower wavenumber in parallel with declined peak (7.3°) intensity by X-ray analysis as temperature increased. Gel strength, gelling temperature and melting temperature of GSSG (47–122 g, 9.30–12.18 °C and 16.87–21.34 °C) were lower than those of CBG (160 g, 22.01 °C and 28.70 °C) and TPSG (151 g, 13.90 °C and 21.34 °C) (p < 0.05). They decreased as temperature elevated in consistent with their gel microstructure with big size voids. These also coincided with their longer relaxation time showed by low field-nuclear magnetic resonance. GSSG50 (62.3 m2/g, 57.5 min) and GSSG55 (55.6 m2/g, 60.8 min) showed higher emulsion activity index and emulsion stability index (p < 0.05) as well as smaller size emulsion droplets than others. Results indicate that GSSG has poor gelling properties, but excellent emulsifying properties. They are greatly affected by extraction temperature. GSSG is proposed to be extracted at a temperature between 45 and 50 °C.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gelatin is a common ingredient used for improving food quality and stability. Most gelatin is produced from mammalian skin or bone. However, animal diseases like bovine spongiform encephalopathy (BSE) and foot and mouth diseases (FMD) cause panic to consumers toward mammalian source gelatin. Religion or cultural might also have some limitation for its application [1, 2]. In contrast, aquatic source gelatin does not have these problems, and to produce gelatin from skin or bone also brings revenue to aquatic food industry.
Properties of gelatin essentially depend on its amino acid composition (AA), protein pattern and functional groups such as –OH or NH2, which could be greatly affected by extraction including pretreatment [3, 4], extraction temperature and time [5, 6]. Different extraction temperatures provide various amount of energy for breaking chemical bonds in collagen, which might lead to different protein patterns and functional groups in final gelatin and might also lead to the involvement of pigments or Maillard reaction products formation. These could provide gelatins with different functional and color properties. Many studies confirmed that gelling properties, emulsifying properties [7, 8] and color properties [6, 9] of fish skin gelatins prepared under different temperature differed.
Chinese giant salamander (Andriasdavidianus) is the largest extant amphibian species in the world [10]. Its intensive farming technologies made great progress in last decade. As a result, production of giant salamander increased sharply in China [11], turning it into a popular delicacy with high economic value. Many featured food products have been developed from its muscle, leaving the skin as a byproduct. As amphibian animal, collagen of giant salamander skin is expected to be different from that of mammals and fish, implying the properties of gelatin from them would be different. There is scarce study providing knowledge of giant salamander skin gelatin (GSSG). This study aimed to understand the extraction temperature effects on physiochemical properties of GSSG so as to facilitate its further exploration. Gelling properties and emulsifying properties of GSSG extracted from different temperatures were evaluated and compared with those of tiger puffer skin gelatin (TPSG) and commercial bovine gelatin (CBG).
Materials and methods
Chemicals and materials
TPSG was prepared using the same method for GSSG extraction as our previous study at 55 °C for 6 h [6]. CBG was purchased from Sangon Biotech Company (Shanghai, China). Skins from Chinese giant salamander with a size of approximate 3.1 ± 0.3 kg was provided by Longtoushan Aquaculture Development Company (Hanzhong, Shaanxi Province, China). Fresh skins were cut into small pieces (0.5 × 0.5 cm2), placed in polyethylene bags and stored at – 20 °C until use.
Gelatin extraction
Non-collagenous proteins in skins were removed by washing with 0.05 M NaOH for 1 h (w/v = 1:10) and the process was repeated one time. Alkali-treated skins were rinsed with deionized water to neutral and soaked using 0.05 M phosphoric acid (2 h, w/v = 1:10). Gelatin was extracted using distilled water at 45, 50, 55, 60 and 65 °C for 6 h. After centrifugation (9000×g for 20 min), supernatant of the extract was collected and lyophilized using a 2KBTES-55 freeze dryer (VirTisCo., Gardiner, NY, USA). GSSG extracted at 45, 50, 55, 60 and 65 °C were named as GSSG45, GSSG50, GSSG55, GSSG60 and GSSG65.
Yield and recovery
Yield and recovery was calculated as Liu et al. [9] using the following equation: Yield (%) = [weight of lyophilized gelatin (g)/weight of initial dry skin (g)] × 100%., in which initial dry skin weight was wet skins dried at 105 °C for 12 h; Recovery (%) = 100% × [Hyp content in dry gelatin (mg/g) × weight of dry gelatin (g)]/[Hyp content in initial dried skin (mg/g) × weight of initial dried skin (g)] × 100%. Hydroxyproline (HYP) content was measured using an A030-3 HYP assay kit (Jiancheng Bioengineering Institute, Nanjing, China) giving result with 2.1% coefficient of variation (CV) and 102% recovery with a lowest detection limit of 0.01 μg/mL. Approximate 50 mg gelatin or dry fish skin was hydrolyzed using 6 M HCl at 100 °C for 5 h. The hydrolyzed samples were incubated with kit solutions and absorbance at 550 nm was measured using a UV-5200 spectrophotometer (Yuanxi Instrument Co., Shanghai, China). HYP content was calculated based on absorbance following kit method.
AA composition
AA composition of gelatin was analyzed as Zhu et al. [12] with modifications. Briefly, samples were hydrolyzed with 6 M HCl at 110 °C for 24 h in a vacuum tube. The hydrolysates were dissolved in water in a volume flask (25 mL) and filtered. Samples were derivatized using Elite-AAK kit and analyzed using a 4.6 × 250 mm C18 column (Elite Analytical Instruments Co., Dalian, China). Results were expressed as number of residues per 1000 residues.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
Protein patterns of gelatin were analyzed using SDS-PAGE. Gelatin was dissolved in buffer (0.5 M Tris–HCl containing 2 g L−1 SDS, 250 g L−1 glycerol and 50 g L−1 β-ME) and incubated in boiling water for 3 min. Protein separation was performed by electrophoresis with a gel composed of 5% stacking gel and 10% running gel at a current of 30 mA using a AE-8135 Mini protein unit (ATTO Corp., Tokyo, Japan). Gel was colored with coomassie blue and destained with methanol and acetic acid.
FTIR and X-ray spectra
Fourier Transform Infrared (FTIR) analysis was performed using a Frontier FTIR/FIR spectrometer (Perkin Elmer Inc., Madison, USA) by the method of Pan et al. [6]. Briefly, dried gelatin powder was mixed with potassium bromide evenly and pressed into a tablet. The spectra in a range of 400–4000 cm−1 were collected and averages for 32 scans at a resolution of 4 cm−1 were. Normalization was done to obtain the same weight so as to enable quantitative comparisons. X-ray analysis was done by the method of Liu et al. [9] using a XRD-7000 X-ray diffractometer (Shimadzu, Tokyo, Japan). Copper Kα was used at 40 kV and 30 mA. The 2q range was set from 5° to 50°
Gel strength and color properties
Gelatin gels were prepared according to the British Standard 757:1975 [13]. Gelatin solution of 66.7 g L−1 was prepared and 120 mL of the solution was placed into a Bloom jar (volume, 150 mL; diameter, 59 mm; height, 85 mm). The samples were stored at 10 °C for 16 h for gel maturation. Gel strength (GS) was measured as the British Standard 757:1975 [13] using a TA.XT.plus texture analyzer (SMS, Surrey, UK) with a load cell of 5 kN equipped with a 1.27 cm diameter flat faced cylindrical Teflon plunger. The maximum force (g) was recorded at a penetration of 4 mm. The speed of the plunger was set at 0.5 mm s−1. Color properties of gel were evaluated using a colorimeter (UltraScan Pro, Hunter Lab Inc., Reston, VA, USA). Gel was wrapped with preservative film, and place on the window of the machine. L*, a* and b* values were recorded.
Gelling temperature (GT) and melting temperature (MT)
GT and MT were determined according to Boran et al. [14] using a Discovery HR-1 rheometer (TA Instruments, New Castle, DE, USA) equipped with a parallel plate with diameter of 35 mm. The gap between them was 1.0 mm. Gelatin solution of 66.7 g L−1 was prepared. The analysis was performed as: scanning rate 0.5 °C min−1, frequency 1 Hz, oscillating applied stress 3 Pa during cooling from 35 to 5 °C and heating from 5 to 35 °C. Temperature, where tan = 1, was calculated as GT or MT.
Microstructure of gel by cryo-scanning electron microscopy
Microstructure of gelatin gel was observed using a cryo-scanning electron microscopy (Cryo-SEM) system composed by a PP3010T cryo-preparation system (Quorum Technologies Ltd, Laughton, UK) and a SU8010 scanning electron microscopy (Hitachi High-Technologies (Shanghai) Co., Shanghai, China). A piece of gel with thickness of 1–2 mm was fixed on a bronze stub and immersed in liquid nitrogen slush prepared under vacuum. The frozen sample was transferred onto a vacuum stage with temperature of – 140 °C immediately and cryo-fractured with an attached knife. The sample was sublimed at – 90 °C for 25 min and sputtered-coated with gold to be conductive. It was transferred onto a stage with temperature of – 140 °C in SEM and observed at an acceleration voltage of 10 kV.
Low field-nuclear magnetic resonance (LF-NMR) analysis of water in gel
Water status in gelatin gel was analyzed as Dong et al. [15] using a MesoMR23-060V-1 NMR (Niumag Electric Corporation, Shanghai, China) equipped with a 0.5 Tesla permanent magnet corresponding to a proton resonance frequency of 23.2 MHz. Gel was measured in a probe at 4 °C for collecting Carr–Purcell–Meiboom–Gill (CPMG) decay signals. Obtained data was analyzed by multi-exponential fitting analysis using attached MultiExpInv analysis software (Niumag Electric Corporation, Shanghai, China). Transverse relaxation times of T21, T22 and T23werecalculated from the peak positions.
Emulsifying properties and microscopic observation of emulsion droplet
Emulsion activity index (EAI) and emulsion stability index (ESI) of gelatin were measured and expressed as Liu et al. [9]. Microscopic observation of emulsion droplet was performed by the method of Abdelmalek et al. [16]. Briefly, mixture of 10 mL soybean oil and 40 mL gelatin solution (1%) was homogenized for 1 min using a homogenizer (Ultraturrax T25 digital, IKA works, Guangzhou, China) at room temperature pH of the mixture was adjusted to 7.0 using 0.2 M NaOH. A drop of emulsions was placed onto a microscope slide, covered with a cover slip and observed using an optical microscope (OLYMPUS-DP72, Olympus Co., Tokyo, Japan).
Statistical analysis
Three independent batch of GSSG for each group were extracted. Analysis of each sample were duplicated. Data were reported as mean ± standard deviation. Comparison of means was done using Duncan’s multiple range tests using the Statistic Package for Social Science (SPSS 16.0, SPSS Inc, Chicago, IL, USA). The significant level was set at p < 0.05.
Results and discussion
Yield and recovery
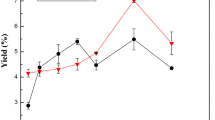
The yield of GSSG was in a range of 33.4–45.2% (Table 1), higher than that of pink perch skin gelatin (27.3%) [17], but lower than that of seabass skin gelatin (51.6–66.4%) [7]. The recovery of GSSG45 was 50.4%, higher than that of channel catfish skin gelatin extracted at 45 °C [18], and it increased to 64.0% in GSSG60, which was higher compared with skin gelatin of chum salmon extracted at 60 °C [9]. Both yield and recovery increased as the elevation of extraction temperature. Covalent and non-covalent bonds vital for stabilizing collagen matrix might be damaged at high temperature, turning triple helix structure into amorphous and facilitating extraction [7]. High temperature might also promote the hydrolysis of amide bonds, contributing to yield increase. Increasing temperature can lead to higher yield, but it could also greatly modify protein pattern and structure of obtained gelatin, affecting its final functionality.
AA composition
Among AA of GSSG (Table 2), glycine occupied the highest proportion (255–257 residues/1000 residues), and it was lower than that of TPSG and CBG (304 and 326 residues/1000 residues). Proline content in GSSG, TPSG and CBG was similar (132–134, 136 and 135 residues/1000 residues). Nevertheless, HYP and imino acid content was lower in GSSG (46–48 and 179–181 residues/1000 residues) than in TPSG (52 and 188 residues/1000 residues) and CBG (69 and 204 residues/1000 residues). In addition, alanine level of GSSG (86–88 residues/1000 residues) was lower than it of TPSG and CBG (111 and 117 residues/1000 residues). Tripeptide Gly-Pro-Y, where the Y position normally occupied by hydroxyproline or alanine [19], plays important role in stabilizing triple helical structure of collagen and its stability has been reported to be proportional to the content of imino acids [16]. Generally, gelatin with high proline, hydroxyproline and alanine content shows better viscoelastic properties than those with low content of them [20]. Above results indicate that the stability of GSSG might be lower than that of CBG and TPSG, which might closely relate with the amphibious living environment and body temperature of giant salamander. GSSG extracted at various temperatures did not show distinct difference in imino acids, different from declined imino acid level due to the involvement of non-collagen protein in chum salmon skin gelatin when extraction temperature increased [9]. Results suggest the purity of obtained GSSG was stable.
It is noted that GSSG contained higher level of leucine, isoleucine, lysine, serine, threonine, tyrosine, valine than TPSG and CBG. The total essential amino acids (EAA) of GSSG (202–204 residues/1000 residues) was much higher than it of the other two (144 and 137 residues/1000 residues). Therefore, GSSG could be considered as good nutritional value protein. Hydrophobic amino acids (HAA) proportion, important to physical properties of gelatin, was lower than it in TPSG and CBG.
Protein pattern
In Fig. 1, TPSG showed clear GSSG45 exhibited clear α1-chain but misty β- and γ-chain. Different from CBG, α1- and α2-chain of TPSG and GSSG45 had lower MW. Thus, three gelatins might have different protein patterns, in accordance with the different living environments of three animals. Since both GSSG55 and TPSG were extracted at 55 °C, the results suggest that the thermal stability of GSSG was lower than it of TPSG.
As temperature increased, band density of α1-, α2- and β-chain in GSSG decreased while more fragments with MW < 97.2 KD showed, especially for GSSG65 whose α1- and α2-chain attenuated distinctly. Pronounced degradation of β-, α1- or α2-chain with simultaneous appearance of low MW peptides have been reported in skin gelatins from clown featherback, bamboo shark and tiger puffer extracted at 65, 60 and 65 °C [6, 8, 21]. High temperature provides a harsh condition that enables the break of inter- or intra-molecular bonds linking collagen chain, forming low MW fragments [22]. Since functional properties of gelatin closely relate with the amount of γ-/α-/β-chains, high MW aggregates and low MW fragments [16], the results imply that extraction temperature could affect GSSG functionalities.
FTIR and X-ray spectra
Amide I band, attributed to C=O stretching vibration coupled with CN stretch and NH bending, reflects the status of triple helix of collagen [23]. It was seen at 655–1656, 1658, 1659–1662, 1659–1662 and 1661–1662 cm−1 in GSSG45, GSSG50, GSSG55, GSSG60 and GSSG65, respectively (Fig. 2a), indicating the loss of triple helix structure as temperature increased. GSSG extracted at high temperature showed many low MW components, in which C=O might be more exposed and reactive [21], leading to the shift to higher wavenumber in amide I. Amide II is originated from out-of-phase combination of CN stretch and in-plane NH deformation of peptide group [23]. It was showed at 1535 and 1534–1536 cm−1 in GSSG45 and GSSG50 but shifted to higher wavenumber in GSSG55, GSSG60 and GSSG65 (1546–1548 cm−1), suggesting the involvement of more NH in hydrogen bonding. Amide III is associated with CN stretching vibrations and NH deformation from amide bonds as well as wagging vibrations of CH2 groups in glycine backbone and proline side-chains [24]. Amide III of GSSG45, GSSG50 and GSSG55 were observed at 1236–1237 cm−1 while it of GSSG60 and GSSG65 were shifted to 1238–1240 cm−1, differed from the stable amide III of TPSG [6]. The amplitudes of amide I, II and III band in GSSG60 and GSSG65 were lower than those in GSSG45, GSSG50 and GSSG55, which could be explained by the loss of triple helix structure and increased disorder [25].
Amide A of GSSG45 was observed at 3355–3358 cm−1, but that of GSSG50, GSSG55, GSSG60 and GSSG65 appeared at lower wavenumber, 3335–3338, 3321–3328, 3323–3327 and 3319–3327 cm−1, respectively. Kaewruang et al. [1] also found a shift to lower wavenumber in amide A when leatherjacket skin gelatin was prepared at high temperature. Amide B band was seen at 2959–2961, 2937–2938, 2934–2938, 2934–2935 and 2927–2929 cm−1 in GSSG45, GSSG50, GSSG55, GSSG60 and GSSG65. Nagarajan et al. [26] reported decreased wavenumber of amide B in squid gelatin extracted with longer time, which could be explained by the interactions of NH3 or CH2 groups between peptide chains. Probably, pronounced degradation of GSSG at high temperature released more free amino to participate in the interactions with reactive groups, resulting decreased wavenumber of amide A and B [27].
Similar as X-ray spectra of skin gelatins from bighead carp [28] and chum salmon [9], two peaks at 7.3° and 21.5° representing triple-helix structure and single left-hand helix chain were observed in GSSG (Fig. 2b). The relative intensity of peak 7.3° was 59% in TPSG and 57% in CBG, and that of GSSG45, 50, 55, 60 and 65 was 57%, 56%, 54%, 48% and 44%, respectively. Intensity of diffraction peak suggests the content of triple helix structure [29]. Therefore, the results indicate more complete triple helix structure in TPSG, CBG than in GSSG45, which is also evidenced by their integrated protein patterns. Decreased peak intensity in GSSG as temperature increased also confirms the loss of triple helix structure. FTIR and X-ray spectra indicate that the secondary structure and functional groups of proteins in GSSG depend much on extraction temperature.
Color properties, GS, GT and MT of gel
In Table 1, most GSSG displayed higher L* value but lower a* and b* value than TPSG and CBG (p < 0.05), suggesting the stronger lightness but weaker redness and yellowness of GSSG. As temperature increased, L* (p < 0.05) and b* value of GSSG increased while a* value decreased, indicating that lightness and yellowness of GSSG were enhanced while redness was attenuated. Enhanced yellowness was also reported in gelatins extracted from seabass skin [7] and puffer skin [6] at high temperature. It is likely that carbonyl groups and free amino groups react to form non-enzymatic browning at high temperature, leading to the increased yellowness [30].
As temperature increased from 45 to 65 °C, GS of GSSG decreased from 137 to 56 g, which were always lower than that of CBG (174 g) and TPSG (159 g) (p < 0.05, Table 1). It could be the low content of imino acids and low MW fragments in GSSG that lead to the low GS. Meanwhile, high temperature might enable the forming of large amount of low MW fragments that could hamper the interaction via inter-junction zone during extraction [31], leading to a low GS. Similar results were found in skin gelatin from clown featherback and tiger puffer [6, 8].
GT (A) and MT (B) of GSSG were in a range of 9.30–12.18 °C and 16.87–21.34 °C, lower than those of TPSG (13.90 °C and 21.34 °C) and CBG (22.01 °C and 28.70 °C) (Fig. 3). Both GT and MT decreased when temperature increased. GT and MT represent the gel forming ability and stability. The results confirm the hypothesis that high temperature led to the formation of low MW fragments (Fig. 1) and affected the secondary structure and functional groups of proteins in GSSG (Fig. 2), which in return hinders the formation of three-dimensional network, lowering gel forming ability and stability [6].
Gel microstructure
Gels prepared from CBG and TPSG showed microstructures with large number of small voids in accordance with their high GS (Fig. 4). Nevertheless, gel from GSSG55 exhibited a matrix with large voids. It is reported that gelatin with high level of hydroxyproline is able to develop strong gel structure due to good hydrogen bond forming ability [20]. TPSG and CBG contained higher content of hydroxyproline than GSSG, which might explain their fine gel microstructures and high GS. On the other hand, protein pattern of GSSG showed more low MW fractions, which might hamper aggregation for network forming [7], leading to a weak gel matrix.
Gel from GSSG45 showed a fine microstructure with small voids. As temperature increased, microstructure of GSSG gel turned coarse with many big voids, coincided with the decreased GS. Similar results were observed in microstructure of gels from skin gelatin of seabass [7] and clown featherback [8] when temperature increased. It is established that gelatin with more high MW peptides (γ-, β-, α1- or α2-chain chains) could develop denser sponge structure than gelatin containing more short chain peptides [32, 33]. Protein pattern of GSSG45 showed clear α1-chains, but these bands faded dramatically as temperature increased, especially for GSSG60. Thus, degradation of these chains into low MW fragments at high temperature hindered the formation of fine microstructure, affecting the final gel properties.
Water in gel
In Fig. 5, three relaxation components, T21, T22 and T23in a range of 2.0–9.3 ms, 10.7–28.5 ms and 231–932 ms were observed in all gels. Compared with CBG and TPSG, longer relaxation time and higher peak area of T21, T22 and T23 were observed in GSSG. Meanwhile, T21 and T23 of GSSG gel exhibited longer relaxation time as temperature increased. T2 relaxation time denotes the status of proton, which is mainly the information of water, in gelatin gel [6]. T21 is ascribed to immobilized water tightly associated with macromolecules while T23 is attributed to trapped water by matrix [34, 35]. Basically, a short relaxation time implies strong water-constraining capability in matrix while a long relaxation time suggests weak water-binding capability [36]. The results indicate that CBG and TPSG gels had higher water binding capacity than GSSG gel, in agreement with their fine microstructure and higher GS. In addition, increased relaxation time also suggests the declined water-binding ability of GSSG extracted at high temperature. Harsh extraction gives more low MW fragments that might hinder the interaction via inner-junction zone, forming a weak network with low water-binding ability and GS [31].
Emulsifying properties
In Fig. 6a, GSSG50 exhibited the highest EAI while GSSG65 showed the lowest EAI (p < 0.05). All GSSG unless GSSG65 showed much higher EAI than CBG and TPSG (p < 0.05), and GSSG50 and GSSG55 also had higher ESI than CBG and TPSG (Fig. 6b), suggesting GSSG had better emulsifying capability and stability than the other two. Short chain peptides with appropriate MW could migrate to interface rapidly and localize at surrounding oil droplets at a fast rate due to good solubility and small size, which could improve emulsifying activity [31]. Further, low MW fragments could provide more charged groups, enhancing electrostatic repulsion to stabilize oil droplets [3]. This is evidenced by more low MW fragments in GSSG than in CBG and TPSG (see Fig. 1). In addition, exposure of nonpolar groups could also facilitate the interactions between peptide chains for stabilizing emulsion droplets. Fairly integrate α1-chain together with more low MW peptides in GSSG50 and GSSG55 might interact with each other to enable the high stability of emulsion droplets. Interestingly, GSSG65 showed poor emulsifying capability and stability. Since it contained huge amount of low MW fragments with charge groups, it might provide too strong repulsion to form elastic film outside oil droplet, resulting in low stability.
In Fig. 6c, GBG and TPSG showed big size droplets while GSSG45 and GSSG50 exhibited small homogenous droplets. Several uneven middle size droplets appeared in GSSG55 and GSSG60, and droplets turned much larger in GSSG65. Increased size implied the coalescence or flocculation of droplets, suggesting the declined emulsion capability. These results are in consistent with the EAI of the gelatins, which confirms excellent emulsifying properties of GSSG and the influence of extraction temperature on it.
Conclusion
GSSG is of high nutritional value with high level of essential amino acid. It contained lower amount of imino acid than CBG and TPSG, which decided its low gel forming ability and stability. However, GSSG50 and GSSG55 had excellent emulsifying activity and stability. Extraction temperature higher than 55 °C generated low MW fragments and modified the secondary structure and functional groups of proteins in GSSG, which hindered the formation gel network, leading to a weak microstructure with low water binding capability and gelling and melting temperature as well as low gel strength. GSSG is proposed to be extracted at a temperature of 45–50 °C for obtaining good functional properties.
References
P. Kaewruang, S. Benjakul, T. Prodpran, S. Nalinanon, Food Biosci. 2, 1–9 (2013)
G. Boran, J.M. Regenstein, J. Food Sci. 74(8), 432–441 (2009)
T. Aewsiri, S. Benjakul, W. Visessanguan, Food Chem. 115(1), 243–249 (2009)
M. Nikoo, S. Benjakul, M. Bashari et al., Food Biosci. 5(5), 19–26 (2014)
I. Kołodziejska, E. Skierka, M. Sadowska, W. Kołodziejski, C. Niecikowska, Food Chem. 107(2), 700–706 (2008)
J. Pan, Q. Li, H. Jia et al., Int. J. Biol. Macromol. 109, 1045–1053 (2018)
S. Sinthusamran, S. Benjakul, H. Kishimura, Food Chem. 152, 276–284 (2014)
P. Kittiphattanabawon, S. Benjakul, S. Sinthusamran, H. Kishimura, LWT - Food Sci. Technol. 66, 186–192 (2016)
Y. Liu, L. Xia, H. Jia et al., J. Sci. Food Agric. 97(15), 5406–5413 (2017)
Z. Qi, Q. Zhang, Z. Wang et al., Vet. Immunol. Immunopathol. 169, 85–95 (2016)
A.A. Cunningham, S.T. Turvey, F. Zhou et al., Oryx 50(2), 265–273 (2015)
B. Zhu, X. Dong, D. Zhou et al., Food Hydrocolloids 28(1), 182–188 (2012)
BSI, Methods for Sampling and Testing Gelatin (Physical and Chemical Methods) (British Standard Institution, BS 757, London, 1975)
G. Boran, S.J. Mulvaney, J.M. Regenstein, J. Food Sci. 75(8), E565–E571 (2010)
X. Dong, Y. Li, Y. Li et al., Food Anal. Methods 10(7), 2207–2216 (2017)
B.E. Abdelmalek, J. Gómez-Estaca, A. Sila et al., LWT - Food Sci. Technol. 65, 924–931 (2016)
J.M. Koli, S. Basu, B.B. Nayak, S.B. Patange, A.U. Pagarkar, V. Gudipati, Food Bioprod. Process. 90(3), 555–562 (2012)
Q. Zhang, Q. Wang, S. Lv et al., Food Biosci. 13, 41–48 (2016)
D.A. Ledward, Gelation of gelatin, in Functional Properties of Food Macromolecules, ed. by J.R. Mitchell, D.A. Ledward (Elsevier Applied Science Publishers, London, 1986), pp. 171–201
M.C. Gómez-Guillén, J. Turnay, M.D. Fernández-Dı́az, N. Ulmo, M.A. Lizarbe, P. Montero, Food Hydrocolloids 16(1), 25–34 (2002)
P. Kittiphattanabawon, S. Benjakul, W. Visessanguan, F. Shahidi, Food Hydrocolloids 24(2–3), 164–171 (2010)
J.H. Muyonga, C.G.B. Cole, K.G. Duodu, Food Chem. 85(1), 81–89 (2004)
J. Bandekar, Biochem. Biophys. Acta 1120(2), 123–143 (1992)
M. Jackson, L.-P.I. Choo, P.H. Watson, W.C. Halliday, H.H. Mantsch, Biochem. Biophys. Acta 1270(1), 1–6 (1995)
J.H. Muyonga, C.G.B. Cole, K.G. Duodu, Food Chem. 86(3), 325–332 (2004)
M. Nagarajan, S. Benjakul, T. Prodpran, P. Songtipya, H. Kishimura, Food Hydrocolloids 29(2), 389–397 (2012)
B.B. Doyle, E.G. Bendit, E.R. Blout, Biopolymers 14(5), 937–957 (1975)
X.-M. Sha, Z.-C. Tu, W. Liu et al., Food Hydrocolloids 36, 173–180 (2014)
A. Bigi, S. Panzavolta, K. Rubini, Biomaterials 25(25), 5675–5680 (2004)
T. Sae-leaw, S. Benjakul, N.M. O'Brien, LWT - Food Sci. Technol. 65, 661–667 (2016)
P. Kaewruang, S. Benjakul, T. Prodpran, Food Chem. 138(2–3), 1431–1437 (2013)
A.M.M. Ali, H. Kishimura, S. Benjakul, Food Hydrocolloids 82, 164–172 (2018)
Z. Khiari, D. Rico, A.B. Martin-Diana, C. Barry-Ryan, J. Mater. Cycles Waste Manag. 19(1), 180–191 (2017)
U. Erikson, I.B. Standal, I.G. Aursand, E. Veliyulin, M. Aursand, Magn. Reson. Chem. 50(7), 471–480 (2012)
H.C. Bertram, A.H. Karlsson, M. Rasmussen, O.D. Pedersen, S. Dønstrup, H.J. Andersen, J. Agric. Food Chem. 49(6), 3092–3100 (2001)
M. Han, P. Wang, X. Xu, G. Zhou, Food Res. Int. 62, 1175–1182 (2014)
Acknowledgements
This work was supported by the National Key R&D Program of China (2016YFD0400700, 2016YFD0400703), Dalian Municipality Youth Science and Technology Star Fund (2018RQ17). J.P. was financially supported by the Ministry of Education, Youth and Sports of the Czech Republic—project CENAKVA (LM2018099), CENAKVA Center Development (CZ.1.05/2.1.00/19.0380) and Biodiversity (CZ.02.1.01/0.0/0.0/16_025/0007370) for analyses in Czech.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pan, J., Lian, H., Shang, M. et al. Physicochemical properties of Chinese giant salamander (Andrias davidianus) skin gelatin as affected by extraction temperature and in comparison with fish and bovine gelatin. Food Measure 14, 2656–2666 (2020). https://doi.org/10.1007/s11694-020-00512-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00512-2