Abstract
Sugarcane juice processing is often accompanied by deterioration of beneficial bioactive compounds and initiation of enzymatic and non-enzymatic browning. Freshly squeezed sugarcane juice was subjected to pressure treatment (300–600 MPa) accompanied by temperatures (30–60 °C) for treatment times of 10–25 min. This study unveiled the potency of high hydrostatic pressure processing (HHP) in reducing the browning index of sugarcane juice by upto 22%, following extraction. The maximum loss of ascorbic acid was reported to be 25% of the initial level, following HHP at 600 MPa/60 °C/25 min. The total phenolics were well retained in the treated juice samples while the antioxidant activity reduced by 10% at 600 MPa/60 °C/25 min. Total destruction of aerobic mesophiles and yeasts and mold was observed at 600 MPa at 30 °C for a treatment time of 20 min. The outcomes of this study unveiled the potency of pressurization in arresting browning, eliminating the microbial load and inhibiting the activity of spoilage enzymes in sugarcane juice in addition to the retention of fresh juice quality. The technology can be adopted as a functional alternative to conventional pasteurization or use of chemical preservatives, generally employed for sugarcane juice processing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fresh sugarcane juice is a very popular beverage in several Asian countries such as India, China, Malaysia and Thailand, mainly due to its refreshing taste and cheap price [1]. The presence of vitamins and minerals made the sugarcane juice a high energy, nutritive drink and is often served as an alternative to refined sugar based drinks [2]. India is the second largest producer of sugarcane in the world, producing about 351.16 MT of sugarcane during the year 2015–2016 [3]. Sugarcane juice contains water (75–85%), non-reducing sugars (10–21%), reducing sugars (0.3–3%), organic substances (0.5–1%), inorganic substances (0.2–0.6%) and nitrogenous substances (0.5–1%) [4]. A 100 mL of the juice contributes 40 kcal of energy, 10 mg of iron and 6 µg of carotene [5]. Hence, there is a high scope for commercialization of sugarcane juice in Indian market as a natural health beverage.
The shelf life of sugarcane juice is limited due to high incidences of microbial contamination and enzymatic reactions, which begins immediately after extraction. Sugarcane juice is highly susceptible to spoilage due to the presence of sugars and high water content [6]. Microorganisms present in the sugarcane juice induces rapid microbial fermentation by conversion of sucrose into organic acid and ethanol, thus imparting sour taste within hours of extraction [7]. Another major problem in sugarcane juice processing is the enzymatic browning due to the activity of polyphenol oxidase and peroxidase [8]. These problems associated with sugarcane juice possess serious challenges in processing and marketing sugarcane based beverages.
Many attempts have been made for stabilization of sugarcane juice either by pasteurization [9] or by the addition of preservatives [10] or by applying hurdle technology [11]. Conventional heat processing of sugarcane juice was sufficient to significantly inactivate the spoilage enzymes in sugarcane juice. However, it was accompanied by the development of undesirable color and jaggery flavor, rendering it unacceptable to the consumers [12]. Application of irradiation in combination with the addition of chemicals had been tried for sugarcane juice preservation [10]. Processing of sugarcane juice with the application of moderate electric field ensured the inactivation of browning enzymes but reported degradation of total flavonoids by upto 40% [13]. Ohmic heating has been a viable technique in reducing the microbial load and enzyme induced changes in the sugarcane juice [14]. However, this technique resulted in loss of ascorbic acid to the tune of upto 33%. Chauhan et al. [3] established the potency of high pressure processing in ensuring the enzymatic degradation and elimination of microbial loads. The high pressure pasteurization of sugarcane juice at 600 MPa for 5 min at ambient temperature has successfully reduced the microbial load and inhibited enzyme activity, while the shelf life of the juice is extended up to 28 days under refrigerated storage [15]. However, no studies have been reported about the associated changes in the bioactive compounds and color of the sugarcane juice, which are significant in ensuring the consumer acceptability of the processed juice.
The U.S. Food and Drug Administration (FDA) have approved the use of high pressure processing as an alternative technology for preservation of food. HPP technology has been successfully applied to fruit juices, jams, jellies, meat products etc. Hence, the present study was conducted to study the effect of high hydrostatic pressure (HHP) processing on the physico-chemical attributes, color and bioactive compounds in the sugarcane juice.
Materials and methods
Sample preparation
Sugarcane (Saccharum officinarum Var. 93A145) was procured from the experimental farm of Agricultural & Food Engineering Department, Indian Institute of Technology Kharagpur, India at a maturity of 10 months. The canes were manually peeled, washed thoroughly with sodium hypochlorite solution and then with fresh water and crushed immediately with laboratory model sugarcane crusher (Make: SGK Industries, Hyderabad). The juice was then filtered using 3-layered muslin cloth for clarification and vacuum packed in low-density polyethylene (LDPE, 80 ± 2 µm) films in packets of 50 mL for HPP treatments. The juice samples were double packed just before the high pressure treatment.
High pressure treatment of sugarcane juice
A full factorial design was used in the study with three independent variables; pressure (P, MPa), temperature (T, °C) and treatment time (t, min). Selected pressure levels were 300, 400, 500 and 600 MPa, whereas the process temperatures were 30, 40, 50 and 60 °C. Their combinations (4 × 4 × 5) were investigated for each response according to the experimental design. The processing time for the pressurization ranged from 10 to 25 min. The packed sugarcane juice samples were immediately pressurized in a batch model high pressure processing system (Model: S-IL-100-250-09-W; Make: Stansted Fluid Power Systems, U.K) having 2-L capacity cylindrical vessel with 100 mm inner diameter and 250 mm depth. The pressure transmitting fluid used was 30% aqueous monopropylene glycol. The target temperature near the sample was maintained by circulating fluid in the jacket surrounding the vessel. The temperature of the immersion medium was measured by a thermocouple and change in temperature of the immersion medium during pressurization was recorded using a programmable logic controller (PLC). A Supervisory Control and Data Acquisition (SCADA) computer was provided which registers all data viz. batch number, process parameters, errors observed, etc. All the samples treated at different parameters combination were cooled using ice bath to avoid post processing effects; analysed for quality attributes and compared with their untreated counterparts. Samples were processed in duplicate and analysed in triplicate (N = 3 × 2 = 6).
Measurement of physico-chemical properties and color of sugarcane juice
The pH, titratable acidity (TA) and total soluble solids (TSS) content of sugarcane juice were determined using the standard methods [16]. The color of the sample is expressed in CIE scale (Commission Internationale de I’Echairages) in terms of L*, a* and b*, where L* denotes Lightness, a* indicates red/green value and b* denotes yellow/blue value. The color values of the sugarcane juice sample were estimated using portable colorimeter (Model: Spectro-guide 45/0 gloss; Make: BYK Gardner, Geretsried, Germany) calibrated against a white and black tile. The overall color difference was computed by total color change (∆E*) as calculated by Eq. (1) [17].
where, \(\Delta L^*{\text{ }}={\text{ }}\left( {{L_{\text{1}}}^* - {L_0}} \right){\text{ }}\Delta a^*{\text{ }}={\text{ }}\left( {{a_{\text{1}}}^* - {a_0}} \right){\text{ }}\Delta b^*{\text{ }}={\text{ }}\left( {{b_{\text{1}}}^* - {b_0}} \right)\)
Whereas, subscript ‘0’ depicts the color value for the reference sample and subscript ‘1’ depicts the color value for the sample being analysed.
The browning index (BI), which is the degree of brown color purity, is a common indicator of browning in food products containing large amount of sugar [18]. The browning index of the sugarcane juice was determined using the Eq. (2) given below [17].
Measurement of ascorbic acid (AA) content
The ascorbic acid (AA, mg/100 mL) content of the sample was determined by visual titration with standardized 2,6-Dichlorophenol-Indophenol dye solution [16]. In brief, the juice sample was dissolved in 3% metaphosphoric acid (HPO3) and 10 mL of this aliquot was titrated against the standardized dye till a pink end point, persisting for more than 15 s.
Measurement of total phenolic content
The ethanolic extract of the juice samples was used for the analysis of total phenolic content (TPC) and antioxidant capacity. The extract was prepared by shaking with 20 mL of 80% ethanol in distilled water for 3 h at ambient temperature (27 ± 1 °C) followed by filtration [16]. TPC in the sample extract was determined by the Folin–Ciocalteu method (FCR Assay) [17]. The TPC values were expressed as Gallic Acid Equivalent (GAE)/100 mL of juice.
Measurement of antioxidant capacity
The antioxidant capacity of the ethanolic extract was measured in terms of its DPPH radical scavenging ability. Fresh DPPH solution was prepared by dissolving 15 mg DPPH in 100 mL methanol. Its absorbance at 517 nm was adjusted, and kept in the dark at − 20 °C until further use. The change in colour of the DPPH solution from purple to yellow, due to the addition of different amounts gallic acid (GA) standards (20–200 µL) on ethanolic extract of juice sample was measured at 517 nm after allowing the solution to stand in dark for 30 min at room temperature. The decrease in absorbance of DPPH after 30 min was calculated and expressed as mg of gallic acid equivalent antioxidant capacity (GAEAC)/100 mL of fresh juice sample [17].
Microbial analysis
The microbial analysis of sugarcane juice was evaluated for aerobic mesophiles (AM), yeasts and mold (YM) and total coliforms (TC) counts using the serial dilution spread plate method according to [19]. The nutrient agar, yeast and mold agar and the violet red bile agar were used for as the growth media for the AM, YM and TC, respectively. All the media were procured from HIMEDIA®, India (HiMedia Laboratories Pvt. Ltd., India). The corresponding incubation conditions for the microbial growth (AM, YM and TC) were 37 °C/24–36 h, 37 °C/18–24 h and 30 °C/5–6 days, respectively. The count was taken as CFU/mL of sample and the detection limit was set at 1 log CFU/mL. All the samples were analysed in duplicate and the mean (n = 4) data expressed as log10 CFU/mL of the sample.
Enzyme activity estimation
The estimation of polyphenol oxidase (PPO) and peroxidase (POD) activities was done using the extract obtained from the sample. The extraction solution was prepared by the method suggested by Swami et al. [20]. PPO assay was performed by adding 1.55 mL of 0.05 M potassium phosphate buffer (pH 7.0) to a mixture containing 1.25 mL of 0.1 M catechol and 200 µL of sugarcane juice extract. The absorbance of the assay mixture was measured at 420 nm for 10 min using UV–Visible spectrophotometer (Model: UV1700; Make: Shimadzu Corp, Kyoto, Japan). The rate of reaction is calculated from the linear slope of activity curves.
POD assay was performed by adding 2.42 mL of 0.05 M potassium phosphate buffer (pH 7.0) to a mixture containing 320 µL of 5% catechol, 160 µL of 0.5% H2O2 and 100 µL of enzyme extract. The absorbance of the mixture was measured for 10 min at 420 nm and percent inactivation of POD was calculated similar to PPO inactivation calculations.
Statistical analysis
Significance tests viz. Fisher’s least significance difference (LSD) and Tukey’s HSD test were carried out at 95% confidence interval for all the attributes in SPSS software version 2.0 (IBM SPSS Statistics, USA). The principal component analysis (PCA) was performed in Origin 2017 (OriginLab Corporation, Northampton, USA).
Results and discussion
Effect of HHP on pH, TSS and titratable acidity
The initial quality attributes of fresh sugarcane juice have been tabulated in Table 1. The pH and titratable acidity (TA) (data not presented) of the sugarcane juice did not show significant variations under all combinations of P–T–t. The °Brix values in the treated samples slightly increased from 19.7 to 23.1 (data not given) with increase in treatment pressure. However, this variation was observed to be statistically insignificant (p < 0.05). This could be attributed to the nondestructive nature of pressure, ensuring the least possibility of the covalent bond breakage within the juice medium [21].
Effect of HHP on color and browning index
The ∆E* values under combined pressure and temperature for the treated samples varied from 0.25 to 5.74 (Table 2). The ∆E* values was observed to increase significantly with increase in temperature and treatment time. Pressurization at lower temperatures (30 and 40 °C) resulted in “noticeable” color change (∆E* < 2.5). With the increase in temperature (50 and 60 °C) and pressure (500 and 600 MPa), the color change was observed to be “well visible” (3.0 < ∆E* < 6.0) [22]. However, the difference in color could be superior or inferior based on the color of reference product (Fig. 1). In case of sugarcane juice this was verified using the BI values.
The BI of the HHP processed sugarcane juice at 30 and 60 °C has been depicted in Fig. 2. The BI of the pressure treated sugarcane juice was significantly lesser than the freshly squeezed juice. The effect was more perceivable at 600 MPa/60 °C/25 min when the BI values of the sugarcane juice reduced by upto 22% in comparison to the fresh juice. The Maillard reaction is one of the most prominent cause of browning in fruit juices containing low ascorbic acid contents [23]. However, retarding effect of pressure on the Maillard reaction at high processing temperatures have also been reported and validated by several authors [24]. While pressure favours the first stage of Maillard reaction involving the formation of the amadori rearrangement products (ARP), it strongly supress further production of the intermediate and brown polymers (2nd stage). This effect is more prominent at pH below 7 [25, 26]. Previous studies have also reported the efficacy of elevated pressures in reducing the browning degree in watermelon juice [27] and grape juice [28]. The HHP processed sugarcane juice appeared brighter than the fresh juice presumably due to the expulsion of air from the tissues due to cell disruption resulting in increased lightness [29].
Effect of HHP on bioactive components
Ascorbic acid
Changes in the ascorbic acid (AA) content is an indicator of the extent of quality changes induced during the processing and storage of fruit products [30]. The AA content of the fresh sugarcane juice was 1.78 ± 0.25 mg/100 mL, whereas in HPP treated samples it varied from 1.33 to 1.74 mg/100 mL. The percentage loss of AA within the entire treatment domain ranged from 2.2 to 24.8% (Fig. 3). The pressure tends to have a stabilizing effect on the AA content in sugarcane juice as indicated by a loss of less than 5% at 300–600 MPa/40 °C. However, at constant level of pressure, increasing the temperature further accelerated the AA degradation indicating the temperature sensitivity of AA. For instance, the HPP treated sample retained 96.7% of AA at 600 MPa/30 °C/25 min, whereas, the corresponding value for 600 MPa/60 °C/25 min is 75.2%. Few studies have suggested that during combined HP/temperature processing of fruit juices, the AA content is significantly affected by the intensity of temperature applied [31, 32]. Similar percentages of reduction in AA has been reported in HPP treated pineapple puree [17] and tomato puree [32].
The stability of AA in the HPP treated fruit juices depends on several factors such as oxygen availability, temperature, light, pH, metal catalysts or ions, the presence of other antioxidants and reducing agents [33]. In the presence of oxygen and at optimum temperature, the AA undergoes oxidation, mostly through the aerobic pathway. Application of temperatures above 50 °C have been shown to affect the functionality of the ascorbic acid to some extent. The AA has also been found to decrease with time due to possible exposure to the heat stresses for prolonged duration. The AA degradation process is further aggravated by the presence of metal ions or chelating agents in the fruit juices produced during pressurization [30]. The detrimental effect of increasing the pressure/temperature/time combination on the AA stability has been elucidated by Taoukis et al. [34]. Perceptible reduction of AA levels has been reported in low acid foods (pH > 4.0) than in high acid foods (pH < 4.0) which further explains the losses induced in the AA content of sugarcane juice (pH 5.1) during pressurization [35].
Total phenolic content
The TPC content in pressure treated sugar cane juice varied between 30.2 and 35.2 mg GAE/100 mL of juice (Table 3). The data tabulated in Table 3 implies that increasing the pressure levels at ambient temperature did not affect the TPC content in sugarcane juice. However, with an increase in temperature (up to 50 °C) and pressure (300–600 MPa) combinedly, there is a significant increase in TPC of juice samples compared with fresh juice. A maximum increase of 10.6% in TPC was obtained at 600 MPa/50 °C/20 min. This could be attributed to higher extractability of some antioxidant compounds at elevated pressure levels due to the disruption of the cell walls [36]. A similar increase of total phenolic content (9.8%) in strawberry puree was observed following pressurization at 600 MPa [37].
From the Table 3, it can be observed that, above 50 °C, total phenolic content in the juice samples decreased significantly in the pressure range of 300–600 MPa. A maximum 5% loss in TPC was observed at 600 MPa/60 °C/25 min. High pressure increase the bio-extractability of phenolic compounds, which are then readily exposed to thermal degradation at high temperature conditions (> 50 °C). A similar trend was also reported in litchi based mixed fruit beverage [38].
Antioxidant capacity
The total antioxidant capacity (TAC) of the fresh sugarcane juice was 4.31 mg GAEAC/100 mL where as in the HPP treated samples, it varied from 3.87 to 4.25 mg GAEAC/100 mL. The antioxidant capacity of the juice was well retained in HPP samples up to 40 °C, at pressure levels of 300, 400, 500 and 600 MPa (Table 4). Retention of antioxidants at mild temperatures (< 40 °C) was reported for tomato puree [39], supporting the data obtained in our study. Huang et al. [15] observed a 10% increase in the TAC of sugarcane juice processed at 600 MPa/30 °C. However, pressurization above 40 °C resulted in a significant loss of TAC in the samples. A maximum loss of 10% was observed at 600 MPa/60 °C/25 min. Upto 18% reduction in the TAC has been reported in mango pulp following processing at 400 MPa//59 °C/16 min [40]. The decrease of total antioxidant capacity during HPP could be directly correlated to AA degradation, as the AA is the main antioxidant compound found in fruit juices [41].
Effect of HHP on microorganisms
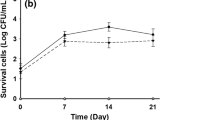
The microbial spoilage in sugarcane juice is accelerated by the aerobic mesophiles, yeasts and mold population, which thrives on the high sugar content in the juice and deters the juice quality. HPP has shown to be highly effective in reducing the microbial load in the treated juice The initial AM, YM and TC population of the fresh sugarcane juice were 6.5, 5.7 and 5.5 log CFU/mL, respectively. The TC were undetectable at pressurization levels as low as 300 MPa (Data not shown). The population of AM and YM were reduced by more than 5 log cycles at 600 MPa/30 °C/20 min ensuring the microbial safety of the juice (Fig. 4). These observations were in agreement with that reported by Chauhan et al. [3]. Among the three micro-organisms studied the coliforms exhibited least resistance to pressure treatment and yeasts and mold showed maximum resistance. Similar results have been previously reported by Huang et al. [15] for high pressure processed sugarcane juice.
The microbicidal effects of high pressure processing could be the outcome of one or more of the following factors, such as, (1) inactivation of proteins/intracellular enzymes (metabolic pathways) (2) cell membrane damage (3) disintegration of ribosomes and/or (4) intracellular pH changes. These effects are often enhanced with increase in processing temperature or decrease in pH (< 4.0) [42].
Effect of HHP on spoilage enzymes
The effect of combined high pressure–temperature treatment on the percentage inactivation of PPO and POD was shown in Fig. 5. The percentage inactivation of PPO ranged from 35% at mild treatment condition of 300 MPa/30 °C/10 min to 92% under extreme condition of 600 MPa /60 °C/25 min. PPO residual activity of 7–11% is sufficient to prevent enzymatic browning during the refrigerated storage of sugarcane juice [43]. This is easily achieved by the combined high pressure–temperature treatment (600 MPa/60 °C/15 min). The efficacy of HHP treatment in inhibiting PPO activity in sugarcane juice had been previously reported [3]. The POD has been inactivated to upto 84% under the intense treatment condition of 600 MPa /60 °C/25 min, which indicates the greater baro-resistance of POD against than that of PPO. It is necessary to point out that in spite of retention of significant enzyme activity than PPO, POD contributes less to discoloration of sugarcane juice under normal conditions, probably due to insufficient levels of hydrogen peroxide [8]. However, inactivation of both the enzymes has to be assured to preserve the appearance and sensory quality of the sugarcane juice.
Principal component analysis
PCA is a highly effective tool in visualizing high dimensional data and grouping correlated variables. The dimensions for nine different parameters have been reduced to two components through the application of PCA (Fig. 6). In the biplot, the principal component 1 (PC1) accounted for 59.5% variation while the PC2 explained 18.13% variability in data. The birotated space plot in Fig. 6 clearly indicates the relationships between various responses and the variations in the experimental conditions. The log cycle reduction in YM, TP, PPO and POD inactivation are heavily loaded on the positive side of the PC1 axis. The AA, TAC and TPC contents are grouped together on the positive side of the PC2 axis. However, the TPC seems to be scattered from the other two responses (AA and TAC). The BI and ΔE* values were scattered in opposite quadrants which could possibly be explained by the opposite trends observed in both attributes, respectively. The correlation matrix illustrates a strong positive correlation between the TAC and AA indicative of the fact that AA contributes to the maximum TAC activity (93%) in sugarcane juice following HPP treatment presumably due to the high scavenging capacity of the latter (Table 5). The PCA plot also suggested the variations in the effects of different treatment conditions employed. It has to be pointed out that all treatment conditions form a cluster on the positive side of PC1 as well as PC2. However, pressurization at 60 °C produced an effect different from all other temperature levels as indicated by the scattered points on the positive side of the PC1 axis.
Biplot obtained after the principal component analysis of the variables in the planes defined by PC1 and PC2 (PC principal component). (PPO polyphenol oxidase, POD peroxidase, E total color difference, BI browning index, TPC total phenol content, TAC antioxidant activity, AA ascorbic acid, AM aerobic mesophiles, YM yeast and mold count)
Conclusions
The findings of this study suggests that HHP is highly effective in reducing the browning of sugarcane juice, which may enhance its consumer acceptability. The pH, TSS and TA of the juice were not significantly affected by high pressure treatment. Besides demonstrating stability of ascorbic acid contents, the HHP treatment retained the total phenolics as well as the anti-oxidant capacity of the sugarcane juice even at temperature levels of 50 °C. The microbial safety of the juice was attained by pressurisation at ambient temperature while the spoilage enzymes (PPO and POD) were substantially inhibited at 600 MPa/60 °C/25 min. PCA analysis revealed a very high correlation between TAC and AA. The study supplements the research conducted on the efficacy of high pressure treatment in processing and preservation of sugarcane juice. The future work will concentrate the stability of HHP treated sugarcane juice during storage.
References
S. Singh, K. Gaikwad, P.K. Omre, Int. J. Agric. Eng. 7, 259 (2014)
H.Y.M. Qudsieh, Y. Salmah, O. Azizah, A.R. Russly, Food Chem. 75, 131 (2001)
O.P. Chauhan, N. Ravi, N. Roopa, S. Kumar, P.S. Raju, J. Food Sci. Technol. 54, 4135 (2017)
M.S. Swaminathan, Food Science, Chemistry and Experimental Foods (Bangalore Print & Publishing Company, Bengaluru, 1987)
K. Parvathy, Proceedings of the scheme for studies on postharvest technology (ICAR), 13, (1983)
T. Krishnakumar, C. Devadas, Beverage Food World 33, 82 (2006)
S. Yusof, L. Shian, A. Osman, Food Chem. 68, 395 (2000)
C.S. Bucheli, S.P. Robinson, J. Agric. Food Chem. 42, 257 (1994)
O.P. Chauhan, D. Singh, S.M. Tyagi, D.K. Balyan, Int. J. Food Prop. 5, 217 (2002)
B.B. Mishra, S. Gautam, A. Sharma, J. Food Sci. 76, M573 (2011)
S. Sankhla, A. Chaturvedi, A. Kuna, K. Dhanlakshmi, Sugar Technol. 14, 26 (2012)
C. Sivasubramanian, J. Pai, Indian Food Packer 48, 51 (1994)
B. Brochier, G.D. Mercali, L.D.F. Marczak, LWT Food Sci. Technol. 74, 396 (2016)
J. Saxena, H.A. Makroo, B. Srivastava, J. Food Process. Eng. 40, e12485 (2017)
H.-W. Huang, Y.H. Chang, C.-Y. Wang, Food Bioprocess Technol. 8, 2483 (2015)
S. Ranganna, Handbook of Analysis and Quality Control for Fruit and Vegetable Products (Tata McGraw-Hill Education, New York, 1986)
S. Chakraborty, P.S. Rao, H.N. Mishra, Innovative Food Sci. Emerg. Technol. 28, 10 (2015)
M.D.P. Buera, R. Lozano, C. Petriella, Die Farbe 32, 318 (1986)
A.P.H. Association, Compendium of Methods for the Microbiological Examination of Foods (APHA, Washington, D.C., 1976)
N.R.S. Hulle, S. Chakraborty, P.S. Rao, Innovative Food Sci. Emerg. Technol. 40, 68 (2017)
X. Chen, W. Qin, L. Ma, F. Xu, P. Jin, Y. Zheng, LWT Food Sci. Technol. 62, 927 (2015)
Z. Cserhalmi, Á Sass-Kiss, M. Tóth-Markus, N. Lechner, Innovative Food Sci. Emerg. Technol. 7, 49 (2006)
N. Babsky, J. Toribio, J. Lozano, J. Food Sci. 51, 564 (1986)
G.A. Ruiz, B. Xi, M. Minor, G. Sala, M. van Boekel, V. Fogliano, M. Stieger, J. Agric. Food Chem. 64, 7208 (2016)
H. Jaeger, A. Janositz, D. Knorr, Pathol. Biol. 58, 207 (2010)
F.J. Moreno, E. Molina, A. Olano, R. López-Fandiño, J. Agric. Food Chem. 51, 394 (2003)
C. Zhang, B. Trierweiler, W. Li, P. Butz, Y. Xu, C.E. Rüfer, Y. Ma, X. Zhao, Food Chem. 126, 254 (2011)
M. Castellari, L. Matricardi, G. Arfelli, G. Carpi, S. Galassi, Rev. de Agaroquimica y Tecnol. de Alimentos 6, 17 (2000)
N. Kaushik, B.P. Kaur, P.S. Rao, Food Sci. Technol. Int. 20, 527 (2014)
S. Tewari, R. Sehrawat, P.K. Nema, B.P. Kaur, J. Food Biochem. 41 (2017)
C. Sánchez-Moreno, L. Plaza, B. de Ancos, M.P. Cano, J. Agric. Food Chem. 51, 647 (2003)
C. Sánchez-Moreno, L. Plaza, B. De Ancos, M.P. Cano, J. Sci. Food Agric. 86, 171 (2006)
M.J. Rodríguez-Roque, B. de Ancos, C. Sánchez-Moreno, M.P. Cano, P. Elez-Martínez, O. Martín-Belloso, J. Funct. Foods 14, 33 (2015)
P. Taoukis, P. Panagiotidis, N. Stoforos, P. Butz, H. Fister, B. Tauscher, High Pressure Food Science, Bioscience and Chemistry (Elsevier, New York, 1998), p. 310
T. Moura, D. Gaudy, M. Jacob, G. Cassanas, Pharm. Acta Helv. 69, 77 (1994)
M. Corrales, S. Toepfl, P. Butz, D. Knorr, B. Tauscher, Innovative Food Sci. Emerg. Technol. 9, 85 (2008)
M.J. Esteve, A. Frigola, I. Citrus, Tree Foresting Sci. Biotechnol. 2, 128 (2008)
L.E. Jayachandran, S. Chakraborty, P.S. Rao, Innovative Food Sci. Emerg. Technol. 28, 1 (2015)
A. Patras, N.P. Brunton, S. Da Pieve, F. Butler, Innovative Food Sci. Emerg. Technol. 10, 308 (2009)
M. Camiro-Cabrera, Z. Escobedo-Avellaneda, B. Salinas-Roca, O. Martín-Belloso, J. Welti-Chanes, Food Bioprocess. Technol. 10, 639 (2017)
B. Júnior, J.F.R.D. Alvarenga, A. Rosenthal, M.C.M.D. Silva, J. Food Process. Technol. 6, 1 (2015)
D. Knorr, K. Reineke, A. Mathys, V. Heinz, R. Buckow, Food Engineering Interfaces (Springer, New York, 2010), p. 325
L.C. Mao, Y.Q. Xu, F. Que, Food Chem. 104, 740 (2007)
Acknowledgements
The first author expresses gratitude to the Acharya N.G. Ranga Agricultural University, A.P, India, for providing sponsorship during the research work. Instrumental facilities and logistics provided by Indian Institute of Technology are highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The manuscript has no conflict of interest and has not been considered for publication elsewhere
Rights and permissions
About this article
Cite this article
Sreedevi, P., Jayachandran, L.E. & Rao, P.S. Browning and bioactive composition of sugarcane juice (Saccharum officinarum) as affected by high hydrostatic pressure processing. Food Measure 12, 1962–1971 (2018). https://doi.org/10.1007/s11694-018-9811-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-018-9811-7