Abstract
Mango (M. indica cv. Datainong) fruit quality rapid determination method based on electronic nose (E-nose) was investigated in this research. E-nose responses to mangoes stored at room temperature were examined for 9 days. Meanwhile, physicochemical and microbiological indexes including firmness, weight loss, surface colour, yellowing rate, pH, total soluble solids, polyphenol oxidase activity and total viable counts were measured to provide quality references for E-nose analysis. Principal component analysis (PCA) and stochastic resonance (SR) were utilized for E-nose data analysis. Results indicated that mango fruit quality decreased sharply during storage. PCA just allowed qualitative quality discrimination, while SNR spectrum using eigen values successfully characterized mango quality. Mango quality predictive model was developed by linear fitting SR eigen values. Validation experiment results demonstrated that the forecasting accuracy of the developed model reached 90%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mango (M. indica cv. Datainong) is an economically fruit that is mainly cultivated in the tropical regions of the world. Due to the abundant nutrients, delicious taste and attractive odor [1, 2], mango is favored by consumers worldwide. However, mango fruit has short shelf life after post-harvest and its quality decreases quickly, as a result of both enzymatic activity and microbial metabolism [3, 4]. Although many efforts, including oxalate treatment [5], bentonite/potassium sorbate coating [6], and pre-harvest sprays of hexanal formulation [7], have been made on mango quality preservation, there are few researches reported on mango fruit quality rapid determination.
Physicochemical examination such as firmness and total soluble solids (TSS) has been commonly utilized for fruit quality analysis. But this method comprises a series of fuzzy operation procedures, which is time-consuming and destructive. Human sensory evaluation provides a non-destructive way for sample analysis. However, the results are often influenced by some human factors, including individual preferences [8]. Recently, much attention has been paid on food quality analysis by instrumental analysis such as gas chromatography (GC), gas chromatography coupled with mass spectrometry (GC/MS), and high performance liquid chromatography (HPLC). Although instrumental analysis can realize computerized control and thereby improve efficiency, the high cost and large-scale equipment makes it not suitable for wide and on-line measurement [9]. To overcome these shortages, researchers have attempted to introduce visible/near infrared (Vis/NIR) spectroscopy into the food field for quality analysis. Vis/NIR spectroscopy allows quick response and non-invasive measurement, but feature data extraction and analysis is still a challenging task [10].
Electronic nose (E-nose) is an electronic olfactory sensing system that chooses volatile gases emitted by samples as analyzing targets [11]. E-nose has a specific sensor array with overlapping sensitivity. Usually, it just takes 2–3 min for one experiment [12], and the whole process can be controlled by a self-made software. After one sampling, a characteristic signal forms and can then be identified by proper pattern recognition algorithms [13, 14]. Due to the advantages including freedom of sample treatment, quick response, and low cost, E-nose has been properly utilized in diverse research fields, particularly in food analysis, including meat products [15, 16], grains [17, 18], milk [19, 20], and oils [21, 22]. Also, the usage of E-nose coupled with chemometrics in fruit quality analysis has been reported. Pan et al. successfully classified strawberry fruits with different decay state and infection type using E-nose combined with PCA [23]. As reported by Wang et al., apple juice spoilage induced by Zygosaccharomyces rouxii was accurately predicted by E-nose combined with partial least squares (PLS) regression [24]. However, there is little information on mango fruit quality rapid determination based on E-nose.
Here, E-nose based mango fruit quality rapid determination was investigated. E-nose responses to mangoes stored at room temperature were examined for 9 days. Meanwhile, physicochemical and microbiological indexes including firmness, weight loss, surface colour, yellowing rate, pH, TSS, polyphenol oxidase (PPO) activity and total viable counts (TVC) were measured to provide quality references for E-nose analysis. Principal component analysis (PCA) and stochastic resonance (SR) were utilized for E-nose data analysis. The relationship between fruit quality indexes and E-nose SR eigen values was modeled. Mango fruit quality predictive model was developed by utilizing SR eigen values and its forecasting accuracy was evaluated.
Materials and methods
Raw materials
Mango samples were purchased from a local fruit market. Samples from the same batch with uniformity of quality (including size, weight and ripening degree) were chosen and transported to the laboratory within an hour. Then mangoes without any mechanical damage were selected for the experiments, and stored at room temperature for 9 days. Each day, 45 samples were randomly taken out for physicochemical, microbiological, and E-nose measurement (five samples for each measurement). In validating experiments, 24 new samples were stored under the same condition.
Physicochemical examination
TA.XT2i Texture Analyzer (Stable Micro Systems, UK) was used for firmness analysis. Flat cylindrical probe p/5 (5 mm in diameter) was set and TPA mode was accepted. Speed in pre- and after-measurement was 3 mm/s. Measurement speed was 1 mm/s. Compression degree was 50%. Residence time interval was 5 s. Load probe type was Auto-0.2 g. Data collection range was 200. Weight loss was measured by Mettler Toledo AL104 electronic balance. Weight loss was calculated as the percentage of weight reduction with respect to the initial weight.
Surface colour was determined by a chromatic meter (TES-135, Taiwan Taishi Electronic Technology Co., Ltd.) and recorded as L*, a*, and b* as CIELab coordinates. Parameters of L*, a*, and b* indicate the lightness (the scale range of 0–100 points from black to white), red (+) or green (–), and yellow (+) or blue (–), respectively. The hue angle (h°), chroma (C), and BI were calculated using the following equations:
Yellowing rate was calculated by the percentage between yellowed samples and all samples. If the color of mango samples appeared light yellow, yellow, and dark yellow, this sample was counted into yellowed samples. In pH measurement, minced mango samples (10 g) were homogenized with 90 ml deionized water and the mixture was filtered. After 30 min, pH of the filtrate were measured using a digital 320 pH metre (Hangzhou Leici Analytical Instruments Company, China). Total soluble solids (TSS) content is one of the most important internal properties that influence the consumer purchasing choice of fresh mango. It is also a key parameter in mango maturity assessment and determination of harvest time.
TSS was determined following the spectral measurement using traditional destructive tests as a reference, according to China standard protocol NY/T 2637-2014 [25]. 250 g of fruit was accurately weighed and then minced by hand with a knife. Mango juice was squeezed using a manual fruit squeezer from the same position with spectra acquisition. The fruit juice was collected for TSS measurement. TSS of the juice was recorded with a temperature correction digital refractometer (WZ113/ATC, China) and expressed in % at 20 °C. PPO was determined by the method presented by Wang et al. [26]. 0.5 ml of enzyme extract was incubated to 2.5 ml of buffered substrate (100 mM sodium phosphate, pH 6.4 and 50 mM catechol), then the change of absorbance at 398 nm was monitored. One unit of PPO activity was defined as the amount of enzyme that caused 0.01 absorbance increase per min per gram of flesh. TVC was examined according to China national standard protocol GB 4789.2, 2010 [27], food microbiological examination: aerobic plate count.
E-nose system and measurement
E-nose consists of three main parts including data acquisition unit (U1), sensor array unit (U2), and power supply unit (U3) (see Fig. 1a). Sensor array contains eight metal oxide semiconductors (MOS) of different chemical compositions and thickness to provide selectivity toward different gases. The selectivity toward volatile compound classes of MOS sensors is indicated by manufacture: S1 (MQ-2, propane), S2 (MQ-3, ethanol), S3 (MQ-4, methane), S4 (MQ-5, propane, butane), S5 (MQ-6, butane), S6 (MQ-7, carbon monoxide), S7 (MQ-8, hydrogen), and S8 (MQ-9, methane, carbon monoxide). Each sensor is installed in independent chamber (see Fig. 1b). MOS sensors rely on changes in conductivity induced by the adsorption of molecules in the gas phase and on subsequent surface reactions. They consist of ceramic substrate coated by metal oxide semiconducting film, and heated by wire resistor. Due to the high temperature (250–500 °C), the volatiles transferred to the surface of the sensors are totally combusted to carbon dioxide and water, leading to a change in the resistance. The high temperature avoids water interference and provides sensors fast response and rapid recovery time. Polytetrafluorethylene (PTFE) material is utilized to fabricate the chamber. Each sensor room is separated, which helps to reduce cross-influence of the gas flow. Sensor response is expressed as sampling voltage (V).
Mango was placed in 200 ml air-tight vial and sealed by sealing membrane. After 30 min equilibration, sampling probe was inserted. After turning on power, washing pump and valve 2 were started. Sampling pump and valve 1 remained off. The air was filtered by active carbon to obtain zero gas, which was utilized for recovering sensor. When sensors’ responses returned the baseline, washing pump and valve 2 were shut off. Then sampling pump and valve 1 were turned on. The volatiles in the headspace were inhaled into gas sensor chamber by sampling pump at a flux speed of 400 ml/min for 40 s. E-nose measurement interval was 0.05 s, and the real-time response signal was recorded. When measurement was over, gas sensors were recovered by zero gas at a flux speed of 1000 ml/min for 600 s, waiting for following measurement. These procedures were all controlled by self-developed software.
SR
SR was proposed by Benzi to explain Earth climate periodical changes [28,29,30]. SR model can be described as follows:
where x is the position of the Brownian particle, t is the time, M and C are adjustable parameters, \(I(t)=S(t)+N(t)\) is an input signal S(t) with an intrinsic noise N(t), ξ(t) is the external noise, and V(x) is the simplest double-well potential with the constants a and b.
Equation (4) can be transformed as
The minimal of V(x) are located at ±x m , where \({x_m}={(a/b)^{1/2}}.\) A potential barrier separates the minimal with the height given by \(\Delta U={a^2}/4b.\) The barrier top is located at \({x_b}=0.\) When three elements of SR interact coherently, the potential barrier can be reduced and the Brownian particle may surmount the energy barrier and enter another potential well. The intensity of signals will increase, which makes it possible that the weak signal can be detected from noise background.
Suppose the input signal is \(I(t)=A\sin (2\pi ft+\varphi ),\) where A is signal intensity, f is signal frequency. D is external noise intensity. SNR is the common quantifier for SR and it can be approximately described as:
This model is used for E-nose data analysis. \(I(t)=A\sin (2\pi ft+\varphi )+ enose\,(t)+N(t)\) denotes an input matrix. It has a sinusoid signal \(A\sin (2\pi ft+\varphi ),\) electronic nose response data \(enose\,(t),\), and intrinsic noise N(t). SNR between the output and input is calculated. This model has been successfully used in food analytical applications [30]. In practical engineering measurement, measured data usually consists of signal and intrinsic noise. If an aimed weak signal is submerged in strong noise, we are not able to detect it. With the help of SR, the energy of intrinsic noise is lowered, and the embedded weak signal is amplified effectively so that the signal can be caught for measurement characterization. In some special measurement, the weak signal is so weak that the aimed signals are still embedded in the noise. Double-layered cascaded SR system is designed to solve this problem. If the measurement signals are still embedded in the noise, the output of the previous bistable system is taken as the latter bistable system. If the input signals are successfully obtained, the exact number of bistable systems is suitable for the information measurement.
Results and discussion
Physicochemical and microbial examination results
Figure 2a shows changes in firmness index. Firmness in day 1 is 84.42 g and decreases fast during storage. After 9 days, it decreases to 33.56 g. When fruit enters into ripening stage, metabolic reactions such as breakdown of cell wall and pectin occurs with the help of enzymatic activity, leading to the decreased firmness [7, 31]. Our results are in consistent with the study of Eyarkai et al. [32]. Weight loss increases during storage (see Fig. 2b). After 9 day’s storage, it reaches 33.322%, indicating that weight loss is a key factor in mango quality loss. According to previous report [7, 32], mango texture profiles, including peel strength, stiffness and flesh firmness, change rapidly during ripening. The changes destroy mango water holding capacity and thereby contribute to fast weight loss. Similar results have also been reported by Nambi et al. [32]. Mango surface colour measurement results are shown in Table 1. The L*, h°, C and BI on day 1 are 70.0, 88.1°, 62.1 and 162.2, respectively. With the increase of storage days, dramatic decreases in L*, h°, C can be found and their final values are 26.3, 73.5° and 12.3, respectively. However, BI presents an increase trend in the first 4 days and then decreases, reaching 126.1 on day 9. Surface colour is closely associated with the contents of colour substances such as phenolic compounds and carotenoids [33]. Colour substances can be oxidized easily with the activity of oxidative enzymes (e.g. PPO), resulting in the changes in surface colour. Mirror results are reported by Zheng et al. [5] and Anusuya et al. [7]. Overall, L*, h° and C are proper colour parameters for mango quality evaluation.
pH examination results are shown in Fig. 2c. pH value on day 1 is 3.60 and suffers a rapid growth in the first 3 days, followed by minor changes in the range of 5.07–5.25. pH is firmly dependent on the content of organic acids such as malic acid and citric acid. Similar changes in pH have also been found by Salinas-Roca et al. [34], who reported a pH increase in fresh-cut mango slices without dip treatments in the first 7 days. TSS content in day 1 is 7.37% and increases rapidly during storage (see Fig. 2d). At the end of storage, it increases to 16.49%. When fruit gets ripening, starch and other macromolecular compounds can be degraded into soluble molecules such as sugars, with the help of enzymes, leading to increase in TSS index [7]. Changes in TSS observed here are in mirror with the results of Zheng et al. [5] and Anusuya et al. [7]. Yellowing rate examination results are displayed in Fig. 2e. Yellowing rate on day 1 is zero and increases dramatically in the following days, reaching 86% on day 9. For most mango cultivars, surface colour changes from dark green to yellowish once they enter into the ripening period as a result of enzymatic reactions [5, 7]. Mirror results are reported by Zheng et al. [5]. PPO is one of the close-related enzymes in most fruits. PPO activity examination results are shown in Fig. 2f. PPO activity in mango flesh on day 1 is 2.8 U/min g and suffers stable increases during storage. After 9 days, it reaches 10.2 U/min g. After harvest, mangoes deteriorate easily due to microbial contamination. To prevent microorganisms from invasion, mango endogenous enzymes such as PPO may be activated [5]. Therefore, PPO increase can be observed during storage. Similarly, Zheng et al. [5] reported an increase in PPO activity in both peel and flesh of mangoes during storage. TVC results are shown in Fig. 3. TVC on day 1 is 3.80 × 104 CFU/g and it increases to 1.12 × 106 CFU/g on day 7. As reported in the literature, the main spoilage microorganisms in mango fruits include Colletotrichum gloeosporioides, Escherichia coli O157:H7, Listeria monocytogenes, Salmonella, molds and yeasts [34,35,36]. After harvest, microorganisms rapidly propagate by consuming nutrients in mango, inducing the growth of TVC. The current results are supported by former studies [34].
E-nose analysis results and mango fruit quality predictive model
Original responses
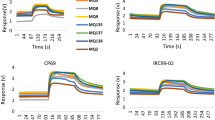
Gas sensors are subjected to baseline drift [12]. It is necessary to validate E-nose sensor array’s stability. E-nose responses to blank sample are also measured during storage according to the method shown in section “E-nose system and measurement”. Zero gas is used as blank sample. Figure 4a shows original responses to blank sample on day 1. Results suggest that sensor array has relatively weak responses and similar results are obtained in the following 8 days. E-nose sensor array exhibits good stability.
E-nose original responses to mango on day 1 are shown in Fig. 4b. The volatile gases existing in the headspace are inhaled into E-nose gas chamber and then sensed by the functional materials settled in sensors. The absorption of functional materials for specific gases induces changes in material’s electrical characteristics. Besides, the sensor array within E-nose setup has eight different gas sensors, and their responses increase with the growth of gas concentrations. So, the signals induced by electrical changes can be utilized to characterize gas concentrations.
The initiative responses of all sensors are close to zero, indicating that the sensor array is in a clean status. With the increase of sampling time, the responses gradually increase and tend to stabilize. A significant increase in voltage can be observed in the sampling interval between 2.5 and 25 s. Finally, sensors S8, S2, S5, and S6 exhibit large response values. S1, S3, and S4’s stable values are much lower, whilst no obvious changes can be observed in S7. E-nose sensor array forms different pattern signals, which provides the analysis basis of E-nose. Next, the saturated values recorded in all sensors are extracted for PCA.
PCA results
PCA results on a two-dimensional plane are shown in Fig. 4c. The first 2 PCs (PC1 and PC2) capture 90.8% of data variance, covering most information of original data. With the increase of storage time, PC1 presents gradual decrease. Mango with different storage time can’t be classified into different categories by PCA.
SR results and mango fruit quality predictive model
Stochastic resonance spectrum calculated by SR model as a function of noise intensity is shown in Fig. 4d. Noise intensity ranges from 40 to 600. SR values at noise intensity of 40 are in the range of 0.22–0.24, and increase rapidly to a peak at noise intensity of about 150, followed by gradual decrease. Mango maximal SNR (Max SR ) values increase during storage period and vary from each other. So, mangoes with different quality can be quantitatively characterized by using Max SR .
In order to explore the relationship between E-nose SR eigen values and mango fruit quality, the Max SR values corresponding to different storage days are extracted. After linear regression, the relationship is developed, as shown in Fig. 4e and Eq. (8).
R = 0.995 indicates that Max SR values have good linearity relation with the mango shelf life. After one-step transform, Eq. (9) is utilized as the mango fruit quality predictive model.
Validation experiment results
To validate the robustness of the developed model [see Eq. (6)], validation experiment is also performed. E-nose responses to mango samples are examined and then inputted into SR system. Afterwards, the Max SR values of mango fruits can be obtained. After inputting Max SR into Eq. (3), the predicted mango storage time can be calculated. Here, 10% is set as the maximal acceptable error. Validation experiment results demonstrate that the forecasting accuracy of this model is 90% (see Table 2). Therefore, E-nose combined with SR provides a rapid and reliable way for mango quality prediction.
Conclusions
Mango fruit quality rapid determination method based on E-nose was investigated here. The results were listed as following:
-
1.
Physicochemical and microbiological analysis suggested that mango fruit quality decreased sharply during storage at room temperature.
-
2.
E-nose sensor array responded to mango fruits with different quality sensitively and stably. The main volatile gases emitted by mango fruit were reducing gases including alkenes, alcohols and carbon monoxide. PCA showed qualitative quality discrimination, while SR MCC spectrum using eigen values quantitatively characterized mango fruit quality.
-
3.
Mango fruit quality predictive model was developed by linear fitting Max SR values, \(Time\,(days)=\frac{{Ma{x_{SR}}+65.96}}{{1.65}}\) (R = 0.991). Validating experiment results demonstrated that the forecasting accuracy of this model is 90%.
The method proposed in this research realizes the goal for mango fruit quality rapid prediction. In comparison with traditional methods (physicochemical and microbiological analysis), E-nose takes some good advantages including non-destructive measurement, quick response, high accuracy, and low cost. It is promising in fruit quality rapid detection.
References
S. Cheema, M. Sommerhalter, Characterization of polyphenol oxidase activity in Ataulfo mango. Food. Chem. 171, 382–387 (2015)
D.S. Sogi, M. Siddiq, K.D. Dolan, Total phenolics, carotenoids and antioxidant properties of Tommy Atkin mango cubes as affected by drying techniques. LWT Food Sci. Tech. 62(1), 564–568 (2015)
D. Prusky, N.T. Keen, Involvement of preformed antifungal compounds and the resistance of subtropical fruits to fungal decay. Plant Dis. 77, 114–119 (1993)
P.E. Zerbini, M. Vanoli, A. Rizzolo, M. Grassi, R.M. de Azevedo Pimentel, L. Spinelli, A. Torricelli, Optical properties, ethylene production and softening in mango fruit. Postharvest Biol. Technol. 101, 58–65 (2015)
X.L. Zheng, L.B. Ye, T.J. Jiang, G.X. Jing, J.R. Li, Limiting the deterioration of mango fruit during storage at room temperature by oxalate treatment. Food Chem. 130, 279–285 (2012)
K. Liu, X.L. Wang, M. Young, Effect of bentonite/potassium sorbate coatings on the quality of mangos in storage at ambient temperature. J. Food Eng. 137, 16–22 (2014)
P. Anusuya, R. Nagaraj, G.J. Janavi, K.S. Subramanian, G. Paliyath, J. Subramanian, Pre-harvest sprays of hexanal formulation for extending retention and shelf-life of mango (Mangifera indica L.) fruits. Sci. Hortic. 211, 231–240 (2016)
R. Desrochers, P. Keane, S. Ellis, K. Dowell, Expanding the sensitivity of conventional analytical techniques in quality control using sensory technology. Food Qual. Prefer. 13, 397–407 (2002)
Y.W. Tang, Z.Y. Gao, S. Wang, X. Gao, J.W. Gao, Y. Ma, X.Y. Liu, J.R. Li, Upconversion particles coated with molecularly imprinted polymers as fluorescence probe for detection of clenbuterol. Biosens. Bioelectron. 71, 44–50 (2015)
N.C.T. Mariani, R.C. da Costa, K.M.G. de Lima, V. Nardini, L.C.C. Júnior, G.H. de Almeida Teixeira, Predicting soluble solid content in intact jaboticaba [Myrciaria jaboticaba (Vell.) O. Berg] fruit using near-infrared spectroscopy and chemometrics. Food Chem. 159, 458–462 (2014)
A. Campagnoli, V. Dell’Orto, Potential application of electronic olfaction systems in feedstuffs analysis and animal nutrition. Sensors 13, 14611–14632 (2013)
M. Peris, L. Escuder-Gilabert, On-line monitoring of food fermentation processes using electronic noses and electronic tongues: a review. Anal. Chim. Acta 804, 29–36 (2013)
K. Persaud, G.H. Dodd, Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 299, 352–355 (1982)
J. Gardner, P. Bartlett, Electronic Noses, Principles and Applications. (Oxford University Press, New York, 1999)
J.S. Vestergaard, M. Martens, P. Turkki, Analysis of sensory quality changes during storage of a modified atmosphere packaged meat product (pizza topping) by an electronic nose system. LWT Food Sci. Technol. 40(6), 1083–1094 (2007)
X.J. Tian, J. Wang, S.Q. Cui, Analysis of pork adulteration in minced mutton using electronic nose of metal oxide sensors. J. Food Eng. 119(4), 744–749 (2013)
R. Paolesse, A. Alimelli, E. Martinelli, C.D. Natale, A. D’Amico, M.G. D’Egidio, G. Aureli, A. Ricelli, C. Fanelli, Detection of fungal contamination of cereal grain samples by an electronic nose. Sens. Actuators B 119(2), 425–430 (2006)
J. Perkowski, M. Buśko, J. Chmielewski, T. Góral, B. Tyrakowska, Content of trichodiene and analysis of fungal volatiles (electronic nose) in wheat and triticale grain naturally infected and inoculated with Fusarium culmorum. Int. J. Food Microbiol. 126, 127–134 (2008)
A.K.G. Kadegowda, L.S. Piperova, R.A. Erdman, Principal component and multivariate analysis of milk long-chain fatty acid composition during diet induced milk fat depression. J. Dairy Sci. 91, 749–759 (2008)
P. Verma, R.D.S. Yadava, Polymer selection for SAW sensor array based electronic noses by fuzzy c-means clustering of partition coefficients: model studies on detection of freshness and spoilage of milk and fish. Sens. Actuators B 209, 751–769 (2015)
L.R. Xu, X.Z. Yu, L. Liu, R. Zhang, A novel method for qualitative analysis of edible oil oxidation using an electronic nose. Food. Chem. 202, 229–235 (2016)
Y. Asikin, G. Maeda, H. Tamaki, M. Mizu, H. Oku, K. Wada, Cultivation line and fruit ripening discriminations of Shiikuwasha (Citrus depressa Hayata) peel oils using aroma compositional, electronic nose, and antioxidant analyses. Food Res. Int. 67, 102–110 (2015)
L.Q. Pan, W. Zhang, N. Zhu, S.B. Mao, K. Tu, Early detection and classification of pathogenic fungal disease in post-harvest strawberry fruit by electronic nose and gas chromatography–mass spectrometry. Food Res. Int. 62, 162–168 (2014)
H.X. Wang, Z.Q. Hu, F.Y. Long, C.F. Guo, Y.H. Yuan, T.L. Yue, Early detection of Zygosaccharomyces rouxii—spawned spoilage in apple juice by electronic nose combined with chemometrics. Int. J. Food Microbiol. 217, 68–78 (2016)
NY/T 2637, Refractometric method for determination of total soluble solids in fruits and vegetables (2014)
Y.S. Wang, S.P. Tian, Y. Xu, G.Z. Qin, H.J. Yao, Changes in the activities of pro- and anti-oxidant enzymes in peach fruit inoculated with Cryptococcus laurentii or Penicillium expansum at 0 or 20 °C. Postharvest Biol. Technol. 34, 21–28 (2004)
GB 4789.2., National food safety standard: food microbiological examination: aerobic plate count (2010)
R. Benzi, A. Sutera, A. Vulpiani, The mechanism of stochastic resonance. J. Phys. A 14(11), L453–L456 (1981)
J.J. Collins, C.C. Chow, T.T. Imhoff, Aperiodic stochastic resonance in excitable systems. Phys. Rev. E 52(4), 3321–3324 (1995)
C. Heneghan, C.C. Chow, J.J. Collins, T.T. Imhoff, S.B. Lowen, M.C. Teich, Information measures quantifying aperiodic stochastic resonance. Phys. Rev. E 54(3), 2228–2231 (1996)
G.B. Seymour, J.E. Taylor, G.A. Tucker, Biochemistry of Fruit Ripening. (Chapman & Hall, London, 1993)
V.E. Nambi, K. Thangavel, K.A. Rajeswari, A. Manickavasagan, V. Geetha, Texture and rheological changes of Indian mango cultivars during ripening. Postharvest Biol. Technol. 117, 152–160 (2016)
F. Charles, V. Vidal, F. Olive, H. Filgueiras, H. Sallanon (2013) Pulsed light treatment as new method to maintain physical and nutritional quality of fresh-cut mangoes. Innov. Food Sci. Emerg. Technol. 18, 190–195
B. Salinas-Roca, R. Soliva-Fortuny, J. Welti-Chanes, O. Martín-Belloso, Combined effect of pulsed light, edible coating and malic acid dipping to improve fresh-cut mango safety and quality. Food Control 66, 190–197 (2016)
X.B. Xu, H.H. Lei, X.Y. Ma, T.F. Lai, H.M. Song, X.Q. Shi, J.K. Li, Antifungal activity of 1-methylcyclopropene (1-MCP) against anthracnose (Colletotrichum gloeosporioides) inpostharvestmangofruit and its possible mechanisms of action. Int. J. Food Microbiol. 241, 1–6 (2017)
M.-J. Kim, C.H. Tang, W.S. Bang, H.-G. Yuk, Antibacterial effect of 405 ± 5 nm light emitting diode illumination against Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella on the surface of fresh-cut mango and its influence on fruit quality. Int. J. Food Microbiol. 244, 82–89 (2017)
Acknowledgements
This work is supported by Public Welfare Technology Application Research Project of Zhejiang Province (Grant No. 2017C31010).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest. Hui Guohua has received research grant from Public Welfare Technology Application Research Project of Zhejiang Province (Grant No. 2017C31010).
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
(If not applicable on the study) Not applicable.
Rights and permissions
About this article
Cite this article
Lihuan, S., Liu, W., Xiaohong, Z. et al. Fabrication of electronic nose system and exploration on its applications in mango fruit (M. indica cv. Datainong) quality rapid determination. Food Measure 11, 1969–1977 (2017). https://doi.org/10.1007/s11694-017-9579-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-017-9579-1