Abstract
Ontogenetic diet shifts in juvenile fishes are sometimes associated with proportional changes to the feeding mechanism. In addition, many piscivorous teleosts transition from invertebrate-prey to fish-prey when the mouth attains a specific diameter. Allometric (disproportionate) growth of the jaws could accelerate a young fish’s ability to reach a critical gape diameter; alternately by opening the lower jaw to a greater degree, a fish might increase gape behaviorally. We investigated the ontogeny of feeding morphology and kinematics in an imperiled piscivore, the Colorado pikeminnow (Ptychocheilus lucius) in a size range of individuals across which a diet shift from invertebrate-prey to prey-fishes is known to occur. We predicted that: (1) the feeding apparatus of the fish would grow proportionally with the rest of the body (isometric growth), that (2) anatomical gape diameter at the known diet transition would be a similar gape diameter to that observed for other piscivorous juvenile fishes (15–20 mm) and (3) feeding kinematic variables would scale isometrically (that is, change in direct proportion to body length) as juvenile pikeminnow became larger. Furthermore, we also asked the question: if changes in feeding morphology and kinematics are present, do the changes in morphology appear to generate the observed changes in kinematics? For juvenile Colorado pikeminnow, the majority of the morphological variables associated with the skull and jaws scale isometrically (that is, proportionally), but seven of eight kinematic variables, including functional gape, scale with negative allometry (that is, they became disproportionately smaller in magnitude). In contrast with the overall trend of isometry, two key aspects of feeding morphology do change with size; the lower jaw of a young Colorado pikeminnow becomes longer (positive allometry), while the head becomes shallower (negative allometry). These findings do not support the hypothesis that morphological ontogenetic changes directly generate changes in feeding kinematics; in fact, allometric jaw growth would, a priori, be expected to generate a larger gape in older fish—which is the opposite of what was observed. We conclude that ontogenetic morphological changes produce a more streamlined cranium that may reduce drag during a rapid, anteriorly directed strike, while concomitant behavioral changes reduce the magnitude of jaw movements—behavioral changes that will facilitate a very rapid opening and closing of the jaws during the gape cycle. Thus, for juvenile pikeminnow, speed and stealth appear to be more important than mouth gape during prey capture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ontogenetic shifts in diet and trophic niche may occur during juvenile life history stages in teleost fishes (Vanicek and Kramer 1969; Schmitt and Holbrook 1984; Keast 1985; Luczkovich et al. 1995; Hernandez and Motta 1997; McCormick 1998) and such dietary shifts are sometimes attributed to size-induced changes to the feeding mechanism (Schmitt and Holbrook 1984; Keast 1985; Hoyle and Keast 1988; Hambright 1991; Mittelbach and Persson 1998). However, as juvenile fish grow larger, it is often assumed that bones and muscles change very little and meet isometric scaling predictions (Hill 1950; Richard and Wainwright 1995; Hernandez 2000), even though isometric growth is rarely observed in juvenile fishes (e.g. Fuiman 1983; but, see also Richard and Wainwright 1995 and Habegger et al. 2011). Deviation from isometry is somewhat surprising because unlike the dramatic morphogenesis and allometric growth seen in larval fish (Fuiman 1983), juveniles do not acquire new anatomical features and are considered to exhibit the adult phenotype (Fuiman 1983; Richard and Wainwright 1995; Cook 1996). In fact, allometric growth of the feeding apparatus may facilitate diet transitions in some teleosts. For example, juvenile sheepshead feed by crushing hard prey between the oral jaws and exhibit positive allometric growth of the jaw and associated muscles (Hernandez and Motta 1997). For this species, a small increase in overall animal length is associated with a proportionally larger jaw and more robust jaw-closing musculature, which translates to a large increase in crushing performance that enables the exploitation of a new food resource (Hernandez and Motta 1997). Thus, functional changes to the feeding apparatus can facilitate or enable the dietary transitions commonly exhibited by juvenile fishes. In the current study, we examine growth of the feeding apparatus in a juvenile piscivorous fish to determine (1) if allometric changes exist and (2) to ascertain if these changes are associated with a known dietary transition.
Juvenile piscivorous fishes are a good model system in which to examine ramifications of morphology on diet because most young piscivores consume small, non-fish prey such as microcrustaceans (see Mittelbach and Persson 1998) and only later transition to a fish-based diet when a certain “size advantage” over the prey has been achieved (Mittelbach and Persson 1998). For piscivores, predator gape-width and gape-depth can limit the size of the prey that can be consumed (Hoyle and Keast 1988; Mittelbach and Persson 1998); in addition, a large gape is considered an advantage for feeding on elusive prey items (Norton 1991). However, it is not clear if mouth-gape undergoes a disproportionate (positive allometric) change as juvenile piscivores grow larger. Positive allometry of the jaw apparatus (a morphological change) could potentially enlarge the gape rapidly and facilitate the transition to fish prey. Alternately, young piscivores may enhance their feeding ability on fish-prey by “choosing” to generate greater lower jaw depression (a behavioral change) during the jaw opening and closing cycle. It is also possible that juvenile piscivores employ a combination of morphological and behavioral changes to facilitate the transition to fish prey.
To address these questions, we quantify morphology and kinematics in an unusual and imperiled cyprinid piscivore, the Colorado pikeminnow (P. lucius). The Colorado pikeminnow (formerly known as the Colorado squawfish) is the largest member of the family Cyprinidae in North America and can reach 1.8 m in total length with a mass of 36 kg or more (Vanicek and Kramer 1969; Minckley 1973). In the wild, pikeminnow smaller than 50 mm total length (TL) primarily feed on insects while pikeminnow larger than 50 mm TL begin to include prey-fishes in their diets (Vanicek and Kramer 1969). As large juveniles (>200 mm TL) and adults, prey-fishes are the only food items found in the stomachs of wild-caught pikeminnow, which suggests that older Colorado pikeminnow are strictly piscivorous. Based on previous work on scaling behavior and anatomy in young piscivorous fishes, we made a suite of predictions. In centrarchids, fish are only included in the diet when individuals reach a critical anatomical gape of 15–20 mm—a size physically large enough to capture and consume fish prey (Wainwright and Richard 1995). In addition, feeding kinematics and morphology change very little across a large size range in a centrarchid piscivore (Richard and Wainwright 1995). Furthermore, the oral-jaw closing lever mechanism scales isometrically in juvenile barracuda (Habegger et al. 2011). Thus, our null hypothesis for pikeminnow is that they grow isometrically until they achieve a gape that is large (or commodious) enough to accommodate fish prey, and we further expect that, when they reach the body size at which they naturally undergo the transition to fish-prey, juvenile pikeminnow will have an anatomical gape in the range of approximately 15–20 mm. In addition, we also expect that feeding kinematic parameters of juvenile pikeminnow will scale isometrically (that is, the movements will scale proportionally with body size) as they grow larger.
In addition, we use this study to address two related questions. First, do changes in feeding mode occur across the known diet transition for Colorado pikeminnow? Changes in feeding mode, from ram-feeding to suction-feeding, during ontogeny have been documented in both larval (Coughlin 1994) and juvenile fishes (Cook 1996). However, for some adult piscivorous fishes such as gar and barracuda, ram feeding is considered the primary feeding mode (Porter and Motta 2004), while in others, such as largemouth bass, suction production plays a key role in prey capture. To address this question, we applied a biomechanical model (Carroll et al. 2004) to determine if suction potential changes as young pikeminnow grow larger. In this analysis, a change in suction potential would suggest that juvenile pikeminnow undergo a change in feeding mode (e.g. from suction-dominated to ram-dominated) as they make the ontogenetic transition from invertebrate-prey to fish-prey. Second, we ask an overarching functional question: do changes in feeding morphology appear to “drive” changes in feeding kinematics? For example, if functional gape becomes proportionally larger (positive allometry), could this change be a direct result of positive allometric growth of the anterior jaws? To address this final question, we employ and modify two existing biomechanical models from the literature (Westneat 2004; Ferry-Graham et al. 2010) to discern if changes in morphology appear to underlie changes in kinematics.
Materials and Methods
Procurement and Care of Study Animals
Colorado pikeminnow (P. lucius) were obtained from captive-spawned adults at Bubbling Ponds Fish Hatchery in Page Springs, Arizona USA. The experimental animals were fed commercial aquaculture food during early life stages. Fish were obtained as juveniles of approximately 20–30 mm total length (TL). Initially, six to seven animals were housed in 19 l community aquaria, but fish were subsequently separated and placed into individual aquaria (37 l) when some pikeminnow grew larger and became more aggressive. Water temperature was maintained at 22–25 C. Fish were fed a diet of commercially available flake foods and occasionally frozen chironomid larvae or frozen adult brine shrimp (Artemia). Animals were held and cared for according to the standards set forth by the Northern Arizona University Institutional Animal Care and Use Committee (protocol number 07–014).
Cranial and Body Morphology
Juvenile pikeminnow, P. lucius, were lightly anesthetized using tricaine methanesulfonate (>300 mg l−1, brand name Finquel, Argent Chemical Laboratories, Redmond WA, USA), buffered with commercial aquarium buffer (“pH 7.0” Aquarium Pharmaceuticals, Incorporated). Once fish were anesthetized, they were removed from the water and placed on a damp paper towel for measurement. A total of 19 Colorado pikeminnow were used for morphological measurements (except functional gape measurements, where only 18 individuals were measured); these fish were not the same individuals as those used for the feeding study (see below), but they encompassed a similar size range (46.6–161.2 mm TL). Seven morphological variables were measured directly from these specimens using digital calipers: (1) anatomical gape, as defined by the linear distance between the tip of the premaxilla and the tip of the lower jaw during manual expansion of the jaws of the anesthetized fish; (2) premaxilla length, as defined by the linear distance from the superior border of the premaxilla to the articulation of the premaxilla with the dentary; (3) head length, as defined by the linear distance from the anterior tip of the snout to the operculum; (4) head width, as defined by the linear distance laterally across the dorsal aspect of the head, measured above the posterior of the suspensorium, behind the eye; (5) head depth, as defined by the linear distance from the dorsal surface of the head to the ventral surface of the head, measured at the most caudal aspect of the suspensorium, just posterior to the eye; (6) lower jaw length; as defined by the linear distance from the tip of the dentary to the articulation of the dentary with the anterior border of the suspensorium; (7) total length, defined as the linear distance from the tip of the snout to the tip of the tail when the tail is dorsoventrally compressed. Upon completion of measurements, fish were placed into a 19 l tank with fresh, de-chlorinated water and an air pump for approximately 5–10 min to recover from anesthesia, after which fish were placed back into their holding tanks.
Feeding Kinematics
To quantify cranial kinematics during feeding, fish were recorded from a lateral view using a Vision Research Phantom high-speed digital-video camera at 1,000 frames−s. Fish were filmed in 37-l aquaria with bubblers or filters turned off to ensure calm water. Prior to the start of filming, a 5 mm grid was placed into the background of each video as a scale reference. Chunks of frozen silversides (fish from the family Atherinidae) were cut into pieces that were scaled to 40–90 % of the functional gape of the fish being filmed; this was done in an effort to simulate “challenging” prey items to obtain maximum cranial movements during a strike. Piscivores feeding in their natural habitat select prey fishes that are on average 45 % of functional gape, but small juvenile fish are known to feed very close to their own functional gape (Luczkovich et al. 1995). Additionally, pike (Esox lucius) select prey fishes that are approximately the same size as their functional gape (Nilsson and Brönmark 2000). Some fishes respond differently to elusive versus non-elusive prey types (see Nemeth 1997): however, we used non-elusive prey for the following reasons: (1) prey could be cut to precise sizes to ensure that each individual P. lucius received a prey item that scaled proportionally with its gape (2) frozen prey did not exhibit escape behaviors and would ensure that the pikeminnow would remain perpendicular to the camera during feeding (3) studies of other piscivores (Micropterus salmoides) documented no difference in suction feeding performance when feeding on elusive prey (fish) and non-elusive prey (Norton and Brainerd 1993), (4) the Colorado pikeminnow in this study were hatchery raised and always fed aggressively on all prey types presented to them, possibly because group-reared fish strive to procure food items before they are captured by other fish housed in the same chamber and (5) a pilot study revealed that pikeminnow responded with a similar behavior to both live and dead prey. In this pilot study, we selected four pikeminnow and offered them a live, juvenile guppy (Poecilia reticulata) and a frozen fish chunk of similar size to the guppy (live and dead prey were presented in a randomized order) and recorded their behavioral response for analysis. For these four fish, two measures of feeding performance, attack velocity and functional gape, did not differ between the two prey types (two paired t tests, both p > 0.05). Pikeminnow fed aggressively on both live and frozen prey items and always captured the frozen prey item in the water column before it could sink to the bottom of the aquarium.
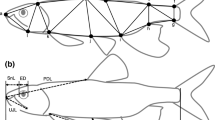
Digital video sequences were converted into individual images and imported into ‘Didge’ image-digitizing software (Cullum 2003) for analysis. Feeding kinematics were recorded for 17 Colorado pikeminnow (range 41.1–101.3 mm TL). Several prey capture sequences were recorded for each fish. From these sequences, one feeding bout where the head and jaw appeared to be maximally expanded during the feeding strike and the fish remained perpendicular to the camera was selected and saved for analysis. Three linear and five angular displacements during feeding were computed from 11 landmark points digitized on the head (Fig. 1c). Angular and linear velocities of lower jaw opening and closing were also calculated from the high-speed video. For each variable, an initial value was determined for time zero, defined here as one frame prior to the onset of mouth opening. Subsequent values calculated for a specific variable were subtracted from the value at time zero to determine the change in that variable over time. The three linear variables were calculated as follows (see Fig. 1c): (1) functional gape, the distance between a point on the anterior tip of the premaxilla (landmark 1) and another on the anterior tip of the dentary (landmark 3); (2) hyoid depression, the distance between a point on the caudal most circumference of the eye (landmark 8) and a point digitized on the ventral aspect of the head directly below the caudal most point on the eye (landmark 11); (3) premaxillary protrusion, the distance between the most rostral point on the circumference of the eye (landmark 9) to the anterior tip of the premaxilla (landmark 1). The five angular measurements of kinematic variables were determined from each image as follows: (1) premaxilla rotation, as defined by a point on the anterior tip of the premaxilla, a point at the angle of the mouth between the premaxilla and dentary (vertex) and a point where the anterior most fin ray of the pelvic fin meets the body (landmarks 1, 2 and 5); (2) hyoid rotation, as defined by a point from the tip of the hyoid (or floor of the buccal cavity directly underneath the caudal-most point on the eye when the hyoid was not visible, this is the point from which the tip of the hyoid is abducted), a point on the caudal bend of the suspensorium (vertex) and a point on the anterior most fin ray of the pelvic fin where it articulates with the body (landmarks 11, 10 and 5); (3) lower jaw rotation, as defined by a point taken on the anterior tip of the dentary, a point at the articulation of the dentary with the suspensorium (vertex) and a point on the anterior-most fin ray of the pelvic fin where that fin articulates with the body (landmarks 3, 4 and 5); (4) gape angle, defined by one point at the anterior tip of the premaxilla, the next at the angle of the mouth between the premaxilla and dentary (this point defines the angle vertex) and the final point at the anterior tip of the dentary (landmarks 1, 2 and 3 respectively); (5) cranial rotation, as measured from a point at the dorsal surface of the neurocranium directly above the center of the eye, a point on the dorsal surface of the body of the fish, directly above the articulation of the pectoral fin (vertex) and a point on the anterior most fin ray of the pelvic fin where it articulates with the body (landmarks 7, 6 and 5).
a Juvenile Colorado pikeminnow, P. lucius, (90.9 mm TL) at time zero (just prior to mouth opening) during the feeding strike. b Juvenile pikeminnow (44.6 mm TL) at time zero. Both scale bars equal to 10 mm. c Juvenile pikeminnow (same individual from 1A) at maximum gape during feeding. Points represent locations of 11 digitized landmarks on the head and body of juvenile pikeminnow. From these landmarks, three linear and five angular kinematic variables were computed. Landmarks are identified as follows: (1) Anterior tip of the premaxilla; (2) Angle of the mouth between the premaxilla and dentary; (3) Anterior tip of the dentary; (4) Articulation of the dentary with the suspensorium; (5) Anterior most fin ray of the pelvic fin where the pelvic fin meets the body; (6) Dorsal surface of the body, directly above the articulation of the pectoral fin with the body (if the body were horizontal); (7) Dorsal surface of the neurocranium, directly above the center of the eye (if the body were horizontal); (8) Caudal most point on the eye (if the body were horizontal); (9) Rostral most point on the eye (if the body were horizontal); (10) Caudal most bend on the suspensorium; (11) Ventral floor of the mouth directly underneath the caudal most point on the eye (if the body were horizontal). Refer to methods for descriptions of how kinematic variables were measured. d Juvenile pikeminnow (same individual from 1B) at maximum gape during feeding
Time to maximum displacement for several kinematic variables was also determined for all 17 Colorado pikeminnow. The timing of kinematic maxima was determined by counting how many frames had elapsed from time zero to the frame where maximum kinematic displacement for that kinematic variable occurred, where each frame represents 1/1,000th of a second, or 1 ms. In this manner, time to maximum displacement was determined for peak gape, peak premaxilla rotation, peak lower jaw rotation, peak hyoid depression and peak cranial rotation. Time to jaw closing was calculated by counting the number of frames from the time of peak lower jaw angle until the lower jaw made contact with the premaxilla.
The four lower jaw velocity variables were computed as follows: (1) angular velocity of opening was calculated by determining the change in the angle of the lower jaw (landmarks 3, 4, and 5) from time zero until the time of peak gape; (2) angular velocity of closing was found by calculating the change in angle (landmarks 3, 4, and 5) from the time of peak gape to the time when the premaxilla and lower jaw came together; (3) linear velocity of opening was calculated by determining the distance moved by the anterior tip of the lower jaw during jaw opening (landmark 3) from time zero until time of peak gape; (4) linear velocity of closing was found by calculating the distance the tip of the lower jaw (landmark 3) moved from the time of peak gape to the time that the lower jaw and the premaxilla came into contact. Both angular and linear velocity variables were computed because the assumptions of isometry are different for angular and linear variables (see ‘scaling analysis’ below).
Scaling Analysis
If isometry is maintained, morphological variables, linear kinematic variables and linear velocity should scale with a slope of one, while angular kinematic variables, angular velocity and time-to-maximum kinematic excursions should scale with a slope of zero (Richard and Wainwright 1995; Hernandez 2000). We used these predictions as our null hypotheses to determine if isometry or allometry characterizes juvenile pikeminnow feeding kinematics and morphology.
All linear, angular, and timing variables were log10 transformed and plotted against the total length of the pikeminnow, which was also log10 transformed. JMP 8 (SAS Institute, Cary, North Carolina) or Sigma Plot 11.0 for Windows (Systat Software, Incorporated, San Jose, California, USA) was used to compute linear regressions for all angular and linear variables on total length. Analysis of variance (ANOVA) was used to determine if slopes that were predicted to be zero under the null hypothesis (angular and timing variables) were significantly different from zero (p < 0.05). T tests described in Sokal and Rohlf (1995) were used to determine if variables that were predicted to have a slope of one under the null hypothesis (linear kinematic and morphological variables) were significantly different from one (p < 0.05).
Morphological Modeling
Functional Gape
We developed a simple model, following a model proposed by Ferry-Graham et al. (2010) to determine how functional gape might change for juvenile P. lucius under three possible growth trajectories: (1) jaw kinematics under an isometric growth trajectory, where the expected functional gape in a large fish is derived from empirical values of jaw morphology, and cranial movements obtained from small specimens free-feeding; (2) empirical values of anatomical gape as measured from live specimens across the size range used in this study; (3) empirical values of functional gape, measured by two points on the tip of the premaxilla and dentary in free-feeding live fish. In the simple model that was used to generate the gape-size trajectory (growth trajectory 1 above), we considered the jaws and functional gape as a triangle, using the length of the premaxilla and lower jaw as two sides of this triangle with the gape angle formed at the vertex of the two lines (Ferry-Graham et al. 2010). The sides of the triangle representing the jaw elements were set at the empirical lengths observed for premaxilla and lower jaw length over the size range of juvenile pikeminnow examined in this study. The law of cosines was used to predict what the functional gape (the side of the triangle opposite the gape angle) should be, assuming the gape angle did not change as juvenile pikeminnow grew larger.
Predicting Suction Production
Carroll et al. (2004) demonstrated that a realistic prediction of subambient buccal pressure generated during suction feeding could be obtained from a small set of morphological variables for centrarchid fishes (bass, crappie and sunfish). This same model has been used in other, more recent studies to estimate the suction generating capacity of many different fishes (see Holzman et al. 2012; Price et al. 2012). Assuming that the suction potential of pikeminnow follows the same form-functional relationship that is seen in centrarchids, any deviation from isometric growth may yield changes to the suction-producing potential of juvenile pikeminnow. Investigation of multiple teleost families reveals that the timing of expansive movements used for suction feeding is similar and strictly regulated, especially for the families centrarchidae and cyprinidae (Gibb and Ferry-Graham 2005). We employed Carroll’s model to investigate how changes in morphology through a juvenile size range of pikeminnow might change the “morphological-suction-potential” or the predicted maximum negative pressure the fish would be able to develop during a suction feeding event. A change in morphological-suction-potential might indicate a shift in feeding mode: for example, if suction potential decreases, this would suggest an increase in reliance on ram-based prey capture. Morphological-suction-potential is calculated as: \( \left[ {PCSA\left( {L_{in} /L_{out} } \right)} \right]/A_{buccal} \) where PCSA is the physiological cross-sectional area of the epaxial muscle, Lin is the length of the in-lever, as measured from the centroid of the epaxial muscle to the articulation of the supracleithrum and posttemporal bones, Lout is the distance from the articulation of the first vertebra to the moment of buccal surface area, and buccal area (Abuccal) is the surface area of the buccal cavity, modeled as a simple cylinder (Carroll et al. 2004).
To determine the morphological-suction-potential in P. lucius juveniles, following the assumptions of this model, the following morphological variables were measured from dead specimens in the lab or euthanized specimens that had been filmed for the kinematic studies (mentioned above): (1) distance from the dorsal surface of the vertebra just above the posterior edge of the operculum (about which rotation of the neurocranium occurs) to the centroid of the epaxial muscle (described in Carroll et al. (2004) as Lin); (2) the distance from the posterior end of the operculum to the tip of the snout, minus half of the buccal length (Lout); (3) the epaxial muscle PCSA and (4) area of the buccal cavity. Lin and Lout variables were measured using a dissecting microscope and digital calipers. The centroid of the epaxial muscle and the PCSA of the epaxial muscle were measured by freezing the fish (after fish were euthanized using tricaine methanesulfonate at a concentration of 400 mg l−1 or more), cutting the fish in cross section at the posterior edge of the operculum, using a razor blade and making a second cross-sectional cut about 1 mm posterior to the first cut, while the fish was still frozen. The cut was made in a similar manner to the cut used by Carroll et al. (2004) to measure PCSA for centrarchid fishes. This procedure yielded a small cross-section of the body of the fish that could then be laid flat (posterior cut-side down) and photographed. Digital images were taken with a Nikon D70 digital camera using a macro lens and a five millimeter scale bar in the frame of the picture for reference. Epaxial muscle PCSA and the centroid of the epaxial muscle were determined using ImageJ (Rasband 2011). The distance from the centroid of the epaxial muscle to the dorsal surface of the vertebrae was measured: this measurement represented the in-lever for cranial rotation, Lin. Post-mortem injection of silicone aquarium sealant into the mouth of the fish allowed us to make a cast of the buccal cavity to obtain buccal area and buccal length. The cast was extracted when the silicone cured and the excess sealant that had filled the gills and operculum was removed. Buccal area was determined by assuming that the buccal cavity could be modeled as a cylinder. It was observed that buccal casts removed from the mouths of the pikeminnow specimens were approximately cylindrical. We calculated the surface area of this cylinder by measuring the length of the buccal cavity from anterior to posterior (the height of the cylinder) and the dorsoventral depth of the buccal cavity at approximately half the length of the buccal cavity (the diameter of the cylinder); surface area was then calculated using the standard geometric formula.
Assuming that the suction potential of pikeminnow follows the same form-functional relationship that is seen in centrarchids, any deviation from isometric growth may yield changes to the suction-producing potential of juvenile pikeminnow.
Mechanical Advantage of The Lower Jaw
We sought to measure mechanical advantage in the lower jaw of juvenile pikeminnow to ascertain if any changes in the jaws ability to move forcefully or rapidly occurred over the size range examined here; mechanical advantage (MA) was measured following Westneat (2004). The dentary and articular bones, the two bones that make up the lower jaw, were dissected out of the juvenile Colorado pikeminnow. The bones were cleaned by placing them in warm water and using forceps to remove the soft tissues. The hot water disarticulated the two bones from one another and split the lower jaw in two halves at the anterior tip. The bones were put back together using a small dab of glue applied with a 30-gauge syringe. Both of the lower jaw halves for each fish were then photographed under a dissecting microscope with a Nikon D70 digital SLR camera. Digital images were imported into ImageJ (Rasband 2011) and the following three measurements were taken [as described in Westneat (2004)]: (1) the out-lever for jaw opening and jaw closing, defined as the distance from the tip of the lower jaw to the quadrate-articular joint at the posterior of the jaw; (2) the closing in-lever, defined as the distance from the quadrate-articular joint to the coronoid process; (3) the opening in-lever, defined as the distance from the quadrate-articular joint to the posteroventral corner of the lower jaw. The jaw opening and closing mechanical advantage is the ratio of the length of the opening in-lever to the length of the out-lever and the ratio of the length of the closing in-lever to the length of the out-lever, respectively. High values of MA (values approaching 1) should enhance the ability of the jaw to produce force during jaw opening or closing; low values (approaching 0) should enhance the ability of the jaw to move rapidly.
Results
Growth Trends of Morphological Variables Characterizing the Head and Mouth of Juvenile Colorado pikeminnow
Of the six morphological variables measured, four of the variables of the cranium and jaws scaled with isometry: their slope was not significantly different from the hypothesized slope of one (Fig. 2). Variables that scaled with isometry were: anatomical gape (slope = 1.089, r2 = 0.911, p value >0.05, Fig. 2a), premaxilla length (slope = 1.089, r2 = 0.945, p value >0.05, Fig. 2b), head length (slope = 0.997, r2 = 0.985, p value >0.05, Fig. 2c) and head width (slope = 0.984, r2 = 0.972, p value >0.05, Fig. 2d). The two morphological variables found to scale allometrically were head depth and lower jaw length, which scaled with negative allometry (slope = 0.896, r2 = 0.963, p value <0.05, Fig. 2e) and positive allometry (slope = 1.188, r2 = 0.969, p value <0.01, Fig. 2f), respectively. This result indicates that the dorsoventral depth of the head became relatively shallower and the lower jaw became relatively longer (compare Fig. 1a, b) as juvenile pikeminnow grew larger.
Morphological variables characterizing the head and mouth of juvenile Colorado pikeminnow, P. lucius (n = 19, except for anatomical gape where n = 18.) P values indicate whether observed slopes (black line) were significantly different from the hypothesized isometric slope of one. Axes are log10-transformed. Anatomical gape, premaxilla length, head length and head width all scaled isometrically, while head depth was found to scale with negative allometry and lower jaw length scaled with positive allometry. Refer to text for details of anatomical measurements
Feeding Kinematics of Juvenile Colorado pikeminnow
After a prey item was placed in the tank, juvenile pikeminnow would employ a quick turn to orient the head and jaws toward the prey. Upon completion of the orientation turn, the pikeminnow would then immediately accelerate toward the food item as it sank toward the bottom. As the pikeminnow accelerated, the dorsal and pectoral fins were adducted and the mouth remained closed or partially closed during the approach. Just before the fish reached the prey item, the mouth rapidly opened and the dorsal and pectoral fins were abducted. Peak gape was reached quickly, within 5 ms in the smallest pikeminnow and within 13 ms in the largest individual. Prey was engulfed when peak gape had been reached or a few milliseconds thereafter. Peak cranial rotation and hyoid depression followed peak gape; peak cranial rotation occurred at 11 ms in the smallest pikeminnow and at 19 ms in the largest while peak hyoid depression occurred at 11 ms in the smallest pikeminnow and at 18 ms in the largest. As all cranial elements began to return to their original resting position, the pikeminnow performed another quick turn and swam away.
In contrast with the isometry observed for the majority of the morphological variables, negative allometry characterized many kinematic variables associated with prey capture (Figs. 3, 4). Seven of eight differed significantly from their predicted slope of zero (for angular variables) or one (for linear variables). Premaxilla rotation was the only angular variable to scale isometrically in the juveniles examined here and its slope was not significantly different from zero (slope = 0.509, r2 = 0.0478, p value = 0.399, Fig. 3a); the premaxilla was rotated 16.9 ± 7.3 degrees (mean ± standard deviation) during feeding, independent of pikeminnow size. The observed slopes of all other angular displacements during feeding indicated that as pikeminnow grew larger, the angular displacements were smaller: hyoid rotation (slope = −1.358, r2 = 0.639, p value <0.001, Fig. 3b), lower jaw rotation (slope = −1.253, r2 = 0.347, p value = 0.013, Fig. 3c), gape angle (slope = −0.526, r2 = 0.337, p value = 0.015, Fig. 3d) and cranial rotation (slope = −0.789, r2 = 0.463, p value = 0.003, Fig. 3e). Similarly, all three linear kinematic variables scaled with a slope that was significantly different from one: functional gape (slope = 0.604, r2 = 0.584, p value <0.05, Fig. 4a), hyoid depression (slope = 0.200, r2 = 0.0582, p value = 0.351, Fig. 4b) and premaxilla protrusion (slope = 0.652, r2 = 0.133, p value = 0.150, Fig. 4c) all demonstrated negative allometry.
Angular kinematic variables measured from high-speed video of feeding events from an ontogenetic size range of juvenile Colorado pikeminnow, P. lucius (n = 17 individuals, 1 video sequence analyzed for each fish). All angular kinematic variables were predicted to scale with a slope of zero. Black lines represent observed slopes of kinematic variables. P values >0.05 indicate slopes which are not significantly different from a slope of zero. Axes are log10-transformed. In general, with the exception of premaxilla rotation, which rotated on average 16.9 ± 7.3 degrees (mean ± standard deviation), smaller juvenile pikeminnow showed greater angular displacements of measured kinematic variables during feeding than larger pikeminnow. Refer to text for details of kinematic measurements
Linear kinematic variables measured from high-speed video of feeding events from an ontogenetic size range of juvenile Colorado pikeminnow, P. lucius (n = 17 individuals, 1 video sequence analyzed for each fish). All linear variables were predicted to scale with an isometric slope of one. Black lines represent observed slopes for kinematic variables. Axes are log10-transformed. In the size range of juvenile pikeminnow examined in this study, the premaxilla was protruded forward during feeding on average 0.7 ± 0.3 mm (mean ± standard deviation). Refer to text for details of kinematic measurements
Angular velocity of jaw opening decreased as pikeminnow grew larger (slope = −1.996, r2 = 0.676, p value <0.001, Fig. 5a) while angular velocity of closing did not correlate with juvenile pikeminnow length (slope = −1.286, r2 = 0.191, p value = 0.079, Fig. 5b). Linear velocity of opening (slope = 0.240, r2 = 0.037, p value = 0.459, Fig. 5c) and linear velocity of lower jaw closing (slope = 0.327, r2 = 0.044, p value = 0.418, Fig. 5d) were also not correlated with pikeminnow total length. In addition, angular velocity of jaw closing did not change over the size range examined here and followed a scaling slope of zero, as predicted by Richard and Wainwright (1995). Linear velocity of both lower jaw opening and closing also scaled with a slope of zero; this is in opposition to the slope of one predicted by Richard and Wainwright (1995) but fits the predictions of Hill (1950).
Angular and linear velocities of lower jaw opening and closing measured during feeding in juvenile Colorado pikeminnow, P. lucius (n = 17). We hypothesized that angular velocities would scale with a slope of zero while linear velocities should scale with a slope of one. Axes are log10-transformed. The only velocity that scaled as predicted was the angular velocity of jaw closing
Four of six elements of the head and jaw took longer to reach maximum displacement as juvenile pikeminnow became larger: time to peak gape (slope = 0.734, r2 = 0.418, p value = 0.005, Fig. 6a), time to peak premaxilla rotation (slope = 0.661, r2 = 0.305, p value = 0.022, Fig. 6b), time to peak lower jaw rotation (slope = 0.742, r2 = 0.405, p value = 0.006, Fig. 6c) and time to peak hyoid depression (slope = 0.391, r2 = 0.297, p value = 0.024, Fig. 6d). No significant linear relationship was found for time to peak cranial rotation (slope = 0.359, r2 = 0.154, p value = 0.119, Fig. 6e). Therefore, peak cranial rotation occurred at 14.2 ± 3.8 ms (mean ± standard deviation) from the onset of the strike. Additionally, time to jaw closing was not found to significantly correlate with pikeminnow total length (slope = 0.277, r2 = 0.042, p value = 0.430, Fig. 6f) indicating that the jaw closed in approximately 6.5 ± 2.4 ms (mean ± standard deviation) independent of total length.
Time to peak kinematic displacement for six kinematic variables tracked during feeding for juvenile Colorado pikeminnow, P. lucius (n = 17). Axes are log10-transformed. All times (except jaw closing) are relative to time zero, where time zero is defined as one frame prior to mouth opening during feeding. It was hypothesized that kinematic timings would not change over our size range of juvenile pikeminnow and thus slopes should not be significantly different from zero. Black lines represent observed slopes for timing variables. In general, kinematic timings indicate that smaller pikeminnow reached maximum excursion of kinematic variables sooner than larger pikeminnow. Time to peak cranial rotation and time to jaw closing were the only variables that were not found to have a significant positive correlation with size, as the slopes were not significantly different from zero. Thus, for the size range of juvenile Colorado pikeminnow examined here, the neurocranium was maximally rotated at 14.2 ± 3.8 ms (mean ± standard deviation) and jaw closing occurred at 6.5 ± 2.4 ms after peak gape
Biomechanical Models: Functional Gape, Suction Potential, and Mechanical Advantage
A model of predicted functional gape (Fig. 7a) illustrates how the gape would change if gape angle were to remain constant as juvenile pikeminnow grow larger. If jaw kinematics were to remain unchanged (dotted line)—that is, if large fish produced the same magnitude of jaw depression as small fish—then larger juvenile pikeminnow would have a much larger functional gape than the observed functional gape (solid line). The anatomical gape, measured from anesthetized pikeminnow by gently spreading the upper and lower jaw with digital calipers, is also included (dashed line). Anatomical gape is larger than functional gape, but not as large as the gape that the fish could hypothetically produce if kinematics increased isometrically as fish grow.
a Predicted functional gape under three possible growth trajectories: (1) jaw kinematics modeled assuming gape angle does not change as juvenile pikeminnow grow larger (dotted line). In this scenario, empirical values of scaling for upper and lower jaw length were used; (2) anatomical gape, as measured by gently spreading the upper and lower jaw apart using calipers (dashed line); (3) functional gape observed in free-feeding juvenile pikeminnow (solid line). It is clear that if kinematics remained constant as juvenile Colorado pikeminnow grow larger, this would result in a much larger functional gape. b Morphological-suction-potential calculated from measured morphological variables on a size range of juvenile Colorado pikeminnow, P. lucius (n = 13). Morphological-suction-potential is a strong indicator of the ability of the fish to generate suction pressure during suction feeding (see Carroll et al. 2004), with higher values of morphological potential correlating to higher values of buccal pressure during feeding. The relationship between morphological-suction-potential and total length for pikeminnow was not significant and indicates that the potential to generate suction pressure changes little over the size range examined. c Mechanical advantage of lower jaw opening and closing, as described by Westneat (2004). Mechanical advantage of opening (closed circles) and mechanical advantage of lower jaw closing (open circles). These results indicate no change in lower jaw mechanical advantage across the size range examined here. d Predicted lower jaw closing time if (1) if jaw kinematics were isometric and did not change as small pikeminnow became larger (dotted line); (2) observed jaw closing time, measured from high-speed video (solid line). If kinematics of jaw opening remained constant, it is clear that as juvenile pikeminnow grew, the lower jaw would take much longer to close
Somewhat surprisingly, the morphological-suction-potential of Colorado pikeminnow, as predicted using the model of Carroll et al. (2004), did not change as fish grew larger (slope = −4.45 × 10−6, r2 = 0.014, p value = 0.699, n = 13, Fig. 7b). Based on their morphology, juvenile pikeminnow do not change in their ability to produce suction pressure during feeding as they grow larger. However, we note that, relative to centrarchids (Carroll et al. 2004), the morphological-suction-potential of juvenile Colorado pikeminnow is extremely low at all sizes.
Mechanical advantage (MA) of lower jaw opening (closed circles, slope = 3.15 × 10−6, r2 = 0.007, p value = 0.710, Fig. 7c) and lower jaw closing (open circles, slope = 2.44 × 10−4, r2 = 0.122, p value = 0.110, Fig. 7c) did not change with total length. Despite the observed positive allometry of the lower jaw (with respect to TL), the in-lever to out-lever ratios were surprisingly consistent across the size range of pikeminnow. This consistency suggests that the entire lower jaw is growing proportionally (with respect to itself) during juvenile development.
Discussion
Most variables quantifying the morphology of the cranium and jaws of juvenile pikeminnow, including anatomical gape, scaled with isometry. Anatomical gape approached 15 mm in the largest juvenile pikeminnow, which is a similar anatomical gape to centrarchid fishes during the dietary transition from invertebrate-prey to fish-prey (Wainwright and Richard 1995). Additionally, the isometry found in mechanical advantage of the pikeminnow lower jaw parallels the isometry seen in the mechanical advantage of Sphyraena barracuda, a marine piscivore (Habegger et al. 2011). However, kinematic excursions of elements of the cranium and jaws during feeding typically scaled with negative allometry: that is, larger juvenile pikeminnow demonstrated reduced kinematic excursions, relative to smaller pikeminnow. We also found that the morphological-suction-potential for suction production did not appear to change as fish grew larger, a result that did not support the hypothesis that fish transition from a more suction-based to a more ram-based feeding behavior as they grew larger. In addition, the unanticipated finding that cranial movements decrease in magnitude (negative allometry), even though most aspects of cranial morphology scale with isometry reveals that pikeminnow undergo negative allometric changes in behavior. These findings raise a suite of new questions. (1) Given the overall trend of isometric growth, what is the significance of the handful of morphological variables that demonstrate positive or negative allometry? (2) Why are kinematic excursions greater in small juveniles, relative to larger juveniles—especially variables related to mouth-gape? (3) What, if anything, can we conclude about the ecological ramifications of this complex suite of anatomical and behavioral changes for pikeminnow in the wild?
As juvenile Colorado pikeminnow grow larger, head depth becomes relatively shallower and jaw length becomes proportionally longer. This overall change in head shape parallels an evolutionary change in cranial morphology exhibited by piscivorous, ram-feeding snakes (Herrel et al. 2008). Herrel and his team of researchers noted that multiple lineages of snakes that feed on fish in the water column via a forward-directed strike have independently converged on a dorsoventrally shallow neurocranium with an elongated jaw (Herrel et al. 2008). Additionally, piscivorous fishes that employ fast, forward directed strikes during feeding (such as barracuda and needlefish, see Porter and Motta 2004), also possess a dorsoventrally flattened head and an elongated jaw. Following Herrel et al. (2008), we posit that the increased jaw length and decreased dorsoventral profile of the head seen in larger juvenile pikeminnow likely serve to reduce hydrodynamic drag during the rapid strike that is necessary to capture potentially elusive prey. A dorsoventrally shallow head and long jaws may be functionally advantageous when rapidly feeding on fast moving prey in the water column, given that several vertebrate taxa with similar diets all show convergence on these morphological characteristics.
Because the midwater invertebrates that serve as the primary prey of young pikeminnow are expected to be captured via a suction-dominated mechanism (smaller prey are more easily drawn into the mouth, see Cook 1996), whereas fish-prey are often consumed via a ram-dominated mechanism (such as that exhibited by barracuda, Porter and Motta 2004), we initially predicted that juvenile pikeminnow would demonstrate reduced reliance on suction production as they grew. In fact, the morphological-suction-potential model (Carroll et al. 2004) for juvenile pikeminnow did not support the hypothesis that a shift in feeding mode occurs, which suggests that the suction component of the strike remains consistent across the size range considered here. However, we note that it is possible that the morphological-suction-potential model does not predict suction ability for this species; in fact, the model has only been empirically validated in centrarchid fishes (bass, crappies and sunfishes). In addition, the morphological-suction-potential model developed by Carroll et al. (2004) assumes that peak buccal pressure and fluid flow into the mouth are tightly linked. However, the relationship between fluid speed at the mouth aperture and buccal pressure varies with morphology in different species of centrarchids (Higham et al. 2006). Therefore, buccal pressure estimates cannot be used to infer fluid speed into the mouth across ontogenetic stages (Higham et al. 2006; Van Wassenbergh et al. 2006). Thus, we cannot exclude the possibility that positive allometric growth of the lower jaw and negative allometry in functional gape may have implications for the flow of water into the mouth, and thus for prey capture dynamics, as pikeminnow grow larger.
However, we suggest that the primary reason that pikeminnow produce smaller magnitude cranial movements and a proportionally smaller mouth-gape as they grow larger is because of the intrinsic trade-off between magnitude of mouth opening and the velocity of jaw closing. If large pikeminnow were to rotate/depress the lower jaw to the same extent as small pikeminnow, this would generate a very large functional gape (dotted line on Fig. 7a); however, extrapolating from empirical values for jaw closing velocity (as measured from the high-speed video), a larger functional gape would take much longer to close (approximately twice as long, see dotted line, Fig. 7d). Interestingly, the jaw closing MA (open circles on Fig. 7c) does not suggest a trend toward a lever system that will increase jaw-closing velocity as juvenile pikeminnow grow. Piscivores such as gar, barracuda, and needlefish, with their extremely elongate jaws (Porter and Motta 2004), have a jaw closing lever system that favors high velocities (but low force, see Westneat 2004; Habegger et al. 2011) and rely on sharp conical teeth to hold fish prey (Porter and Motta 2004). Ontogenetic measurements of mechanical advantage of jaw-closing in S. barracuda reveals isometric scaling (Habegger et al. 2011) and is thought to reflect little to no change in mechanical demands of prey capture, as juvenile and adult S. barracuda are piscivorous. Changes in mechanical advantage of the jaw-closing lever to favor high force production are typically seen during ontogeny of durophagous organisms (for example Archosargus probatocephalus, see Hernandez and Motta 1997), in which proportionally more hard-shelled prey are incorporated into the diet as animals grow larger. In contrast, the MA of the juvenile pikeminnow lower-jaw closing-lever suggests a compromise between force production and velocity, perhaps because the jaws must close rapidly and the prey may be seized between the anterior jaws during feeding. During the course of this study, juvenile pikeminnow were occasionally observed grabbing the prey between the jaws (pers. obs.), however, live fish have elongate bodies that may be more likely to extend out the sides of the mouth than the cube-shaped model prey items used here.
We undertook this study with the expectation that a very large mouth opening enhances the capture of elusive prey-fishes by piscivores. We examined morphology and behavior in juvenile pikeminnow across a dietary transition with the expectation that either morphology or behavior of the fish (or both) would change to enable the fish to produce a very large mouth-gape. However, the actual changes that occur in this unusual and imperiled cyprinid predator during juvenile development tell a very different story. Morphology changes as fish grow to produce a more streamlined cranium that may reduce drag during a rapid, anteriorly directed strike. Concomitant behavioral changes reduce the magnitude of the jaw movements, which appears to facilitate a very rapid opening and closing of the jaws during the gape cycle—a key aspect of the strike for capturing prey within the buccal cavity or entrapping it between the jaws. Thus for juvenile pikeminnow, in contrast to what has been suggested for other piscivores (Hoyle and Keast 1988; Mittelbach and Persson 1998), speed and stealth during the strike appear to be much more important when feeding than the size of the mouth opening.
References
Carroll, A. M., Wainwright, P. C., Huskey, S. H., Collar, D. C., & Turingan, R. G. (2004). Morphology predicts suction feeding performance in centrarchid fishes. The Journal of Experimental Biology, 207, 3873–3881.
Cook, A. (1996). Ontogeny of feeding morphology and kinematics in juvenile fishes: A case study of the cottid fish Clinocottus analis. The Journal of Experimental Biology, 199, 1961–1971.
Coughlin, D. J. (1994). Suction prey capture by clownfish larvae (Amphiprion perideraion). Copeia, 1994(1), 242–246.
Cullum, A. J. (2003). Didge-image digitizing software. Version 2.3.
Ferry-Graham, L. A., Hernandez, L. P., Gibb, A. C., & Pace, C. (2010). Unusual kinematics and jaw morphology associated with piscivory in the poeciliid, Belonesox belizanus. Zoology, 113, 140–147.
Fuiman, L. A. (1983). Growth gradients in fish larvae. Journal of Fish Biology, 23, 117–123.
Gibb, A. C., & Ferry-Graham, L. (2005). Cranial movements during suction feeding in teleost fishes: Are they modified to enhance suction production? Zoology, 108, 141–153.
Habegger, M. L., Motta, P. J., Huber, D. R., Deban, S. M. (2011). Feeding biomechanics in the Great Barracuda during ontogeny. Journal of Zoology, 283(1), 63–72.
Hambright, K. D. (1991). Experimental analysis of prey selection by largemouth bass: Role of predator mouth width and prey body depth. Transactions of the American Fisheries Society, 120, 500–508.
Hernandez, L. P. (2000). Intraspecific scaling of feeding mechanics in an ontogenetic series of zebrafish, Danio rerio. The Journal of Experimental Biology, 203, 3033–3043.
Hernandez, L. P., & Motta, P. J. (1997). Trophic consequences of differential performance: Ontogeny of oral jaw-crushing performance in the sheepshead, Archosargus probatocephalus (Teleostei, Sparidae). Journal of Zoology, 243, 737–756.
Herrel, A., Vincent, S. E., Alfaro, M. E., Van Wassenbergh, S., Vanhooydonck, B., & Irschick, D. J. (2008). Morphological convergence as a consequence of extreme functional demands: Examples from the feeding system of natricine snakes. Journal of Evolutionary Biology, 21, 1438–1448.
Higham, T. E., Day, S. W., & Wainwright, P. C. (2006). The pressures of suction feeding: The relation between buccal pressure and induced fluid speed in centrarchid fishes. The Journal of Experimental Biology, 209, 3281–3287.
Hill, A. V. (1950). The dimensions of animals and their muscular dynamics. Science Progress, 38, 209–230.
Holzman, R., Collar, D. C., Mehta, R. S., & Wainwright, P. C. (2012). An integrative modeling approach to elucidate suction-feeding performance. The Journal of Experimental Biology, 215, 1–13.
Hoyle, J. A., & Keast, A. (1988). Prey handling time in two piscivores, Esox americanus vermiculatus and Micropterus salmoides, with contrasting mouth morphologies. Canadian Journal of Zoology, 66, 540–542.
Keast, A. (1985). The piscivore feeding guild of fishes in small freshwater ecosystems. Environmental Biology of Fishes, 12(2), 119–129.
Luczkovich, J. J., Norton, S. F., & Gilmore, R. G. (1995). The influence of oral anatomy on prey selection during the ontogeny of two percoid fishes, Lagodon rhomboides and Centropomus undecimalis. Environmental Biology of Fishes, 44, 79–95.
McCormick, M. I. (1998). Ontogeny of diet shifts by a microcarnivorous fish, Cheilodactylus spectabilis: Relationship between feeding mechanics, microhabitat selection and growth. Marine Biology, 132, 9–20.
Minckley, W. L. (1973). Fishes of arizona. Arizona Game and Fish Department.
Mittelbach, G. G., & Persson, L. (1998). The ontogeny of piscivory and its ecological consequences. Canadian Journal of Fisheries and Aquatic Sciences, 55, 1454–1465.
Nemeth, D. H. (1997). Modulation of buccal pressure during prey capture in Hexagrammos decagrammus (Teleostei: Hexagrammidae). The Journal of Experimental Biology, 200, 2145–2154.
Nilsson, P. A., & Brönmark, C. (2000). Prey vulnerability to a gape-size limited predator: Behavioural and morphological impacts on northern pike piscivory. Oikos, 88, 539–546.
Norton, S. F. (1991). Capture success and diet of cottid fishes: The role of predator morphology and attack kinematics. Ecology, 72(5), 1807–1819.
Norton, S. F., & Brainerd, E. L. (1993). Convergence in the feeding mechanics of ecomorphologically similar species in the Centrarchidae and Cichlidae. The Journal of Experimental Biology, 176, 11–29.
Porter, H. T., & Motta, P. J. (2004). A comparison of strike and prey capture kinematics of three species of piscivorous fishes: Florida gar (Lepisosteus platyrhincus), redfin needlefish (Strongylura notata), and great barracuda (Sphyraena barracuda). Marine Biology, 145, 989–1000.
Price, S. A., Tavera, J. J., Near, T. J., & Wainwright, P. C. (2012). Elevated rates of morphological and functional diversification in reef-dwelling haemulid fishes. Evolution, 1–12.
Rasband, W. S. (2011). ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA. http://imagej.nih.gov/ij/, 1997–2011.
Richard, B. A., & Wainwright, P. C. (1995). Scaling the feeding mechanism of largemouth bass (Micropterus salmoides): Kinematics of prey capture. The Journal of Experimental Biology, 198, 419–433.
Schmitt, R. J., & Holbrook, S. J. (1984). Ontogeny of prey selection by black surfperch Embiotoca jacksoni (Pisces: Embiotocidae): The roles of fish morphology, foraging behavior and patch selection. Marine Ecology Progress Series, 18, 225–239.
Sokal, R. R., & Rohlf, F. J. (1995). Biometry. New York: WH Freeman and Company.
Van Wassenbergh, S., Aerts, P., & Herrel, A. (2006). Hydrodynamic modelling of aquatic suction performance and intra-oral pressures: Limitations for comparative studies. Journal of the Royal Society, Interface, 3, 507–514.
Vanicek, C. D., & Kramer, R. H. (1969). Life history of the Colorado squawfish, Ptychocheilus lucius, and the Colorado chub, Gila robusta, in the Green River in Dinosaur National Monument, 1964–1966. Transactions of the American Fisheries Society, 98(2), 193–208.
Wainwright, P. C., & Richard, B. A. (1995). Predicting patterns of prey use from morphology of fishes. Environmental Biology of Fishes, 44, 97–113.
Westneat, M. W. (2004). Evolution of levers and linkages in the feeding mechanisms of fishes. Integrative and Comparative Biology, 44, 378–389.
Acknowledgments
David Ward of the Arizona Game and Fish Department was instrumental in providing us with juvenile pikeminnow for this study. This research was supported by the following grants: National Science Foundation IOS-0726001 to ACG and Science Foundation Arizona CAA 0057-07 to ACG.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Standards
The experiments performed in this study complied with all U.S. laws regarding housing, care and handling of research animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burnette, M.F., Gibb, A.C. Do Changes in Morphology and Prey-Capture Movements Facilitate a Dietary Transition in Juvenile Colorado pikeminnow, Ptychocheilus lucius?. Evol Biol 40, 261–275 (2013). https://doi.org/10.1007/s11692-012-9207-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-012-9207-2