Abstract
Over evolutionary time, predator-prey interactions have shaped and constrained functional and behavioral traits of piscivorous fishes. The endangered Colorado Pikeminnow Ptychocheilus lucius, a large endemic piscivore of the Colorado River Basin, encounters a substantially altered prey base that differs in behaviors and morphologies compared to the historical suite of native prey. To assess physical limitations of Colorado Pikeminnow predation, we conducted a feeding experiment with two species of nonnative prey (spined and despined Channel Catfish Ictalurus punctatus and Red Shiner Cyprinella lutrensis) and quantified scaling of cranial morphology in this predator. In our predation experiments, Colorado Pikeminnow (215–312 mm total length) consumed both spined and despined Channel Catfish as well as Red Shiner but only consumed prey less than 20% of the predator’s total length. Previous feeding trials using smaller Colorado Pikeminnow, with native and nonnative prey species, indicated they consumed prey up to 35% of their total length, suggesting relative prey size limits may decrease as this predator grows. Morphological measurements also suggested relative prey size suitability may decrease as Colorado Pikeminnow become larger, with head depth and width demonstrating isometric scaling at small sizes and shifting to negative allometry as fish get larger. Together, these data suggest an ontogenetic shift in the head morphology of Colorado Pikeminnow may decrease the relative size of prey available to these predators. In severely altered systems, understanding trophic characteristics that limit overall predator resource availability will be critical for conservation of piscivorous fishes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selective pressures stemming from predator-prey interactions likely shape and constrain phenotypic characteristics of predators (Garland et al. 1990; Agrawal 2001; Costa et al. 2015; Yamamichi and Ellner 2016). Yet, rapid changes have occurred within aquatic systems through the introduction of nonnative fish species (Cucherousset and Olden 2011). In these altered systems it has been common to study predator-prey interactions with nonnative piscivorous fishes (Clarkson et al. 2005; Mueller 2005; Franssen et al. 2014a; Propst et al. 2014). Less studied, however, are cases with native fishes as predators and nonnative species as prey (Carlsson et al. 2009). Ultimately, predicting how physical characteristics resulting from historical complex functional tradeoffs have affected predator–prey interactions will be important for quantifying the current trophic structure of aquatic systems (Schmitz et al. 2004; Preisser et al. 2005; Baldridge and Smith 2008; Holzman et al. 2011).
Cyprinid piscivores are particularly interesting because these fishes seem poorly adapted for predation as they lack jaw teeth, have a relatively small pharyngeal cavity, and lack a true stomach (Nelson 1994; De Graaf et al. 2010). Moreover, within this highly abundant and geographically wide-ranging family of fishes, specialization in piscivory is rare (Nelson 1994; De Graaf et al. 2010). Of these rare specialists, the Colorado Pikeminnow (Ptychocheilus lucius), entirely piscivorous as an adult and the largest cyprinid native to North America, evolved as the top predator in the Colorado River system (Vanicek and Kramer 1969; Portz and Tyus 2004). Indeed, the predatory pressure this cyprinid piscivore exerted is hypothesized to have been so great as to drive the evolution of large nuchal humps in two Colorado River Basin prey species (Portz and Tyus 2004).
By 1967, populations of Colorado Pikeminnow declined to such an extent the species was federally listed as endangered (USFWS 1967). Concurrent with the decline of this top predator, the composition and abundance of potential prey fishes also changed through introduction and establishment of 62 nonnative fishes and declines in the 28 native species (Tyus and Saunders 2000; Olden et al. 2006). While the size ranges of introduced and native fishes generally overlap (Olden et al. 2006), it is difficult to quantify current prey resources for Colorado Pikeminnow as prey availability likely varies spatiotemporally and is dependent on both predator and prey life-stages. Nonetheless, quantifying phenotypic constraints to prey consumption will be a first step in understanding trophic interactions in non-coevolved systems as well as providing more realistic and precise parameters for use in bioenergetic modeling, a tool often used to support conservation and recovery (Stergiou and Karpouzi 2002; USFWS 2002; Miller and Lamarra 2006; Wirtz 2012; SJRIP 2016).
Understanding how life-history traits, especially in systems with altered prey bases, impact the trophic interactions of Colorado Pikeminnow is difficult due to our limited understanding of factors constraining piscivory through ontogeny. Like most piscivorous fishes, Colorado Pikeminnow undergo an ontogenetic diet shift with a switch from feeding on small invertebrates to piscivory at about 200 mm total length (Vanicek and Kramer 1969). For some piscivores, gape size may constrain the suite and size of available prey (Nilsson and Bronmark 2000) and for Colorado Pikeminnow prey consumption has been assumed to be a function of gape proportional to predator length (Portz and Tyus 2004). But successful consumption could be more dependent on feeding biomechanics like those which result in increasing suction potential (Norton 1991; Shadwick and Lauder 2006) or those which afford hydrodynamics efficiencies (Burnette and Gibb 2013). Yet, our current understanding of how growth and morphology may affect successful Colorado Pikeminnow prey consumption is lacking.
To date, the few feeding experiments conducted indicate limits to prey consumption may not be constant over a size range of Colorado Pikeminnow. At a size in which Colorado Pikeminnow are still likely omnivorous (<200 mm TL), Franssen et al. (2007) demonstrated Colorado Pikeminnow consumed fish prey up to 35% of the Colorado Pikeminnow’s body length. The suite of prey species used by Franssen et al. (2007) was three native and one nonnative fish species - Red Shiner, Cyprinella lutrensis. Pimental et al. (1985) offered larger Colorado Pikeminnow (388–510 mm TL) three other nonnative prey species, Channel Catfish (Ictalurus punctatus) –spined and despined– and two nonnative trout Onchorynchus spp. In this instance Colorado Pikeminnow, on average, only consumed prey up to 20% of the Colorado Pikeminnow’s body length. Together, these feeding experiments indicated a possible reduction in the relative size of prey available to Colorado Pikeminnow as they grow.
A mechanistic understanding of changes in feeding ecology as a function of ontogenetic development may be derived by assessing changes in morphology. Burnette and Gibb (2013) found negative allometric scaling of head depth in small Colorado Pikeminnow (<200 mm TL). Although this may suggest relative prey size availability changes through ontogeny, negative allometric growth did not result in decreased suction potential (Burnette and Gibb 2013) and this scaling could also benefit a transition to a more ram-based feeding strategy (Porter and Motta 2004; Herrel et al. 2008). However, in both the Colorado Pikeminnow morphological assessment and feeding experiments, inferences into changes in predator-prey size consumption due to physical constraints were limited as those investigations did not include the size range at which an ontogenetic diet shift to piscivory is presumed to occur (~ 200 mm TL; Vanicek and Kramer 1969).
To help understand physical constraints to trophic interactions in non-coevolved predator-prey systems we investigated consumption of nonnative prey by Colorado Pikeminnow over size ranges at which an important ontogenetic transition occurs (i.e., shift to piscivory) and assessed whether morphological changes in feeding structures occurred which could physically limit the size of available of prey. We conducted a feeding experiment quantifying the maximum relative prey size of Channel Catfish and Red Shiner consumed by Colorado Pikeminnow (> 200 mm TL). These prey species were chosen because: 1) results could be compared to prior feeding experiments (Pimental et al. 1985; Franssen et al. 2007); 2) both species are abundant in the San Juan River as prey where a population of Colorado Pikeminnow persists - Channel Catfish is the most abundant large-bodied and Red Shiner the most abundant of the small-bodied nonnative species (Franssen et al. 2014b; Franssen et al. 2016a); and 3) the anti-predator defense (spined dorsal and pectoral fins) of Channel Catfish may be a deterrent to consumption or threat to Colorado Pikeminnow survival when ingested (Pimental et al. 1985; Ryden and Smith 2002). We paired the feeding experiment with an assessment of morphological scaling, to identify potential physical cranial limitations of Colorado Pikeminnow piscivory, over a range of sizes in which an ontogenetic transition to these prey species would occur.
Methods
Colorado Pikeminnow, and prey sources, and housing
Colorado Pikeminnow were obtained from US Fish and Wildlife Service Southwestern Native Aquatic Resource and Recovery Center (Southwestern Native ARRC), Dexter, NM. Those used during the feeding experiment (n = 41) averaged 325 mm TL (range = 248–375 mm TL). Channel Catfish (n = 276) used as prey averaged 25 mm TL when first acquired from Inks Dam National Fish Hatchery, Burnet, TX and Red Shiner (n = 115) averaged 35 mm TL when collected from the Rio Grande in Albuquerque, NM. All fishes were housed at the US Fish and Wildlife Service New Mexico Fish and Wildlife Conservation Office (NMFWCO), Albuquerque, NM.
Colorado Pikeminnow feeding trials
We conducted experiments in 0.75 m3 tanks connected through a recirculating water system. Three arenas (0.25 m3) were delineated in each tank and pelleted feed was withheld from Colorado Pikeminnow for 72 h prior to each experiment. Both Colorado Pikeminnow and prey fishes were anesthetized using the label-recommended dose of Tricaine methanesulfonate (MS222) and length (standard [SL] and total [TL]) was measured prior to each experimental pairing. Predator and prey remained in an arena for 4.5 d with no other feed offered. Observations were made each morning and evening to determine if a predation event had occurred and the health status of Colorado Pikeminnow.
We conducted four different predation trials (Table 1). Trials 1 and 2 consisted of a single Colorado Pikeminnow and single Channel Catfish per arena; trials 3 (Channel Catfish) and 4 (Red Shiner) consisted of three Colorado Pikeminnow and three prey fish in each arena as observations indicated Colorado Pikeminnow fed more often when competitors were present (Pimental et al. 1985, personal communications Manuel Ulibarri, Southwestern Native ARRC). Prior to being offered to Colorado Pikeminnow, we removed the dorsal and pectoral spines from half the Channel Catfish using a scalpel to evaluate potential effects of spines on size specific susceptibility to consumption as well as mortality related to consumption of spined individuals (Ryden and Smith 2002). Each trial occurred over a three-week period with a month between trials to allow prey fish time to grow (i.e., change prey-predator size ratios; Table 1). Trial 1 consisted of 41 Colorado Pikeminnow randomly offered a spined or despined Channel Catfish, whereas trial 2 consisted of only 38 of the 41 Colorado Pikeminnow (Table 1). In trials 3 and 4, 36 of the Colorado Pikeminnow were offered prey. Channel Catfish offered in trial 3 were all either spined or despined in each arena. The type of Channel Catfish (spined or despined) offered to individual Colorado Pikeminnow (identified through a passive integrated transponder) was switched from one trial to the next to diminish the effect of any learned behaviors. Red Shiner was offered to Colorado Pikeminnow in trial 4 not only to determine if this nonnative prey species would be consumed, but also to assess whether Colorado Pikeminnow developed an antagonistic behavior to consuming prey in general; a possible response to being offered spined Channel Catfish in prior trials. Red Shiner offered in trial 4 was similar in size to Channel Catfish used in trials 1 and 2 (Table 1).
To allow a comparison to feeding experiments by Pimental et al. (1985) and Franssen et al. (2007), prey to predator length ratios were calculated for all feeding trials. In trials 1 and 2, the prey–predator ratio was simply calculated between the two fishes. For trials 3 and 4, the ratio was calculated as the mean length of the three Colorado Pikeminnow and the mean length of the three prey fish. To assess the ontogenetic effects on the potential of a predation event, we qualitatively compared the prey–predator size ratios from our feeding trials with those conducted by Franssen et al. (2007) and Pimental et al. (1985). To assess whether Channel Catfish spines had an effect on consumption we applied a two-tailed independent samples t-test (assuming equal variances) of consumed spined and despined Channel Catfish in trials 1–3.
Ontogenetic changes in Colorado Pikeminnow cranial morphology
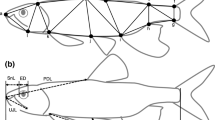
We used 223 individuals [live and those preserved from Franssen et al. 2016b] to quantify morphological changes through ontogeny in Colorado Pikeminnow. The caudal fin from preserved specimens had been removed, thus SL was measured for all specimens. Preserved specimens ranged in size from 96 to 213 mm SL while live fish ranged in size from 215 to 312 mm SL. We made five cranial measurements on each fish using linear distances: (1) premaxilla length – anterior portion of the premaxilla bone to its articulation with the dentary bone; (2) lower jaw – anterior to posterior length of dentary bone; (3) head length – anterior portion of the snout to the posterior margin of the operculum; (4) head depth – measured posterior to the eye; and (5) head width – laterally across the dorsal surface of the head, measured posterior to the eye (Fig. 1). Standard length was measured as the linear distance anterior of the snout to the posterior end of the mid-lateral portion of the hypural plate. Measurements on live fish were made after each fish had been anesthetized using the label recommended dose of MS222. Using digital calipers, two observers measured each fish independently and these measurements were averaged for each fish.
Regression-based analyses of cranial measurements and length were conducted to test for non-linear scaling of cranial variables and identify isometric versus allometric scaling of traits. We used segmented linear regression with the package ‘segmented’ (in the R statistical language; R Development Core Team 2015) to assess if a statistical nonlinear ‘break’ could be identified in each trait as fish grew. If no breaks between Colorado Pikeminnow length and trait measured was detected, we applied a simple linear regression and assessed allometry from 95% confidence intervals of estimated slopes. Isometric scaling is indicated when the slope is not significantly different from one and allometric scaling when slopes are significantly different from one (negative if <1 and positive if >1). All variables were log10 transformed prior to analyses to linearize variables.
Results
Colorado Pikeminnow feeding trials
Overall, Colorado Pikeminnow consumed Channel Catfish whose prey–predator size ratio was ≤0.17 (Table 2 and Fig. 2).There was no difference in the size of consumed spined and despined Channel Catfish (t11 = 0.60, p = 0.56) and Colorado Pikeminnow did not demonstrate apparent negative effects from consuming Channel Catfish such as choking or mortality. Of 41 possible predation events in trial 1, 26% of the Channel Catfish offered were consumed (six spined and five despined). The mean prey–predator size ratio for consumed (mean = 0.12; SD = 0.02) and uneaten fish (mean = 0.13; SD = 0.02) was similar. In trial 2, the mean overall prey–predator size ratio in the arenas increased to 0.19 (range = 0.15–0.25) and of 38 prey offered, two despined Channel Catfish were consumed. The prey–predator size ratio for these two predation events was 0.15 and 0.17. In trial 2, two despined Channel Catfish, found dead with presumed bite marks, were not considered consumed. In trial 3, where three Colorado Pikeminnow and three Channel Catfish were paired in each arena, no predation events occurred. The mean prey–predator size ratio among arenas was 0.22 (SD = 0.01, range = 0.20–0.27).
Relationship between Colorado Pikeminnow total length and prey-predator size ratio for consumed (red symbols) and uneaten prey (open symbols). Panel (a) data redrawn from Franssen et al. (2007) includes four species of prey fish (Bluehead Sucker, Flannelmouth Sucker, Speckled Dace, and Red Shiner); panel (b) results from the feeding experiment reported herein; and panel (c) data redrawn from average sizes of predator and prey in Pimental et al. (1985) trials which included three prey species (Channel Catfish with and without spines, Rainbow Trout, and Rio Grande Cutthroat Trout)
In the fourth trial, when Red Shiner were presented as a novel prey type, a total of six fish were consumed. The arena with the largest Red Shiner consumed had a prey–predator size ratio of 0.19 (Table 2). The mean ratios of consumed and uneaten Red Shiner was 0.16 (SD = 0.01) and 0.17 (SD = 0.02), respectively. The prey–predator size range of consumed (0.15–0.19) Red Shiner and those left uneaten (0.13–0.21), overlapped.
Ontogenetic changes in Colorado Pikeminnow cranial morphology
Of the five variables measured, three traits changed linearly while two indicated an ontogenetic change in scaling as fish increased in size. Premaxilla and lower jaw measurements changed linearly and were slightly positively allometric (slope = 1.03 [1.01–1.06, 95% CI] and slope = 1.04 [1.02–1.07], respectively), while head length was isometric (Fig. 3). Conversely, head depth and width exhibited a nonlinear ‘break’ in scaling as a function of standard length. For head depth, the estimated length at which the breakpoint occurred was 147 mm SL (135–160 95% CI). The slope of the first segment indicated isometric scaling, whereas the second segment indicated negative allometric scaling (slope = 0.75 [0.71–0.79]). Head width showed similar trends, as the first segment demonstrated isometry and the second, negative allometry (slope = 0.66 [0.58–0.74]). The ontogenetic change in scaling of head width occurred, on average, at a greater length than for depth, as the estimated breakpoint for head width was 171 mm SL (155–181 95% CI).
Linear and segmented regressions of Colorado Pikeminnow cranial measurements are shown as a function of standard length (log10 transformed). Slope (b1) and 95% confidence interval around each slope shown. A segment break was significant for head depth and width (slopes and 95% confidence interval for each segment, S1 and S2). For these traits horizontal line denotes upper and lower 95% confidence interval for estimated segment break point (closed circle)
Discussion
Consumption of spined Channel Catfish did not visibly harm Colorado Pikeminnow and consumption of both Channel Catfish, whether spined or despined, and Red Shiner, appeared to be limited by a function of the size ratio between prey and predator. Our results were similar to feeding trials by Pimental et al. (1985) whose larger Colorado Pikeminnow (388–510 mm TL) consumed spined Channel Catfish without negative consequences and prey were consumed, on average, at size ratios between 0.14–0.20. However, as it pertains to size ratios of consumed prey, our results were dissimilar to feeding trials using smaller Colorado Pikeminnow (Franssen et al. 2007; mean = 110–199 mm TL) where prey were consumed at a ratio as large as 0.35. Whereas Franssen et al. (2007) used three native prey species, they also offered nonnative Red Shiner. This nonnative species was consumed at a higher prey-predator size ratio (0.35) than observed for the larger Colorado Pikeminnow (248–375 mm TL) used in this study (0.19). The data sets of Pimental et al. (1985) and Franssen et al. (2007), as well as our study suggest that as Colorado Pikeminnow grow, the relative prey size they selectively consume, decreases.
Constraining prey consumption to a size ratio of <0.19 was generally unexpected for this top predator given some piscivorous fishes can consume prey at size ratios as great as 0.70–0.89 (Winemiller and Kelso-Winemiller 1994; Scharf et al. 2000). However, Colorado Pikeminnow may be more similar to other specialized piscivorous cyprinids which limit prey consumption to a prey-predator size ratio ≤ 0.26 (De Graaf et al. 2008). The reduction in the smaller relative size of prey consumed by Colorado Pikeminnow as they grow might reflect evolutionary constraints or reduced evolutionary pressures to consume proportionally large prey over ontogeny.
The physical basis for this ontogenetic change in relative prey size may derive from Colorado Pikeminnow cranial structures shifting from isometry to negative allometry as fish increase in size. When an ontogenetic shift to piscivory is presumed to occur (~200 mm TL), the heads of Colorado Pikeminnow became disproportionately shallow and narrow as compared to the length of the head (scaled isometrically) and the premaxilla and lower jaw (both exhibited positive allometry). These results are slightly different from those of Burnette and Gibb (2013) who used smaller Colorado Pikeminnow (47–161 mm TL) and found three traits scaled isometrically (premaxilla, head length, and head width) and the other two scaled allometrically (head depth scaled negatively and lower jaw length positively). Together, these data indicate Colorado Pikeminnow undergo complex ontogenetic changes to their head morphology that could affect size-specific availability of prey by altering physical or behavioral relative gape limitations.
The ontogenetic changes which result in a dorsoventrally flattened head in larger Colorado Pikeminnow may reflect evolutionary trade-offs between form and function. While negative allometry of some cranial traits could reduce the relative size of prey consumed or decrease bite force (Herrel et al. 2001; Herrel and O’Reilly 2005), a streamlined cranium is also produced, potentially reducing hydrodynamic drag allowing for faster strikes used in ram-based feeding (Porter and Motta 2004; Herrel et al. 2008). Another trade-off could occur if a dorsoventrally flattened head also decreases a fish’s bite angle, which could benefit these predators by reducing stresses on the skull (Bourke et al. 2008). Negative allometry may also result in increased stealth and ability to rapidly open and close the jaw (Burnette and Gibb 2013). Understanding these performance-based trade-offs in Colorado Pikeminnow as it transitions to piscivory will likely require additional assessments such as shape (geometric morphometrics) and structural force (finite element) analyses which could be important to explain these patterns.
As with the other Colorado Pikeminnow feeding and morphological investigations conducted using hatchery fish (Pimental et al. 1985; Franssen et al. 2007; Burnette and Gibb 2013), we assumed fish cultured in the hatchery reflect natural behaviors and morphological variation of the species. It is not unusual for fishes reared in artificial environments to limit consumption and feed less efficiently as compared to wild fish (Sundstrom and Johnsson 2001) and Colorado Pikeminnow used in our feeding experiment consumed few fish overall. However, the purpose of this investigation was not to quantify the consumption rate of nonnative prey. Colorado Pikeminnow used during this investigation could be demonstrating morphological variation related to captive rearing as phenotypic plasticity in head, jaw, and fin characteristics has been observed in other fishes held in captivity (Imre et al. 2002; Wringe et al. 2015). Future efforts to assess limits to consumption by wild Colorado Pikeminnow and variation of head morphology will likely need to assess the transferability of our findings using hatchery fish to natural populations.
The ontogenetic change in Colorado Pikeminnow’s feeding apparatus was likely evolutionarily advantageous, but morphological or behavioral constraints to relative prey size availability could be detrimental in systems where the composition and abundance of suitably-sized prey has been altered. Understanding how these life-history characteristics and others may interact with contemporary environmental conditions to impede recovery will be critical. For many imperiled species, especially top predators such as Colorado Pikeminnow, bioenergetic modeling can be conducted to understand how a species’ trophic ecology and contemporary conditions interact to exert pressures on or limit population growth (Petersen et al. 2008). Yet, the capability of bioenergetics modeling to predict the trophic and biotic structure of aquatic systems is based on accurate assumptions of predator-prey interactions (Stergiou and Karpouzi 2002; Wirtz 2012). For Colorado Pikeminnow, our data suggest relative prey size availability, whether native or nonnative, will vary over different ontogenetic stages and once fully piscivorous, Colorado Pikeminnow may be constrained to consumption of prey less than a quarter its length.
References
Agrawal AA (2001) Phenotypic plasticity in the interactions and evolution of species. Science 294:321–326
Baldridge AK, Smith LD (2008) Temperature constraints on phenotypic plasticity explain biogeographic patterns in predator trophic morphology. Mar Ecol Prog Ser 365:25–34
Bourke J, Wroe S, Moreno K, McHenry C, Clausen P (2008) Effects of gape and tooth position on bite force and skull stress in the dingo (Canis lupus dingo) using a 3-dimensional finite element approach. PLoS One 3:e2200
Burnette MF, Gibb AC (2013) Do changes in morphology and prey–capture movements facilitate a dietary transition in juvenile Colorado Pikeminnow, Ptychocheilus lucius? Evol Biol 40:261–275
Carlsson NOL, Sarnelle O, Stayer DL (2009) Native predators and exotic prey – an acquired taste? Front Ecol Evol 7:525–532
Clarkson RW, Marsh PC, Stefferud SE, Stefferud JE (2005) Conflicts between native fish and nonnative sport fish management in the southwestern United States. Fisheries 30:20–27
Costa M, Hauzy C, Loeuille N, Méléard S (2015) Stochastic eco–evolutionary model of a prey–predator community. J Math Biol 72:573–622
Cucherousset J, Olden JD (2011) Ecological impacts of non-native freshwater fishes. Fisheries 36:215–230
De Graaf M, Dejen E, Osse JWM, Sibbing FA (2008) Adaptive radiation of Lake Tana’s Labeobarbus species flock (Pisces, Cyprinidae). Mar Freshw Res 59:391–407
De Graaf M, Van de Weerd GH, Osse JMW, Sibbing FA (2010) Diversification of prey capture techniques among the piscivores in Lake Tana’s (Ethiopia) Labeobarbus species flock (Cyprinidae). Afr Zool 45:32–40
Franssen NR, Gido KB, Propst DL (2007) Flow regime affects availability of native and nonnative prey of an endangered predator. Biol Conserv 138:330–340
Franssen NR, Davis JE, Ryden DW, Gido KB (2014a) Fish community responses to mechanical removal of nonnative fishes in a large southwestern river. Fisheries 39:352–363
Franssen NR, Gilbert EI, Propst DL (2014b) Effects of longitudinal and lateral stream channel complexity on native and non-native fishes in an invaded desert stream. Freshw Biol 60:16–30
Franssen NR, Durst SL, Gido KB, Ryden DW, Lamarra V, Propst DL (2016a) Long-term dynamics of large-bodied fishes assessed from spatially intensive monitoring of a managed desert river. River Res Appl 32:348–361
Franssen NR, Gilbert EI, James AP, Davis JE (2016b) Isotopic tissue turnover and discrimination factors following a laboratory diet switch in Colorado Pikeminnow (Ptychocheilus lucius). Can J Fish Aquat Sci 74:265–272
Garland T, Bennett AF, Daniels CB (1990) Heritability of locomotor performance and its correlates in a natural population. Experientia 46:530–533
Herrel A, O’Reilly JC (2005) Ontogenetic scaling of bite force in lizards and turtles. Physiol Biochem Zool 11:31–42
Herrel A, Damme RV, Vanhooydonck B, Vree FD (2001) The implications of bite performance for diet in two species of lacertid lizards. Can J Zool 79:662–670
Herrel A, Vincent SE, Alfaro ME, Wassenbergh SV, Vanhooydonck B, Irschick DJ (2008) Morphological convergence as a consequence of extreme functional demands: examples from the feeding system of natricine snakes. J Evol Biol 21:1438–1448
Holzman R, Collar DC, Mehta RS, Wainwright PC (2011) Functional complexity can mitigate performance trade–offs. Am Nat 177:E69–E83
Imre I, McLaughlin RL, Noakes DLG (2002) Phenotypic plasticity in brook charr: changes in caudal fin induced by water flow. J Fish Biol 61:1171–1181
Miller JM and Lamarra VA (2006) San Juan River population model documentation and report. San Juan River basin recovery implementation program, U.S. Fish and Wildlife Service, Albuquerque, New Mexico
Mueller GA (2005) Predatory fish removal and native fish recovery in the Colorado River Mainstem. Fisheries 30:10–19
Nelson JS (1994) Fishes of the world. New York. Wiley, New York
Nilsson PA, Bronmark C (2000) Prey vulnerability to a gape–size limited predator: behavioural and morphological impacts on northern pike piscivory. Oikos 88:539–546
Norton SF (1991) Capture success and diet of cottid fishes: the role of predator morphology and attack kinematics. Ecology 72:1807–1819
Olden JD, Poff NL, Bestgen KR (2006) Life-history strategies predict fish invasion and extirpation in the Colorado River basin. Ecol Monogr 76:25–40
Petersen JH, DeAngelis DL, Paukert CP (2008) An overview of methods for developing bioenergetics and life history models for rare and endangered species. Trans Am Fish Soc 137:244–253
Pimental R, Bulkle R, Tyus H (1985) Choking of Colorado squawfish, Ptychocheilus lucius (Cyprinidae), on channel catfish, Ictalurus punctatus (Ictaluridae), as a cause of mortality. Southwest Nat 30:154–158
Porter HT, Motta PJ (2004) A comparison of strike and prey capture kinematics of three species of piscivorous fishes: Florida gar Lepisosteus platyrhincus, Redfin needlefish Strongylura notata, and great barracuda Sphyraena barracuda. Mar Biol 145:989–1000
Portz D, Tyus H (2004) Fish humps in two Colorado River fishes: a morphological response to cyprinid predation? Environ Biol Fish 71:233–245
Preisser EL, Bolnick DI, Benard MF (2005) Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology 86:501–509
Propst DL, Gido KB, Whitney JE, Gilbert EI, Pilger TJ, Monie AM, Paroz YM, Wick JM, Monzingo JA, Meyer DA (2014) Efficacy of mechanically removing nonnative predators from a desert stream. River Res Appl 31:692–703
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Ryden DW, Smith JR (2002) Colorado Pikeminnow with a channel catfish lodged in its throat in the San Juan River, Utah. Southwest Nat 47:92–94
San Juan River Basin Recovery Implementation Program (SJRIP) (2016) Long–range plan. San Juan River basin recovery implementation program, U.S. Fish and Wildlife Service, Albuquerque, New Mexico
Scharf FS, Juanes F, Rountree RA (2000) Predator size–prey size relationships of marine fish predators: interspecific variation and effects of ontogeny and body size on trophic–niche breadth. Mar Ecol Prog Ser 208:229–248
Schmitz OJ, Krivan V, Ovadia O (2004) Trophic cascades: the primacy of trait–mediated indirect interactions. Ecol Lett 7:153–163
Shadwick RE, Lauder GV (2006) Fish biomechanics. In: Hoar WS, Randall DJ, Farrell AP (eds) Fish physiology, vol 23. Academic Press, San Diego
Stergiou KI, Karpouzi VS (2002) Feeding habits and trophic levels of Mediterranean fish. Rev Fish Biol Fish 11:217–254
Sundstrom LF, Johnsson JJ (2001) Experience and social environment influence the ability of young brown trout to forage on live novel prey. Anim Behav 61:249–255
Tyus HM, Saunders JF (2000) Nonnative fish control and endangered fish recovery: lessons from the Colorado River. Fisheries 25:17–24
U.S. Fish and wildlife service (USFWS) (1967) Office of the Secretary native fish and wildlife endangered species. Federal Register 48:32(11 March 1967):4001
U.S. Fish and Wildlife Service (USFWS) (2002) Colorado Pikeminnow Ptychocheilus lucius recovery goals: amendment and supplement to the Colorado squawfish recovery plan. U.S. fish and wildlife service, Mountain–prairie region, Denver, Colorado
Vanicek CD, Kramer RH (1969) Life history of the Colorado squawfish, Ptychocheilus lucius, and the Colorado chub, Gila robusta, in the Green River in dinosaur National Monument, 1964–1966. Trans Am Fish Soc 98:193–208
Winemiller KO, Kelso-Winemiller LC (1994) Comparative ecology of the African pike, Hepsetus odoe, and tigerfish, Hydrocynus forskahlii, in the Zambezi River floodplain. J Fish Biol 45:211–225
Wirtz KW (2012) Who is eating whom? Morphology and feeding type determine the size relation between planktonic predators and their ideal prey. Mar Ecol Prog Ser 445:1–12
Wringe BF, Fleming IA, Purchase CF (2015) Rapid morphological divergence of cultured cod of the Northwest Atlantic from their source population. Aquac Environ Interact 7:167–177
Yamamichi M, Ellner SP (2016) Antagonistic coevolution between quantitative and Mendelian traits. Proc R Soc London, Ser B 283:20152926
Acknowledgements
We thank S. Walker, Assistant Project Leader Inks Dam National Fish Hatchery, who provided Channel Catfish used in feeding experiments and M. Ulibarri, Supervisor Southwestern Native ARRC, who provided Colorado Pikeminnow. We thank personnel from NMFWCO, which included but was not limited to, A. Dean, W. Furr, D. Myer, C. Kitcheyan, and R. Ulibarri who assisted in fish husbandry, feeding experiments, and morphological assessment. We also thank A. Snyder, Division of Fishes Collections Manager Museum of Southwestern Biology, for accession of Colorado Pikeminnow specimens. We thank T. Diver for the Colorado Pikeminnow photographs. Lastly we thank, M. Dela Cruz and D. Propst for helpful comments on prior versions of this manuscript. Experimental use of federally listed Colorado Pikeminnow was conducted with U.S. Fish and Wildlife Service Permit No. TE676811-3. Mention of MS222 and R statistical language does not constitute endorsement by U.S. Fish and Wildlife Service.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gilbert, E.I., Durst, S.L., James, A.P. et al. Cranial morphological scaling and relative prey size limitations for a native predator in an invaded system. Environ Biol Fish 101, 1067–1076 (2018). https://doi.org/10.1007/s10641-018-0760-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-018-0760-0