Abstract
Human brain development has been studied intensively with neuroimaging. However, little is known about how genes influence developmental brain trajectories, even though a significant number of genes (about 10,000, or approximately one-third) in the human genome are expressed primarily in the brain and during brain development. Interestingly, in addition to showing differential expression among tissues, many genes are differentially expressed across the ages (e.g., antagonistic pleiotropy). Age-specific gene expression plays an important role in several critical events in brain development, including neuronal cell migration, synaptogenesis and neurotransmitter receptor specificity, as well as in aging and neurodegenerative disorders (e.g., Alzheimer disease or amyotrophic lateral sclerosis). In addition, the majority of psychiatric and mental disorders are polygenic, and many have onsets during childhood and adolescence. In this review, we summarize the major findings from neuroimaging studies that link genetics with brain development, from infancy to young adulthood. Specifically, we focus on the heritability of brain structures across the ages, age-related genetic influences on brain development and sex-specific developmental trajectories.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
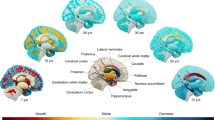
Human brain development involves a cascade of processes that starts 2 weeks post conception, when the first cell differentiates. These processes interact with each other and have different dynamics and termination times: some are completed at birth, whereas others continue to develop throughout the lifespan (Fig. 1). By 3–4 weeks of gestation, the neural tube is formed, which then differentiates into the full nervous system between 4 to 12 weeks. Cells proliferate and give rise to neurons. From 12 to 20 weeks, these neurons migrate along the radial cells to their final destination and form the cortex (Rakic 1990). Between 24 weeks into gestation to 4 weeks after birth, the first apoptosis occurs, reducing the number of neurons by half. Concurrently, myelination begins at 29 weeks of gestation in the brain stem and develops primarily from inferior to superior and from posterior to anterior directions (Volpe 2000; Huppi and Inder 2001). By early childhood, most of the axons are myelinated, although myelination continues through adolescence and across the second and third decades of life (Yakovlev and Lecours 1967; Benes et al. 1994; Blakemore 2012; Lebel et al. 2012). Synaptic proliferation and organization starts at about 20 weeks of gestation, and synapses continue to proliferate from birth to 2 years after birth (Huttenlocher 1979; Huttenlocher and de Courten 1987). However, a regional reorganization occurs most predominantly during childhood and adolescence (Blakemore 2012).
Overall, the brain grows rapidly during fetal life through childhood, and matures more slowly during adolescence and young adulthood until its development plateaus and/or follows a declining trajectory with aging. After birth, brain development primarily depends on individual experiences (“self-organization”) (Lewis 2005), along with many other variables (Andersen 2003). However, of the whole human genome, 10,000 genes (approximately one-third) are expressed only during brain development (Johnson et al. 2009; Zhang et al. 2011). Change in gene expression is fastest in human brain tissue during fetal development, slows down through childhood and adolescence, stabilizes through adulthood, then speeds up again after age 50, with distinct redirection of expression changes prior to birth and in early adulthood (Colantuoni et al. 2011). Genes with high expression during fetal development have the greatest decreased expression in the aging cortex, whereas genes with low expression during fetal development show increased expression in aging and neurodegeneration (Colantuoni et al. 2011). Recently, an increasing number of studies have attempted to examine the complex interactions between genes, environment and the developing brain. The cumulative data clearly indicates that brain development is under tight genetic control across the lifespan.
Recent studies using brain imaging techniques confirmed that the brain develops throughout childhood and young adulthood, and that the various brain structures follow different trajectories of development and maturation (Gogtay et al. 2004; Westlye et al. 2010; Lebel et al. 2012). Since genes influence developmental trajectories, neuroimaging provides a powerful, non-invasive tool to investigate phenotypic variations during brain development in relation to genetic differences and age or sex-specific gene-expression.
Techniques commonly used to study brain development
While electroencephalography (EEG) was mainly used to study babies and young children, magnetic resonance imaging (MRI) and functional MRI (fMRI) are widely used for brain research because of its high resolution, multiple advances in image analysis, and the large range of image contrasts that they provide. Structural MRI provides information on brain morphometry, such as cortical thickness or area, and cortical and subcortical volumes. Using specific stimulation paradigms, functional MRI (fMRI) can yield information on functional networks dedicated to a particular task and their connectivity. Similarly, resting-state-fMRI (rs-fMRI) or task-free fMRI can provide an estimate for the functional connectivity in the brain at rest (without an activation paradigm). These task-free brain activities appear to reflect coordinated and intrinsic spontaneous fluctuations in brain activity at baseline (Gusnard and Raichle 2001). Rs-fMRI provides insight into the unprompted activity that is naturally produced within the brain, which subsequently promotes communication across regions.
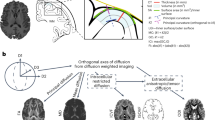
White matter (WM) involves various networks across brain regions and is important for efficiency and integration of information between cortical regions. WM integrity can be assessed using diffusion tensor imaging (DTI), which provides fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD) or axial diffusivity (AD) in specific regions of interest (ROIs) or the whole brain.
Neurochemicals that are present in large millimolar quantities can be assessed using proton magnetic resonance spectroscopy (1H-MRS), which is a non-invasive MR technique that can measure levels of N-Acetyl Aspartate (NAA, neuronal marker), total Creatine (tCr, metabolic marker), total Choline (tCho, cell-membrane marker), and Myo-inositol (mI, glial marker). However, quantitative MRS has lower spatial resolution and requires advanced sequences and post-processing compared to structural MRI, making it less commonly used in the clinical setting. However, MRS can provide insights into the chemistry underlying structural changes, and chemical alterations often precede structural abnormalities during disease progression.
Neuroimaging techniques have yielded valuable data on trajectories of the developing brain and made it possible to evaluate the role of environment and genetics in normal brain development.
Studies of genetic influences on brain development
In this review, we will focus on genetic influence on “normal” or typical brain development from neonates to young adults and will summarize studies conducted in pediatric populations linking brain imaging to genetics. We will concentrate specifically on age-by-gene or gene-by-heritability interactions, since age-related changes in heritability may be linked to the timing of gene expression. Knowledge of when a given brain structure is particularly sensitive to genetic or environmental influences during development may also have important educational and therapeutic implications.
We performed a search in PubMed®, limited to “human subjects” and combinations of the following keywords: brain morphometry, DTI, MR Spectroscopy, fMRI, heritability, genetic, children, adolescents, brain development, age, age-related, and sexual dimorphism. The studies queried were included in the review if they were published in English in a peer-reviewed journal and involved the investigation of genetic factors. Conference abstracts, case studies or qualitative studies were excluded. If available, information on the number of subjects, average age and age range of the sample, type of analysis, and heritability estimates with their 95 % confidence intervals were extracted from the papers.
Heritability in brain development: family and twin studies
Heritability is the proportion of the observed variation in a particular trait that can be attributed to inherited genetic factors. It is measured by estimating the relative contributions of genetic and non-genetic factors to the total variation of that trait in a population. Although heritability estimates are difficult to compare across studies, for a given trait, heritability (h2) of less than 0.30 can be considered low, between 0.30 and 0.60 is moderate and above 0.60 is high. Family- and twin-based studies allow the evaluation of separate and combined effects of genetic and environmental contributions on any trait. Monozygotic (MZ) and dizygotic (DZ) twins share the same or very similar family environments if the twins are raised together. MZ twins are almost genetically identical while DZ twins share only half their genes, like non-twin siblings. Univariate studies evaluate differential correlations of a particular phenotype in MZ and DZ twins to estimate the heritability of a trait (Plomin et al. 1994), whereas multivariate studies can analyze the links between genetic and environmental effects on specific regions of interest in the brain. Contributions of additive genetic (A), common or shared environmental (C), and unique environmental (E) effects on variations of a phenotype can be modeled using structural equation modeling (SEM). SEM allows for parameter estimations, while the Falconer method uses correlation analysis to test whether genetic or environmental factors contribute significantly to individual differences (parameter calculation). SEM can also be used to determine whether the traits defined by neuroimaging are heritable, and how heritability can explain brain development.
Brain morphometry-studies on heritability
Brain morphometry, especially cortical volume and thickness, has been the most studied MR-based phenotype for heritability (Table 1). Structural MRI provides images of the brain that can be processed to produce quantitative measures of brain size (volume or thickness) and shape (including surface area). Because MRI does not involve ionizing radiation, it is ideally suited for repeated measurements during development to chart brain growth trajectories. Two major longitudinal studies evaluated heritability of brain morphometric measures over time. The first study is the Brain SCALE study (Brain Structure and Cognition: an Adolescent Longitudinal Twin Study into the Genetic Etiology of Individual Difference, Netherlands); it evaluated pre-adolescents at 9 and 12 years of age with psychometric, neuropsychological, and neuroimaging measures (van Soelen et al. 2012a, b). The second study, conducted by investigators from the Child Psychiatry Branch of the National Institute of Mental Health (USA), followed children and adolescents from 5 to 18 years of age (Wallace et al. 2006; Lenroot et al. 2009; Ordaz et al. 2010). In these youths, heritability of global brain and intracranial volumes were similar to those reported in adults. Heritability of total gray matter was lower in neonates (56 %) compared to 5–18 year old children (~77–85 %) (Wallace et al. 2006; Giedd et al. 2007; Peper et al. 2008) and adults (82 %) (Baaré et al. 2001). Heritability of total white matter volume in neonates (85 %) was similar to that in 5–18 year old children (85 %) (Peper et al. 2008; Wallace et al. 2006) and in adults (87 %) (Baaré et al. 2001). However, within the age range from 5 to 18 years, heritability of total gray matter decreased with age, while that of total white matter increased with age (Wallace et al. 2006; Giedd et al. 2007). Cerebellar volumes showed increasing heritability with age, from low (17 %) in neonates to moderate (49 %) in children (Wallace et al. 2006) and high (>60 %) in adults (Wright et al. 2002). In contrast, lateral ventricle volumes showed a higher heritability (71 %) in neonates (Gilmore et al. 2010) than in children (~30–35 %) (Wallace et al. 2006; Schmitt et al. 2007; Peper et al. 2009) and in adults (<10 %) (Chou et al. 2009).
Heritability of the total cortical thickness was less in 6 and 8 years old children (65–71 %) (Yoon et al. 2010; van Soelen et al. 2012b, 2013) than that in 12 and 14 years old adolescents (80–81 %) (van Soelen et al. 2012b; Yang et al. 2012; van Soelen et al. 2013) or in mid-aged adults (81 %) (Panizzon et al. 2009). In 8 year old children, overall cortical thickness in the frontal, temporal, parietal and occipital lobes exhibited moderate to high heritability (53–72 %), whereas regional thickness of the lobules in the inferior and superior parietal and inferior temporal cortices showed low heritability (<30 %) (Yoon et al. 2010). In adolescents, the thickness of the prefrontal cortex, including bilateral dorsolateral and superior frontal regions, and the middle temporal and supramarginal cortices, had heritability greater than 75 % (Schmitt et al. 2009; Lenroot et al. 2009; Yang et al. 2012). As in adults (Tramo et al. 1998; Joshi et al. 2011), differences in heritability were observed between hemispheres in adolescents in multiple frontal regions (Schmitt et al. 2009; Yang et al. 2012). In 9 and 12 years old children, heritability of cortical thinning was predominantly found in superior and middle frontal regions, superior temporal cortices, cingulate, sensorimotor cortices, and primary visual and lateral occipital cortices (van Soelen et al. 2012b). Inferior regions of the primary motor and sensory cortex in the frontal and postgyral cortices that are associated with facial recognition functions showed a higher heritability in early development compared to other brain regions, and an increasing heritability with age (Lenroot et al. 2009; van Soelen et al. 2013). From later childhood through adolescence, heritability increased most markedly in dorsolateral prefrontal, superior parietal and temporal cortices, and in language-related regions in the left hemisphere including Broca and Wernicke’s areas (Lenroot et al. 2009; van Soelen et al. 2013).
Overall, primary sensory regions that mature first had higher heritability than later maturing brain regions, such as the frontal cortex (van Soelen et al. 2013). However, late maturing regions showed an increase in heritability with age until 18 years and stabilized thereafter, whereas the heritability of primary sensory regions was stable across the age range of 21–27 and 51–59 years (Rimol et al. 2010; Joshi et al. 2011).
White matter integrity-studies for heritability
In the last two decades, neuroimaging studies revealed the importance of white matter in several psychiatric and neurodegenerative disorders. Although genetics may play a role in WM integrity during brain development, heritability in WM integrity has not yet been examined extensively across the lifespan. Most studies to date involved adults since the peak maturation of the WM occurs at the age range of 25–40 years. However, three longitudinal twin studies examined the heritability of WM integrity with age in infants (Chen et al. 2009) and adolescents (Chiang et al. 2011; Brouwer et al. 2012), while two other studies evaluated heritability cross-sectionally in neonates at 40 days post birth (Geng et al. 2012) and at 9 years of age (Brouwer et al. 2010).
These studies show that heritability of FA and RD in the whole WM was higher in neonates (FA = 60 % and RD = 53 %) (Chen et al. 2009; Geng et al. 2012) than in children (for FA ~ 54 %) (Brouwer et al. 2010, 2012; Chiang et al. 2011) and adults (FA = 52 % and RD = 37 %) (Kochunov et al. 2010). Likewise, AD heritability was moderate (57 %) in neonates (Geng et al. 2012), but lower (33–46 %) in children (Brouwer et al. 2010, 2012) and not significant in adults (Kochunov et al. 2010). AD reflects the axonal organization and is often used to assess for possible injury to fiber bundles (Budde et al. 2007), and therefore may provide a sensitive measure of environmental influences in the brain during development.
Regional heritability (for FA and AD) varied with maturation status. Heritability was typically high in WM structures that mature earlier (Lee et al. 2009; Kochunov et al. 2010; Geng et al. 2012). However, at any age, heritability of FA in the corpus callosum was higher in the genu, which develops later than the splenium, but FA heritability for both genu and splenium decreased at the same rate over time (Brouwer et al. 2010; Kochunov et al. 2010; Brouwer et al. 2012).
During maturation, changes in RD may reflect axonal myelin synthesis and proliferation of glial cells, while changes in FA may reflect fiber organization, but is also influenced by changes in RD (Partridge et al. 2004). Therefore, more than one diffusion measure is required to fully characterize the maturation process (Table 2).
Functional brain networks-studies of heritability
Functional MRI (fMRI) and electroencephalography (EEG) allow the measurement of brain function and activity. To our knowledge, only one study examined heritability of the functional brain networks using fMRI in the younger population (van den Heuvel et al. 2013). Connectivity density is defined as the total number of brain connections, and cortical network efficiency is assessed by the temporal dependency of neuronal activation patterns of anatomically separated brain regions. Independent of the connectivity density, heritability of the global cortical network efficiency was moderate (42 %) at 12 years of age (van den Heuvel et al. 2013), and higher (60 %) in adults (Fornito et al. 2011).
EEG showed low heritability (11–27 %) of frontal asymmetry in 9 to 10 year old children (Gao et al. 2009), similar to that in adults (Coan and Allen 2003; Anokhin et al. 2006; Smit et al. 2008). In contrast, EEG alpha power was highly heritable (70–85 %) in children (Gao et al. 2009; van Baal et al. 2001), and in older adolescents and adults (van Baal et al. 1996). EEG alpha power scores increased from 9 to 10 years of age (Gao et al. 2009) and from 15 to 17 years of age (McGuire et al. 1998), despite these narrow age ranges. Although these studies involved small sample sizes, the findings suggest that heritability of global functional brain connectivity increases during brain development, while heritability of the architecture and maturation in the frontal cortex is relatively stable across the age range.
Magnetic resonance spectroscopy (MRS) studies of heritability
Brain metabolite concentrations measured by 1H-MRS change throughout the lifespan, with the greatest changes occurring early (Kreis et al. 1993; Pouwels et al. 1999) and late in life (Chang et al. 1996). Only one study examined the heritability of brain metabolites and how they change with age (Batouli et al. 2012). In elderly twins (65–88 years), only NAA levels showed a high heritability (72 %) in the posterior cingulate cortex (Batouli et al. 2012): sex appeared to have no significant effects on heritability. More studies on the heritability of these brain metabolites in different brain regions throughout the life span should be performed.
Epigenetics in brain development
Epigenetics is the study of gene expression that has been modified by various factors, such as DNA methylation, histone modifications, microRNAs, and long noncoding RNAs, typically in response to environmental exposures. Modified gene expression may last across the lifespan or even become heritable (Weaver et al. 2004). Histone and DNA methylation are epigenetic phenomena that change in the human brain from childhood to adulthood, suggesting that the epigenetic pattern may modify brain development. However, only one study examined the global cytosine methylation of DNA with age in humans (Siegmund et al. 2007). From 17 weeks of gestation to 104 years of age, DNA methylation levels increased in genes that show decreased mRNA expression. Emerging data from studies on gene-by-environment-by-age interactions of DNA methylation show that epigenetic effects may influence processes such as fetal programming, in which events that occurred during gestation may last across the lifespan. Stable and long-lasting epigenetic alterations in the brain also have been suggested to cause some psychiatric disorders (Feinberg 2007; Gardner et al. 2007; Simmons 2009), leading to the hypothesis that many adult diseases with epigenetic underpinnings have fetal origins (Bezek et al. 2008; Doherty et al. 2009). Therefore, epigenetics may shape human behaviors, and environmental events may have heritable impact on the genes.
An ongoing twin study aims to unravel influences in epigenetic mechanisms throughout the lifespan (Tan et al. 2013). This study should improve our understanding of age-by-gene and age-by-epigenetic interactions, and how molecular pathways are modified during brain development.
Gene specific expression in brain development
Brain development depends on finely controlled expression of genes. Developmental changes in gene expression are well established in animal models, but data are rare in humans and even rarer in the pediatric population. Genome-wide association studies (GWAS) use a non-hypothesis-driven approach to interrogate genetic variants across the whole genome. To our knowledge, only one study to date used this approach in a healthy pediatric population and showed that the genotype for DOK5, encoding for a protein mediating neurite outgrowth and activation of the MAP kinase pathway, modifies the activation of the amygdala during face processing in children (9–18 years, (Liu et al. 2010)). Other studies examined candidate genes based on prior evidence and biological plausibility. Most of these studies showed genetic influences on brain morphometry and brain activation (i.e. fMRI after a specific task), while only three studies reported on the relationship between genetic variants and DTI measures (Pacheco et al. 2009; Thomason et al. 2010; Sundram et al. 2010).
The most studied genes in pediatric populations are those encoding the dopamine inactivator catechol-O-methyl transferase (COMT), neurotrophin, brain derived neurotrophic factor (BDNF, (Casey et al. 2009; Knickmeyer et al. 2013; Mueller et al. 2013)), the schizophrenia candidate gene neuregulin (NRG1, (Knickmeyer et al. 2013)), and the serotonin transporter (5-HTT, (Pacheco et al. 2009; Lau et al. 2009; Wiggins et al. 2012)). Amongst these, the COMT genotype was the only one found to influence brain morphometry (Raznahan et al. 2011; Knickmeyer et al. 2013), white matter integrity (Thomason et al. 2010; Sundram et al. 2010) and brain connectivity (Mechelli et al. 2009). A longitudinal study in healthy children additionally demonstrated that a greater COMT Val158Met Val allele dose is associated with a decrease in the cortical thickness loss in the prefrontal cortex from 9 to 22 years of age (Raznahan et al. 2011). Genetic variants in the genes APOE, DISC1, GAD1, GPCD1, ESR1, DYX1C1, DCD2, KIAA0319, HOXA1, DTNBP1, OXTR, MAOA, IL-6, MET and PER2 were also found with each gene being associated with a particular neuroimaging phenotypes, raising the possibility that they may be involved in brain development (Shaw et al. 2007; Reiman et al. 2009; Furman et al. 2011; Tognin et al. 2011; Darki et al. 2012; Hedrick et al. 2012; Raznahan et al. 2012; Forbes et al. 2012; Ziermans et al. 2012; Knickmeyer et al. 2013). However, these studies need replication in independent cohorts, in part to evaluate for possible racial and ethnic differences, since the study populations were primarily Caucasian. Moreover, potential interactions between genetic variants, sex, and age need to be investigated further.
Sexual dimorphism in brain development
Evidence from historic post-mortem studies to recent meta-analyses indicate that the brain is structurally, metabolically, and functionally sexually dimorphic (Sacher et al. 2013). In adults, men have larger brains and volumes of gray and white matter than women (Peters 1991; Passe et al. 1997; Good et al. 2001; Luders et al. 2005), while women have greater gray matter-to-white matter ratios than men (Gur et al. 1991; Nopoulos et al. 2000; Goldstein et al. 2001; Allen et al. 2003; Luders et al. 2005; Chen et al. 2007). The size of white matter structures, such as the corpus callosum, differs by gender although controversies existed since the first description (Oppenheim et al. 1987; Holloway et al. 1993; Bishop and Wahlsten 1997; Dorion et al. 2001). Brain activation patterns show gender differences during a variety of cognitive tasks (Gur and Gur 1990; Gur et al. 1995, 2000; Speck et al. 2000; Weiss et al. 2003; Bell et al. 2006). However, sexual dimorphism of brain measurements in adults can be biased by multiple factors, such as environment and most obviously height differences (Dekaban 1978; Fausto-Sterling and Balaban 1993). Cumulative mean height within the first 15 years of life varies by only 1 % between boys and girls, and girls are taller from 10 to 13.5 years old (Kuczmarski et al. 2002). Therefore, studying sexual dimorphism on brain measures across the age span from birth to young adulthood has the advantage of identifying factors that could influence gender-specific differences, and when (e.g., perinatal, childhood, adolescence) and why (e.g., gene specific, hormonal, environmental) these differences arise. This section provides a comprehensive review on how structural and functional neuroimaging deciphers genetic-by-gender-by-age interactions on the developing brain.
Sexual dimorphism in brain morphometry
The earliest sexual dimorphism observed in life is that of head circumference, which was assessed by prenatal ultrasound as early as the second trimester of pregnancy (Joffe et al. 2005). Although it is the least heritable, the cerebellum is the most sexually dimorphic brain structure. Boys have larger total cerebellar volume than girls and the magnitude of sex-differences varies significantly with age: 10 % at 8 years and 13 % at 20 years of age (Tiemeier et al. 2010). Likewise, the volumes of cortical gray and white matter are generally larger in boys than in girls (Fig. 2a). Cortical gray matter volumes increase somewhat more rapidly with age in boys than in girls: 6–7.5 % larger in neonates and children, and ~10 % larger during adolescence (Reiss et al. 1996; Caviness et al. 1996; Giedd et al. 1996, 1999; Lenroot et al. 2007; Gilmore et al. 2007). Gender-specific differences in cortical white matter volume are greater in adults than in children and adolescents: 15–17 % differences were observed from childhood to young adolescents (De Bellis et al. 2001; Perrin et al. 2008, 2009). Boys have greater rates of age-dependent increase in total white matter volume than girls (De Bellis et al. 2001; Lenroot et al. 2007). Regionally, sexual dimorphism of developmental trajectories is observed more for the frontal and occipital lobes (gray and white matter), whereas no sex-differences in developmental trajectories were found for the temporal and parietal lobes (Giedd et al. 1996, 1997; Lenroot et al. 2007; Sowell et al. 2007). Between the ages of 4 to 18 years, age-dependent increases in the amygdala, caudate and putamen volumes were observed only in boys (e.g., amygdala in boys increases by 53 %) while an age-dependent increase of the hippocampal volume was found only in girls (Filipek et al. 1994; Giedd et al. 1996, 1997; Sowell et al. 2002; Lenroot et al. 2007; Neufang et al. 2009).
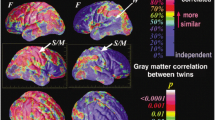
Multiple imaging modalities illustrating sexual dimorphism in the brain across the lifespan. a Across the ages 7–20 years, girls (red) have smaller volumes of white and frontal gray matter than boys (blue). Compared to girls, boys showed a greater age-related increase in white matter volume, and a steeper age-related decline in the frontal gray matter (adapted from Lenroot et al. 2007). b Heritability accounts for up to 80 % of the variation in fractional anisotropy in adult males (top row, red color voxels). (Adapted from Chiang et al. 2011). c Age-by-sex interaction on homotopic resting state functional connectivity (rsFC). Statistical maps are visualized as six hemispheric surfaces (showing cortical regions) and six axial slices (showing subcortical regions). The top scatter plot shows opposite brain activation patterns in males (red) and females (blue) in the dorsolateral prefrontal cortex (top); the opposite is true for the amygdala (bottom scatter plot) (Adapted from Zuo et al. 2010). d Interactions between age and sex, or genetic influence of testosterone levels, on relative white matter (WM) volume. Top panels: Between the ages of 11–20 years, boys show steeper age-dependent increase in relative WM volume (corrected total brain volume) compared to girls. Bottom panels: Boys with the more efficient short AR gene and higher plasma levels of bioavailable testosterone had larger relative WM volumes. (Adapted from Perrin et al. 2008)
Sexual dimorphism for cerebral asymmetry is already present at birth as a result of genetic programming, but the pattern of cerebral asymmetry changes after birth, suggesting that asymmetry may depend on shared environment as well as genetic factors. In neonates, cerebral asymmetry was more pronounced in girls than in boys in the prefrontal and occipital regions (Gilmore et al. 2007; Tanaka et al. 2012), while little consistency exists in sex-related and hemispheric differences in regional cortical volumes (Good et al. 2001; Raz et al. 2004). However, the left-greater than-right asymmetry in fetuses and neonates (Hering-Hanit et al. 2001; Gilmore et al. 2007; Ratnarajah et al. 2013) is opposite to that observed in older children (Caviness et al. 1996; Giedd et al. 1996; Reiss et al. 1996; Matsuzawa et al. 2001) and in adults (Nopoulos et al. 2000; Toga and Thompson 2003; Raz et al. 2004).
In contrast, intracranial volume is consistently larger in boys than in girls, starting with neonates (+2–9 %) (Gilmore et al. 2007; Qiu et al. 2013) and continues to be larger in adolescents and in adults (+11.9 to14.6 %) (Blatter et al. 1995; Gur et al. 1999; Raz et al. 2004). Neither the volumes of the lateral ventricle or corpus callosum show a gender-by-age interaction during the development, with boys having larger ventricle volumes but girls showing larger corpus callosum relative to the total brain volume across the age span (Giedd et al. 1996; Lenroot et al. 2007).
One of the most striking findings of theses pediatric studies is that most adult patterns of sexual dimorphism are already present at birth and are therefore primarily under genetic control (inherited). In contrast, gender-specific changes in brain structures that develop after birth typically occur at puberty, suggesting that the neuroanatomical development in certain brain regions is mediated by physiological processes associated with puberty (e.g. hormonal changes). However, no direct measures of puberty were evaluated in the majority of these studies.
Sexual dimorphism in white matter integrity
Findings on sexual dimorphism for DTI metrics are controversial and inconsistent. Two studies found gender differences in FA, RD and AD across the lifespan (Westlye et al. 2010; Lebel et al. 2012), two found no gender differences in DTI parameters in young children; (Gilmore et al. 2007; Geng et al. 2012), and others reported mixed results based on narrow age ranges of the subject populations (Abe et al. 2002; Ota et al. 2006; Schneiderman et al. 2007; Hsu et al. 2008; Schmithorst et al. 2008; Bava et al. 2011; Chiang et al. 2011). Only one study examined age-related differences in sexual dimorphism of the WM integrity during childhood and adolescence (Schmithorst et al. 2008). In neonates, sexual dimorphism was not observed for any of the DTI (FA/RD/AD) parameters in the basal ganglia and thalamus (Qiu et al. 2013). In children and adolescents, higher FA values in the caudate and thalamus were found in boys than in girls (Menzler et al. 2011; Pal et al. 2011), suggesting that sexual dimorphism of anisotropy in the deep GM structures arises after birth. Girls from 5 to 18 years old displayed a trend toward increased FA with age only in the right hemisphere (arcuate fasciculus, frontal lobe and occipito-temporo-parietal white matter) while boys displayed this trend only in the left hemisphere frontal lobe (Schmithorst et al. 2008). Girls also showed a greater MD increase with age compared to boys in associative regions (Schmithorst et al. 2008). In young adults, men have higher FA than women in the genu and the splenium of the corpus callosum (Westerhausen et al. 2004; Chiang et al. 2011), while women have higher FA in the middle and superior occipital gyri (Chiang et al. 2011). In the genu and splenium of the corpus callosum, the external and internal capsules and the superior fronto-occipital fasciculus, genetic influences account for approximately 80 % of the variation in FA in men (Fig. 2b) (Chiang et al. 2011). No gender differences in brain structures were found for RD (Perrin et al. 2009; Bava et al. 2011) across childhood and adolescence, suggesting that myelination is not sufficient to characterize white matter disparities.
Sexual dimorphism during brain development was also found using magnetization transfer imaging (MTI), which provides estimates of the macromolecular content (e.g. myelin content) in white matter tissue. Age-dependent decreases in MT were found across adolescence in boys only (Perrin et al. 2008, 2009).
Overall, findings from these DTI and MTI studies suggest that sexual dimorphism for WM is in part under genetic control, but may also be influenced by microenvironmental factors (e.g. hormones) that ultimately change the brain structure.
Sexual dimorphism in functional brain networks
Sexual dimorphism on functional MRI was reported in only three studies of healthy children and adolescents (Zuo et al. 2010; Wu et al. 2013; Ratnarajah et al. 2013). However, multiple fMRI studies in adults found evidence for sexual dimorphism in emotional perception and memory, fear conditioning and visuospatial abilities (Sacher et al. 2013). While no age-by-gender interactions in global network parameters were found, girls and boys have different developmental patterns of the functional brain networks associated with visuospatial function (left cuneus and left superior parietal gyrus) and emotion processing (left rectus gyrus). Boys showed an age-related increase, while girls showed age-related decrease, in the regional nodal properties in the left superior parietal gyrus (Wu et al. 2013). No gender differences in structural connectivity asymmetry was found in neonates (Ratnarajah et al. 2013) despite the rapid synaptic proliferation in neonates (Gilmore et al. 2007; Qiu et al. 2013).
Across the age span of 7 to 85 years, resting state functional connectivity (rsFC) increased with age in boys but decreased with age in girls in the dorsolateral prefrontal cortex (DLPFC) (Fig. 2c) (Zuo et al. 2010). However, in the amygdala, an opposite pattern was observed, with an age-related decrease in rsFC in boys but an age-related increase in girls (Zuo et al. 2010). These findings demonstrate gender differences in the developmental trajectory of functional homotopy (i.e. the high degree of synchrony in spontaneous activity between geometrically corresponding interhemispheric regions) in the amygdala and within the DLPFC (Zuo et al. 2010).
Sexual dimorphism in the developmental trajectories of functional connectivity may underlie sex differences in the functional maturation of these specific networks, and ultimately the sex-related cognitive differences in children and adolescents.
Sexual dimorphism on magnetic resonance spectroscopy
Few MRS studies evaluated brain development in relation to genetic factors. Multiple studies showed that brain metabolites change rapidly early in life and then more subtly and linearly after puberty (Kreis et al. 1993; Pouwels et al. 1999; Kadota et al. 2001; Goldstein et al. 2009). To date, only one MRS study evaluated sexual dimorphism across the lifespan (4–88 years), using the NAA/Cho ratio in the centrum semiovale (Kadota et al. 2001). Boys exhibited a steeper age-related increase in NAA/Cho in the white matter than girls, and the age-dependent trajectory of the NAA/Cho peaked 1 to 3 years earlier in boys than in girls (Kadota et al. 2001). Cerebral laterality in WM NAA/Cho ratios also differed by sex: greater NAA/Cho ratios were found in the right compared to the left WM in boys, but not in girls. In adults, age-related changes in brain metabolites were reported but considerable heterogeneity of metabolite concentrations across studies were found (Kadota et al. 2001; Baker et al. 2008; Maudsley et al. 2009; Chang et al. 1996). Note also that metabolite ratios are difficult to interpret since it is unclear what proportion of the age-dependent increase in NAA/Cho reflects age-dependent increase in NAA, because Cho concentration also increases with age during adolescence (Cloak et al. 2011). Therefore, future MRS studies investigating metabolite changes related to age and sex, or the additional influence of genetic factors, should measure metabolite concentrations rather than ratios.
Dosage of genes on the X-chromosome
A different approach to study sex-specific genetic influences on brain development is to evaluate individuals with sex-chromosomal abnormalities, such as boys with XXY (e.g. Klinefelter syndrome) or 49,XXXXY syndrome, or girls with the commonly studied Turner syndrome (X-monosomy). Individuals with Klinefelter syndrome have reduced gray matter in the insula, temporal gyrus, amygdala, cingulate and hippocampus (Giedd et al. 1996; Patwardhan et al. 2002), while 49,XXXXY boys have smaller total brain size and abnormal development of the white matter with a thinner corpus callosum and multifocal WM lesions (Blumenthal et al. 2013). Brain morphometry studies show that girls with Turner syndrome (TS) have larger volumes of the amygdala and orbitofrontal cortex (Good et al. 2003), as well as larger superior temporal gyrus (Kesler et al. 2003). They also have smaller volumes in parieto-occipital regions and parietal lobes (Murphy et al. 1993; Reiss et al. 1995; Brown et al. 2002, 2004), and reduced FA in parieto-occipital regions along the superior longitudinal fasciculus (Holzapfel et al. 2006). TS girls also have abnormal patterns of brain activation in frontal and parietal cortices and subcortical regions, such as the caudate during visuospatial tasks (Kesler et al. 2004), executive function (Haberecht et al. 2001; Hart et al. 2006) and arithmetic tasks (Kesler et al. 2006).
Altogether, these results suggest that the dosage of genes on the X chromosome plays an important role in determining the development of brain structures (gray and white matter) and brain function.
Sex steroid receptors and hormone levels
Gender differences are also observable at the molecular level, although there is a scarcity of human studies (Lenroot et al. 2007; Schmithorst and Holland 2007). Adolescent brain development is mediated by both sex steroids: androgens (MacLusky et al. 2006) and estrogens (Galea et al. 2006). Sex steroid hormones are involved in brain “organization” that first establish sexual dimorphism of brain morphology leading to sexual behavior and physiology in adulthood, and in brain “activation” to further maintain gender-specific behaviors in adulthood (Cooke et al. 1998). However, the role of sex steroids and hormones on brain development was investigated only by a few neuroimaging studies.
In girls, estradiol levels were negatively associated with global gray matter volumes and regionally with the inferior frontal gyrus (Peper et al. 2009). In contrast, testosterone levels correlated positively with gray matter volumes in boys (Peper et al. 2009). Testosterone levels also correlated with white matter volumes depending on the androgen receptor (AR) genotype: boys with the more “efficient” (short) AR gene had greater WM volume than those with the less “efficient” (long) AR gene (Fig. 2d) (Perrin et al. 2008). Similarly, boys with the more “efficient” AR gene had a less-steep age-related loss in cortical thickness at the inferior parietal lobule. However, girls with the more “efficient” short AR gene had more rapid thinning of the left inferior frontal gyrus (Raznahan et al. 2010). Since the left inferior frontal gyrus is involved in language and impulse-control domains, the more rapid thinning of this brain region suggests earlier maturation in the girls. This longitudinal study of cortical thickness in changes adolescents showed that the AR gene contributes to sexual dimorphism during brain development. Additionally, manipulation of androgens early in life may have functional consequences on cortical function. For example, object discrimination, a task associated with the prefrontal cortex, is performed better in non-human primate adolescent males and androgen-exposed females relative to unexposed normal females (Clark and Goldman-Rakic 1989). In contrast, pubertal increases in sex hormone levels attenuated pre-pulse inhibition in rhesus macaques (Morris et al. 2010).
To our knowledge, only one study investigated the role of non-steroid hormones on brain development and its role on sexual dimorphism (Peper et al. 2008). Luteinizing hormone (LH) is one of the first endocrine markers of puberty, and was positively associated with larger white matter volume in the left cingulum, middle temporal gyrus (left and right) and splenium of the corpus callosum, as well as with higher WM density in the left anterior cingulum (Peper et al. 2008). Interestingly, moderate to high heritability in relation to LH levels were found in the splenium of the corpus callosum (39 %) and the left cingulum (43–65 %) (Peper et al. 2008). However, since LH stimulates the Leydig cells to produce testosterone, these associations may in part be due to the effects of testosterone on brain development and on heritability.
Taken together, both non-steroid and steroid sex hormones may play important roles in sexual dimorphism of brain morphometry. Changes or abnormalities in the levels of these hormones may therefore contribute to the susceptibility of neuropsychiatric disorders that are known to emerge at puberty and to have gender-specific prevalence rates.
Conclusion and future studies
This review highlights the important contributions of neuroimaging in understanding the role of genetics on brain development, and how genetics influence brain developmental trajectories. Future studies can be augmented by linking genetics and neuroimaging with biomarkers and cognitive neuroscience, in order to define more reliable endophenotypes. The emergence of multiple datasets also highlights several methodological issues that lead to difficulties in interpreting or comparing findings across studies. Some of these methodological issues include the selection of diverse human subject populations, the various neuroimaging acquisition parameters, differences in behavioral assessments, and the complex statistical comparisons. Resolving these issues and sharing of protocols across studies might lead to shared data resources that ultimately could provide a better understanding of normal brain development. Having a normative neuroscience database across the ages would provide a critically important foundation for elucidating how genes might influence brain disorders across the lifespan.
Finally, genetic effects are not static but may change with age and be modified by environment. However, most human studies were conducted cross-sectionally, and the few longitudinal studies across wider age ranges are limited by relatively small sample sizes. Overall, studies on age-related genetic influences on brain development are scarce and needed to improve our understanding of the developing brain. Knowledge derived from genetic influences on brain development and aging may lead to new strategies for more personalized therapeutic interventions, especially for the neuropsychiatric disorders that emerge at puberty and for many neurodegenerative disorders that occur with aging.
References
Abe, O., Aoki, S., Hayashi, N., Yamada, H., Kunimatsu, A., Mori, H., et al. (2002). Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. Neurobiology of Aging, 23(3), 433–441.
Allen, J. S., Damasio, H., Grabowski, T. J., Bruss, J., & Zhang, W. (2003). Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. NeuroImage, 18(4), 880–894.
Andersen, S. L. (2003). Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews, 27(1–2), 3–18.
Anokhin, A. P., Golosheykin, S., Sirevaag, E., Kristjansson, S., Rohrbaugh, J. W., & Heath, A. C. (2006). Rapid discrimination of visual scene content in the human brain. Brain Research, 1093(1), 167–177. doi:10.1016/j.brainres.2006.03.108.
Baare, W. F., Hulshoff Pol, H. E., Boomsma, D. I., Posthuma, D., de Geus, E. J., Schnack, H. G., et al. (2001). Quantitative genetic modeling of variation in human brain morphology. Cerebral Cortex, 11(9), 816–824.
Baker, E. H., Basso, G., Barker, P. B., Smith, M. A., Bonekamp, D., & Horska, A. (2008). Regional apparent metabolite concentrations in young adult brain measured by (1)H MR spectroscopy at 3 Tesla. Journal of Magnetic Resonance Imaging, 27(3), 489–499. doi:10.1002/jmri.21285.
Batouli, S. A., Sachdev, P. S., Wen, W., Wright, M. J., Suo, C., Ames, D., et al. (2012). The heritability of brain metabolites on proton magnetic resonance spectroscopy in older individuals. NeuroImage, 62(1), 281–289. doi:10.1016/j.neuroimage.2012.04.043.
Bava, S., Boucquey, V., Goldenberg, D., Thayer, R. E., Ward, M., Jacobus, J., et al. (2011). Sex differences in adolescent white matter architecture. Brain Research, 1375, 41–48. doi:10.1016/j.brainres.2010.12.051.
Bell, E. C., Willson, M. C., Wilman, A. H., Dave, S., & Silverstone, P. H. (2006). Males and females differ in brain activation during cognitive tasks. NeuroImage, 30(2), 529–538. doi:10.1016/j.neuroimage.2005.09.049.
Benes, F. M., Turtle, M., Khan, Y., & Farol, P. (1994). Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry, 51(6), 477–484.
Bezek, S., Ujhazy, E., Dubovicky, M., & Mach, M. (2008). Nongenomic memory of foetal history in chronic diseases development. Neuro Endocrinology Letters, 29(5), 620–626.
Bishop, K. M., & Wahlsten, D. (1997). Sex differences in the human corpus callosum: myth or reality? Neuroscience and Biobehavioral Reviews, 21(5), 581–601.
Blakemore, S. J. (2012). Imaging brain development: the adolescent brain. NeuroImage, 61(2), 397–406. doi:10.1016/j.neuroimage.2011.11.080.
Blatter, D. D., Bigler, E. D., Gale, S. D., Johnson, S. C., Anderson, C. V., Burnett, B. M., et al. (1995). Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. AJNR - American Journal of Neuroradiology, 16(2), 241–251.
Blumenthal, J. D., Baker, E. H., Lee, N. R., Wade, B., Clasen, L. S., Lenroot, R. K., et al. (2013). Brain morphological abnormalities in 49, XXXXY syndrome: a pediatric magnetic resonance imaging study. Neuroimage (Amst), 2, 197–203. doi:10.1016/j.nicl.2013.01.003.
Brouwer, R. M., Mandl, R. C., Peper, J. S., van Baal, G. C., Kahn, R. S., Boomsma, D. I., et al. (2010). Heritability of DTI and MTR in nine-year-old children. NeuroImage, 53(3), 1085–1092. doi:10.1016/j.neuroimage.2010.03.017.
Brouwer, R. M., Mandl, R. C., Schnack, H. G., van Soelen, I. L., van Baal, G. C., Peper, J. S., et al. (2012). White matter development in early puberty: a longitudinal volumetric and diffusion tensor imaging twin study. PLoS One, 7(4), e32316. doi:10.1371/journal.pone.0032316.
Brown, W. E., Kesler, S. R., Eliez, S., Warsofsky, I. S., Haberecht, M., Patwardhan, A., et al. (2002). Brain development in Turner syndrome: a magnetic resonance imaging study. Psychiatry Research, 116(3), 187–196.
Brown, W. E., Kesler, S. R., Eliez, S., Warsofsky, I. S., Haberecht, M., & Reiss, A. L. (2004). A volumetric study of parietal lobe subregions in Turner syndrome. Developmental Medicine and Child Neurology, 46(9), 607–609.
Budde, M. D., Kim, J. H., Liang, H. F., Schmidt, R. E., Russell, J. H., Cross, A. H., et al. (2007). Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magnetic Resonance in Medicine, 57(4), 688–695. doi:10.1002/mrm.21200.
Casey, B. J., Glatt, C. E., Tottenham, N., Soliman, F., Bath, K., Amso, D., et al. (2009). Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience, 164(1), 108–120. doi:10.1016/j.neuroscience.2009.03.081.
Caviness, V. S., Jr., Kennedy, D. N., Richelme, C., Rademacher, J., & Filipek, P. A. (1996). The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cerebral Cortex, 6(5), 726–736.
Chang, L., Ernst, T., Poland, R. E., & Jenden, D. J. (1996). In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sciences, 58(22), 2049–2056.
Chen, X., Sachdev, P. S., Wen, W., & Anstey, K. J. (2007). Sex differences in regional gray matter in healthy individuals aged 44–48 years: a voxel-based morphometric study. NeuroImage, 36(3), 691–699. doi:10.1016/j.neuroimage.2007.03.063.
Chen, Y., Zhu, H., Shen, D., An, H., Gilmore, J., & Lin, W. (2009). Mapping growth patterns and genetic influences on early brain development in twins. Medical Image Computing and Computer-Assisted Intervention, 12(Pt 2), 232–239.
Chiang, M. C., McMahon, K. L., de Zubicaray, G. I., Martin, N. G., Hickie, I., Toga, A. W., et al. (2011). Genetics of white matter development: a DTI study of 705 twins and their siblings aged 12 to 29. NeuroImage, 54(3), 2308–2317. doi:10.1016/j.neuroimage.2010.10.015.
Chou, Y. Y., Lepore, N., Chiang, M. C., Avedissian, C., Barysheva, M., McMahon, K. L., et al. (2009). Mapping genetic influences on ventricular structure in twins. NeuroImage, 44(4), 1312–1323. doi:10.1016/j.neuroimage.2008.10.036.
Clark, A. S., & Goldman-Rakic, P. S. (1989). Gonadal hormones influence the emergence of cortical function in nonhuman primates. Behavioral Neuroscience, 103(6), 1287–1295.
Cloak, C. C., Alicata, D., Chang, L., Andrews-Shigaki, B., & Ernst, T. (2011). Age and sex effects levels of choline compounds in the anterior cingulate cortex of adolescent methamphetamine users. Drug and Alcohol Dependence, 119(3), 207–215. doi:10.1016/j.drugalcdep.2011.06.017.
Coan, J. A., & Allen, J. J. (2003). Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology, 40(1), 106–114.
Colantuoni, C., Lipska, B. K., Ye, T., Hyde, T. M., Tao, R., Leek, J. T., et al. (2011). Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature, 478(7370), 519–523. doi:10.1038/nature10524.
Cooke, B., Hegstrom, C. D., Villeneuve, L. S., & Breedlove, S. M. (1998). Sexual differentiation of the vertebrate brain: principles and mechanisms. Frontiers in Neuroendocrinology, 19(4), 323–362. doi:10.1006/frne.1998.0171.
Darki, F., Peyrard-Janvid, M., Matsson, H., Kere, J., & Klingberg, T. (2012). Three dyslexia susceptibility genes, DYX1C1, DCDC2, and KIAA0319, affect temporo-parietal white matter structure. Biological Psychiatry, 72(8), 671–676. doi:10.1016/j.biopsych.2012.05.008.
De Bellis, M. D., Keshavan, M. S., Beers, S. R., Hall, J., Frustaci, K., Masalehdan, A., et al. (2001). Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex, 11(6), 552–557.
Dekaban, A. S. (1978). Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Annals of Neurology, 4(4), 345–356. doi:10.1002/ana.410040410.
Doherty, S. P., Grabowski, J., Hoffman, C., Ng, S. P., & Zelikoff, J. T. (2009). Early life insult from cigarette smoke may be predictive of chronic diseases later in life. Biomarkers, 14(Suppl 1), 97–101. doi:10.1080/13547500902965898.
Dorion, A. A., Capron, C., & Duyme, M. (2001). Measurement of the corpus callosum using magnetic resonance imaging: analyses of methods and techniques. Perceptual and Motor Skills, 92(3 Pt 2), 1075–1094.
Fausto-Sterling, A., & Balaban, E. (1993). Genetics and male sexual orientation. Science, 261(5126), 1257. author reply 1259.
Feinberg, A. P. (2007). Phenotypic plasticity and the epigenetics of human disease. Nature, 447(7143), 433–440. doi:10.1038/nature05919.
Filipek, P. A., Richelme, C., Kennedy, D. N., & Caviness, V. S., Jr. (1994). The young adult human brain: an MRI-based morphometric analysis. Cerebral Cortex, 4(4), 344–360.
Forbes, E. E., Dahl, R. E., Almeida, J. R., Ferrell, R. E., Nimgaonkar, V. L., Mansour, H., et al. (2012). PER2 rs2304672 polymorphism moderates circadian-relevant reward circuitry activity in adolescents. Biological Psychiatry, 71(5), 451–457. doi:10.1016/j.biopsych.2011.10.012.
Fornito, A., Zalesky, A., Bassett, D. S., Meunier, D., Ellison-Wright, I., Yucel, M., et al. (2011). Genetic influences on cost-efficient organization of human cortical functional networks. Journal of Neuroscience, 31(9), 3261–3270. doi:10.1523/JNEUROSCI.4858-10.2011.
Furman, D. J., Chen, M. C., & Gotlib, I. H. (2011). Variant in oxytocin receptor gene is associated with amygdala volume. Psychoneuroendocrinology, 36(6), 891–897. doi:10.1016/j.psyneuen.2010.12.004.
Galea, L. A., Spritzer, M. D., Barker, J. M., & Pawluski, J. L. (2006). Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus, 16(3), 225–232. doi:10.1002/hipo.20154.
Gao, Y., Tuvblad, C., Raine, A., Lozano, D. I., & Baker, L. A. (2009). Genetic and environmental influences on frontal EEG asymmetry and alpha power in 9-10-year-old twins. Psychophysiology, 46(4), 787–796. doi:10.1111/j.1469-8986.2009.00815.x.
Gardner, J. L., Craven, L., Turnbull, D. M., & Taylor, R. W. (2007). Experimental strategies towards treating mitochondrial DNA disorders. Bioscience Reports, 27(1–3), 139–150. doi:10.1007/s10540-007-9042-3.
Geng, X., Prom-Wormley, E. C., Perez, J., Kubarych, T., Styner, M., Lin, W., et al. (2012). White matter heritability using diffusion tensor imaging in neonatal brains. Twin Research and Human Genetics, 15(3), 336–350. doi:10.1017/thg.2012.14.
Giedd, J. N., Blumenthal, J., Jeffries, N. O., Castellanos, F. X., Liu, H., Zijdenbos, A., et al. (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience, 2(10), 861–863. doi:10.1038/13158.
Giedd, J. N., Castellanos, F. X., Rajapakse, J. C., Vaituzis, A. C., & Rapoport, J. L. (1997). Sexual dimorphism of the developing human brain. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 21(8), 1185–1201.
Giedd, J. N., Schmitt, J. E., & Neale, M. C. (2007). Structural brain magnetic resonance imaging of pediatric twins. Human Brain Mapping, 28(6), 474–481. doi:10.1002/hbm.20403.
Giedd, J. N., Snell, J. W., Lange, N., Rajapakse, J. C., Casey, B. J., Kozuch, P. L., et al. (1996). Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex, 6(4), 551–560.
Gilmore, J. H., Lin, W., Prastawa, M. W., Looney, C. B., Vetsa, Y. S., Knickmeyer, R. C., et al. (2007). Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. Journal of Neuroscience, 27(6), 1255–1260. doi:10.1523/JNEUROSCI.3339-06.2007.
Gilmore, J. H., Schmitt, J. E., Knickmeyer, R. C., Smith, J. K., Lin, W., Styner, M., et al. (2010). Genetic and environmental contributions to neonatal brain structure: a twin study. Human Brain Mapping, 31(8), 1174–1182. doi:10.1002/hbm.20926.
Gogtay, N., Giedd, J. N., Lusk, L., Hayashi, K. M., Greenstein, D., Vaituzis, A. C., et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 101(21), 8174–8179. doi:10.1073/pnas.0402680101.
Goldstein, G., Panchalingam, K., McClure, R. J., Stanley, J. A., Calhoun, V. D., Pearlson, G. D., et al. (2009). Molecular neurodevelopment: an in vivo 31P-1H MRSI study. Journal of International Neuropsychological Society, 15(5), 671–683. doi:10.1017/S1355617709990233.
Goldstein, J. M., Seidman, L. J., Horton, N. J., Makris, N., Kennedy, D. N., Caviness, V. S., Jr., et al. (2001). Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex, 11(6), 490–497.
Good, C. D., Johnsrude, I., Ashburner, J., Henson, R. N., Friston, K. J., & Frackowiak, R. S. (2001). Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage, 14(3), 685–700. doi:10.1006/nimg.2001.0857.
Good, C. D., Lawrence, K., Thomas, N. S., Price, C. J., Ashburner, J., Friston, K. J., et al. (2003). Dosage-sensitive X-linked locus influences the development of amygdala and orbitofrontal cortex, and fear recognition in humans. Brain, 126(Pt 11), 2431–2446. doi:10.1093/brain/awg242.
Gur, R. C., Alsop, D., Glahn, D., Petty, R., Swanson, C. L., Maldjian, J. A., et al. (2000). An fMRI study of sex differences in regional activation to a verbal and a spatial task. Brain and Language, 74(2), 157–170. doi:10.1006/brln.2000.2325.
Gur, R. C., Mozley, L. H., Mozley, P. D., Resnick, S. M., Karp, J. S., Alavi, A., et al. (1995). Sex differences in regional cerebral glucose metabolism during a resting state. Science, 267(5197), 528–531.
Gur, R. C., Mozley, P. D., Resnick, S. M., Gottlieb, G. L., Kohn, M., Zimmerman, R., et al. (1991). Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proceedings of the National Academy of Sciences of the United States of America, 88(7), 2845–2849.
Gur, R. C., Turetsky, B. I., Matsui, M., Yan, M., Bilker, W., Hughett, P., et al. (1999). Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. Journal of Neuroscience, 19(10), 4065–4072.
Gur, R. E., & Gur, R. C. (1990). Gender differences in regional cerebral blood flow. Schizophrenia Bulletin, 16(2), 247–254.
Gusnard, D. A., & Raichle, M. E. (2001). Searching for a baseline: functional imaging and the resting human brain. Nature Review Neuroscience, 2(10), 685–694. doi:10.1038/35094500.
Haberecht, M. F., Menon, V., Warsofsky, I. S., White, C. D., Dyer-Friedman, J., Glover, G. H., et al. (2001). Functional neuroanatomy of visuo-spatial working memory in Turner syndrome. Human Brain Mapping, 14(2), 96–107.
Hart, S. J., Davenport, M. L., Hooper, S. R., & Belger, A. (2006). Visuospatial executive function in Turner syndrome: functional MRI and neurocognitive findings. Brain, 129(Pt 5), 1125–1136. doi:10.1093/brain/awl046.
Hedrick, A., Lee, Y., Wallace, G. L., Greenstein, D., Clasen, L., Giedd, J. N., et al. (2012). Autism risk gene MET variation and cortical thickness in typically developing children and adolescents. Autism Research, 5(6), 434–439. doi:10.1002/aur.1256.
Hering-Hanit, R., Achiron, R., Lipitz, S., & Achiron, A. (2001). Asymmetry of fetal cerebral hemispheres: in utero ultrasound study. Archives of Disease in Childhood - Fetal and Neonatal Edition, 85(3), F194–F196.
Holloway, R. L., Anderson, P. J., Defendini, R., & Harper, C. (1993). Sexual dimorphism of the human corpus callosum from three independent samples: relative size of the corpus callosum. American Journal of Physical Anthropology, 92(4), 481–498. doi:10.1002/ajpa.1330920407.
Holzapfel, M., Barnea-Goraly, N., Eckert, M. A., Kesler, S. R., & Reiss, A. L. (2006). Selective alterations of white matter associated with visuospatial and sensorimotor dysfunction in turner syndrome. Journal of Neuroscience, 26(26), 7007–7013. doi:10.1523/JNEUROSCI.1764-06.2006.
Hsu, J. L., Leemans, A., Bai, C. H., Lee, C. H., Tsai, Y. F., Chiu, H. C., et al. (2008). Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. NeuroImage, 39(2), 566–577. doi:10.1016/j.neuroimage.2007.09.017.
Huppi & Inder. (2001). Magnetic resonance techniques in the evaluation of the perinatal brain: recent advances and future directions. Semin Neonatol, 6(2), 195–210. doi:10.1053/siny.2001.0039.
Huttenlocher, P. R. (1979). Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Research, 163(2), 195–205.
Huttenlocher, P. R., & de Courten, C. (1987). The development of synapses in striate cortex of man. Human Neurobiology, 6(1), 1–9.
Joffe, T. H., Tarantal, A. F., Rice, K., Leland, M., Oerke, A. K., Rodeck, C., et al. (2005). Fetal and infant head circumference sexual dimorphism in primates. American Journal of Physical Anthropology, 126(1), 97–110. doi:10.1002/ajpa.20035.
Johnson, M. B., Kawasawa, Y. I., Mason, C. E., Krsnik, Z., Coppola, G., Bogdanovic, D., et al. (2009). Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron, 62(4), 494–509. doi:10.1016/j.neuron.2009.03.027.
Joshi, A. A., Lepore, N., Joshi, S. H., Lee, A. D., Barysheva, M., Stein, J. L., et al. (2011). The contribution of genes to cortical thickness and volume. Neuroreport, 22(3), 101–105. doi:10.1097/WNR.0b013e3283424c84.
Kadota, T., Horinouchi, T., & Kuroda, C. (2001). Development and aging of the cerebrum: assessment with proton MR spectroscopy. AJNR - American Journal of Neuroradiology, 22(1), 128–135.
Kesler, S. R., Blasey, C. M., Brown, W. E., Yankowitz, J., Zeng, S. M., Bender, B. G., et al. (2003). Effects of X-monosomy and X-linked imprinting on superior temporal gyrus morphology in Turner syndrome. Biological Psychiatry, 54(6), 636–646.
Kesler, S. R., Haberecht, M. F., Menon, V., Warsofsky, I. S., Dyer-Friedman, J., Neely, E. K., et al. (2004). Functional neuroanatomy of spatial orientation processing in Turner syndrome. Cerebral Cortex, 14(2), 174–180.
Kesler, S. R., Menon, V., & Reiss, A. L. (2006). Neuro-functional differences associated with arithmetic processing in Turner syndrome. Cerebral Cortex, 16(6), 849–856. doi:10.1093/cercor/bhj028.
Knickmeyer, R. C., Wang, J., Zhu, H., Geng, X., Woolson, S., Hamer, R. M., et al. (2013). Common Variants in Psychiatric Risk Genes Predict Brain Structure at Birth. Cerebral Cortex. doi:10.1093/cercor/bhs401.
Kochunov, P., Glahn, D. C., Lancaster, J. L., Winkler, A. M., Smith, S., Thompson, P. M., et al. (2010). Genetics of microstructure of cerebral white matter using diffusion tensor imaging. NeuroImage, 53(3), 1109–1116. doi:10.1016/j.neuroimage.2010.01.078.
Kreis, R., Ernst, T., & Ross, B. D. (1993). Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magnetic Resonance in Medicine, 30(4), 424–437.
Kuczmarski, R. J., Ogden, C. L., Guo, S. S., Grummer-Strawn, L. M., Flegal, K. M., Mei, Z., et al. (2002). 2000 CDC Growth Charts for the United States: methods and development. Vital and Health Statistics, 11(246), 1–190.
Lau, J. Y., Goldman, D., Buzas, B., Fromm, S. J., Guyer, A. E., Hodgkinson, C., et al. (2009). Amygdala function and 5-HTT gene variants in adolescent anxiety and major depressive disorder. Biological Psychiatry, 65(4), 349–355. doi:10.1016/j.biopsych.2008.08.037.
Lebel, C., Gee, M., Camicioli, R., Wieler, M., Martin, W., & Beaulieu, C. (2012). Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage, 60(1), 340–352. doi:10.1016/j.neuroimage.2011.11.094.
Lee, A. D., Lepore, N., Brun, C., Chou, Y. Y., Barysheva, M., Chiang, M. C., et al. (2009). Tensor-based analysis of genetic influences on brain integrity using DTI in 100 twins. Medical Image Computing and Computer-Assisted Intervention, 12(Pt 1), 967–974.
Lenroot, R. K., Gogtay, N., Greenstein, D. K., Wells, E. M., Wallace, G. L., Clasen, L. S., et al. (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage, 36(4), 1065–1073. doi:10.1016/j.neuroimage.2007.03.053.
Lenroot, R. K., Schmitt, J. E., Ordaz, S. J., Wallace, G. L., Neale, M. C., Lerch, J. P., et al. (2009). Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Human Brain Mapping, 30(1), 163–174. doi:10.1002/hbm.20494.
Lewis, C. (2005). Self-organizing individual differences in brain development. Developmental Review, 25(3–4), 252–277. doi:10.1016/j.dr.2005.10.006.
Liu, X., Akula, N., Skup, M., Brotman, M. A., Leibenluft, E., & McMahon, F. J. (2010). A genome-wide association study of amygdala activation in youths with and without bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 49(1), 33–41.
Luders, E., Narr, K. L., Thompson, P. M., Woods, R. P., Rex, D. E., Jancke, L., et al. (2005). Mapping cortical gray matter in the young adult brain: effects of gender. NeuroImage, 26(2), 493–501. doi:10.1016/j.neuroimage.2005.02.010.
MacLusky, N. J., Hajszan, T., Prange-Kiel, J., & Leranth, C. (2006). Androgen modulation of hippocampal synaptic plasticity. Neuroscience, 138(3), 957–965. doi:10.1016/j.neuroscience.2005.12.054.
Matsuzawa, J., Matsui, M., Konishi, T., Noguchi, K., Gur, R. C., Bilker, W., et al. (2001). Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cerebral Cortex, 11(4), 335–342.
Maudsley, A. A., Domenig, C., Govind, V., Darkazanli, A., Studholme, C., Arheart, K., et al. (2009). Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI). Magnetic Resonance in Medicine, 61(3), 548–559. doi:10.1002/mrm.21875.
McGuire, K. A., Iacono, K. J., & McGue, M. (1998). Genetic influences on the spontaneous EEG: an examination of 15-year-old and 17-year-old twins. Developmental Neuropsychology, 14, 7–18.
Mechelli, A., Tognin, S., McGuire, P. K., Prata, D., Sartori, G., Fusar-Poli, P., et al. (2009). Genetic vulnerability to affective psychopathology in childhood: a combined voxel-based morphometry and functional magnetic resonance imaging study. Biological Psychiatry, 66(3), 231–237. doi:10.1016/j.biopsych.2009.01.033.
Menzler, K., Belke, M., Wehrmann, E., Krakow, K., Lengler, U., Jansen, A., et al. (2011). Men and women are different: diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. NeuroImage, 54(4), 2557–2562. doi:10.1016/j.neuroimage.2010.11.029.
Morris, R. W., Fung, S. J., Rothmond, D. A., Richards, B., Ward, S., Noble, P. L., et al. (2010). The effect of gonadectomy on prepulse inhibition and fear-potentiated startle in adolescent rhesus macaques. Psychoneuroendocrinology, 35(6), 896–905. doi:10.1016/j.psyneuen.2009.12.002.
Mueller, S. C., Aouidad, A., Gorodetsky, E., Goldman, D., Pine, D. S., & Ernst, M. (2013). Gray matter volume in adolescent anxiety: an impact of the brain-derived neurotrophic factor Val(66)Met polymorphism? Journal of the American Academy of Child and Adolescent Psychiatry, 52(2), 184–195. doi:10.1016/j.jaac.2012.11.016.
Murphy, D. G., DeCarli, C., Daly, E., Haxby, J. V., Allen, G., White, B. J., et al. (1993). X-chromosome effects on female brain: a magnetic resonance imaging study of Turner’s syndrome. Lancet, 342(8881), 1197–1200.
Neufang, S., Specht, K., Hausmann, M., Gunturkun, O., Herpertz-Dahlmann, B., Fink, G. R., et al. (2009). Sex differences and the impact of steroid hormones on the developing human brain. Cerebral Cortex, 19(2), 464–473. doi:10.1093/cercor/bhn100.
Nopoulos, P., Flaum, M., O’Leary, D., & Andreasen, N. C. (2000). Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Research, 98(1), 1–13.
Oppenheim, J. S., Lee, B. C., Nass, R., & Gazzaniga, M. S. (1987). No sex-related differences in human corpus callosum based on magnetic resonance imagery. Annals of Neurology, 21(6), 604–606. doi:10.1002/ana.410210615.
Ordaz, S. J., Lenroot, R. K., Wallace, G. L., Clasen, L. S., Blumenthal, J. D., Schmitt, J. E., et al. (2010). Are there differences in brain morphometry between twins and unrelated singletons? A pediatric MRI study. Genes, Brain, and Behavior, 9(3), 288–295. doi:10.1111/j.1601-183X.2009.00558.x.
Ota, M., Obata, T., Akine, Y., Ito, H., Ikehira, H., Asada, T., et al. (2006). Age-related degeneration of corpus callosum measured with diffusion tensor imaging. NeuroImage, 31(4), 1445–1452. doi:10.1016/j.neuroimage.2006.02.008.
Pacheco, J., Beevers, C. G., Benavides, C., McGeary, J., Stice, E., & Schnyer, D. M. (2009). Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. Journal of Neuroscience, 29(19), 6229–6233. doi:10.1523/JNEUROSCI.0896-09.2009.
Pal, D., Trivedi, R., Saksena, S., Yadav, A., Kumar, M., Pandey, C. M., et al. (2011). Quantification of age- and gender-related changes in diffusion tensor imaging indices in deep grey matter of the normal human brain. Journal of Clinical Neuroscience, 18(2), 193–196. doi:10.1016/j.jocn.2010.05.033.
Panizzon, M. S., Fennema-Notestine, C., Eyler, L. T., Jernigan, T. L., Prom-Wormley, E., Neale, M., et al. (2009). Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex, 19(11), 2728–2735. doi:10.1093/cercor/bhp026.
Partridge, S. C., Mukherjee, P., Henry, R. G., Miller, S. P., Berman, J. I., Jin, H., et al. (2004). Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. NeuroImage, 22(3), 1302–1314. doi:10.1016/j.neuroimage.2004.02.038.
Passe, T. J., Rajagopalan, P., Tupler, L. A., Byrum, C. E., MacFall, J. R., & Krishnan, K. R. (1997). Age and sex effects on brain morphology. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 21(8), 1231–1237.
Patwardhan, A. J., Brown, W. E., Bender, B. G., Linden, M. G., Eliez, S., & Reiss, A. L. (2002). Reduced size of the amygdala in individuals with 47, XXY and 47, XXX karyotypes. American Journal of Medical Genetics, 114(1), 93–98.
Peper, J. S., Brouwer, R. M., Schnack, H. G., van Baal, G. C., van Leeuwen, M., van den Berg, S. M., et al. (2008). Cerebral white matter in early puberty is associated with luteinizing hormone concentrations. Psychoneuroendocrinology, 33(7), 909–915. doi:10.1016/j.psyneuen.2008.03.017.
Peper, J. S., Schnack, H. G., Brouwer, R. M., Van Baal, G. C., Pjetri, E., Szekely, E., et al. (2009). Heritability of regional and global brain structure at the onset of puberty: a magnetic resonance imaging study in 9-year-old twin pairs. Human Brain Mapping, 30(7), 2184–2196. doi:10.1002/hbm.20660.
Perrin, J. S., Herve, P. Y., Leonard, G., Perron, M., Pike, G. B., Pitiot, A., et al. (2008). Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. Journal of Neuroscience, 28(38), 9519–9524. doi:10.1523/JNEUROSCI.1212-08.2008.
Perrin, J. S., Leonard, G., Perron, M., Pike, G. B., Pitiot, A., Richer, L., et al. (2009). Sex differences in the growth of white matter during adolescence. NeuroImage, 45(4), 1055–1066. doi:10.1016/j.neuroimage.2009.01.023.
Peters, M. (1991). Sex differences in human brain size and the general meaning of differences in brain size. Canadian Journal of Psychology, 45(4), 507–522.
Plomin, R., Owen, M. J., & McGuffin, P. (1994). The genetic basis of complex human behaviors. Science, 264(5166), 1733–1739.
Pouwels, P. J., Brockmann, K., Kruse, B., Wilken, B., Wick, M., Hanefeld, F., et al. (1999). Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatric Research, 46(4), 474–485. doi:10.1203/00006450-199910000-00019.
Qiu, A., Fortier, M. V., Bai, J., Zhang, X., Chong, Y. S., Kwek, K., et al. (2013). Morphology and microstructure of subcortical structures at birth: a large-scale Asian neonatal neuroimaging study. NeuroImage, 65, 315–323. doi:10.1016/j.neuroimage.2012.09.032.
Rakic, P. (1990). Principles of neural cell migration. Experientia, 46(9), 882–891.
Ratnarajah, N., Rifkin-Graboi, A., Fortier, M. V., Chong, Y. S., Kwek, K., Saw, S. M., et al. (2013). Structural connectivity asymmetry in the neonatal brain. NeuroImage, 75, 187–194. doi:10.1016/j.neuroimage.2013.02.052.
Raz, N., Gunning-Dixon, F., Head, D., Rodrigue, K. M., Williamson, A., & Acker, J. D. (2004). Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiology of Aging, 25(3), 377–396. doi:10.1016/S0197-4580(03)00118-0.
Raznahan, A., Greenstein, D., Lee, Y., Long, R., Clasen, L., Gochman, P., et al. (2011). Catechol-o-methyl transferase (COMT) val158met polymorphism and adolescent cortical development in patients with childhood-onset schizophrenia, their non-psychotic siblings, and healthy controls. NeuroImage, 57(4), 1517–1523. doi:10.1016/j.neuroimage.2011.05.032.
Raznahan, A., Lee, Y., Stidd, R., Long, R., Greenstein, D., Clasen, L., et al. (2010). Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proceedings of the National Academy of Sciences of the United States of America, 107(39), 16988–16993. doi:10.1073/pnas.1006025107.
Raznahan, A., Lee, Y., Vaituzis, C., Tran, L., Mackie, S., Tiemeier, H., et al. (2012). Allelic variation within the putative autism spectrum disorder risk gene homeobox A1 and cerebellar maturation in typically developing children and adolescents. Autism Research, 5(2), 93–100. doi:10.1002/aur.238.
Reiman, M., Parkkola, R., Lapinleimu, H., Lehtonen, L., & Haataja, L. (2009). Interleukin-6–174 and −572 genotypes and the volume of deep gray matter in preterm infants. Pediatric Research, 65(1), 90–96. doi:10.1203/PDR.0b013e31818bbfac.
Reiss, A. L., Abrams, M. T., Singer, H. S., Ross, J. L., & Denckla, M. B. (1996). Brain development, gender and IQ in children. A volumetric imaging study. Brain, 119(Pt 5), 1763–1774.
Reiss, A. L., Mazzocco, M. M., Greenlaw, R., Freund, L. S., & Ross, J. L. (1995). Neurodevelopmental effects of X monosomy: a volumetric imaging study. Annals of Neurology, 38(5), 731–738. doi:10.1002/ana.410380507.
Rimol, L. M., Panizzon, M. S., Fennema-Notestine, C., Eyler, L. T., Fischl, B., Franz, C. E., et al. (2010). Cortical thickness is influenced by regionally specific genetic factors. Biological Psychiatry, 67(5), 493–499. doi:10.1016/j.biopsych.2009.09.032.
Sacher, J., Neumann, J., Okon-Singer, H., Gotowiec, S., & Villringer, A. (2013). Sexual dimorphism in the human brain: evidence from neuroimaging. Magnetic Resonance Imaging, 31(3), 366–375. doi:10.1016/j.mri.2012.06.007.
Schmithorst, V. J., & Holland, S. K. (2007). Sex differences in the development of neuroanatomical functional connectivity underlying intelligence found using Bayesian connectivity analysis. NeuroImage, 35(1), 406–419. doi:10.1016/j.neuroimage.2006.11.046.
Schmithorst, V. J., Holland, S. K., & Dardzinski, B. J. (2008). Developmental differences in white matter architecture between boys and girls. Human Brain Mapping, 29(6), 696–710. doi:10.1002/hbm.20431.
Schmitt, J. E., Lenroot, R. K., Ordaz, S. E., Wallace, G. L., Lerch, J. P., Evans, A. C., et al. (2009). Variance decomposition of MRI-based covariance maps using genetically informative samples and structural equation modeling. NeuroImage, 47(1), 56–64. doi:10.1016/j.neuroimage.2008.06.039.
Schmitt, J. E., Wallace, G. L., Rosenthal, M. A., Molloy, E. A., Ordaz, S., Lenroot, R., et al. (2007). A multivariate analysis of neuroanatomic relationships in a genetically informative pediatric sample. NeuroImage, 35(1), 70–82. doi:10.1016/j.neuroimage.2006.04.232.
Schneiderman, J. S., Buchsbaum, M. S., Haznedar, M. M., Hazlett, E. A., Brickman, A. M., Shihabuddin, L., et al. (2007). Diffusion tensor anisotropy in adolescents and adults. Neuropsychobiology, 55(2), 96–111. doi:10.1159/000104277.
Shaw, P., Lerch, J. P., Pruessner, J. C., Taylor, K. N., Rose, A. B., Greenstein, D., et al. (2007). Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurology, 6(6), 494–500. doi:10.1016/S1474-4422(07)70106-0.
Siegmund, K. D., Connor, C. M., Campan, M., Long, T. I., Weisenberger, D. J., Biniszkiewicz, D., et al. (2007). DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PLoS One, 2(9), e895. doi:10.1371/journal.pone.0000895.
Simmons, R. A. (2009). Developmental origins of adult disease. Pediatric Clinics of North America, 56(3), 449–466. doi:10.1016/j.pcl.2009.03.004. Table of Contents.
Smit, D. J., Stam, C. J., Posthuma, D., Boomsma, D. I., & de Geus, E. J. (2008). Heritability of “small-world” networks in the brain: a graph theoretical analysis of resting-state EEG functional connectivity. Human Brain Mapping, 29(12), 1368–1378. doi:10.1002/hbm.20468.
Sowell, E. R., Peterson, B. S., Kan, E., Woods, R. P., Yoshii, J., Bansal, R., et al. (2007). Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cerebral Cortex, 17(7), 1550–1560. doi:10.1093/cercor/bhl066.
Sowell, E. R., Trauner, D. A., Gamst, A., & Jernigan, T. L. (2002). Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental Medicine and Child Neurology, 44(1), 4–16.
Speck, O., Ernst, T., Braun, J., Koch, C., Miller, E., & Chang, L. (2000). Gender differences in the functional organization of the brain for working memory. Neuroreport, 11(11), 2581–2585.
Sundram, F., Campbell, L. E., Azuma, R., Daly, E., Bloemen, O. J., Barker, G. J., et al. (2010). White matter microstructure in 22q11 deletion syndrome: a pilot diffusion tensor imaging and voxel-based morphometry study of children and adolescents. Journal of Neurodevelopmental Disorders, 2(2), 77–92. doi:10.1007/s11689-010-9043-6.
Tan, Q., Christiansen, L., Thomassen, M., Kruse, T. A., & Christensen, K. (2013). Twins for epigenetic studies of human aging and development. Ageing Research Reviews, 12(1), 182–187. doi:10.1016/j.arr.2012.06.004.
Tanaka, C., Matsui, M., Uematsu, A., Noguchi, K., & Miyawaki, T. (2012). Developmental trajectories of the fronto-temporal lobes from infancy to early adulthood in healthy individuals. Developmental Neuroscience, 34(6), 477–487. doi:10.1159/000345152.
Thomason, M. E., Dougherty, R. F., Colich, N. L., Perry, L. M., Rykhlevskaia, E. I., Louro, H. M., et al. (2010). COMT genotype affects prefrontal white matter pathways in children and adolescents. NeuroImage, 53(3), 926–934. doi:10.1016/j.neuroimage.2010.01.033.
Tiemeier, H., Lenroot, R. K., Greenstein, D. K., Tran, L., Pierson, R., & Giedd, J. N. (2010). Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. NeuroImage, 49(1), 63–70. doi:10.1016/j.neuroimage.2009.08.016.
Toga, A. W., & Thompson, P. M. (2003). Mapping brain asymmetry. Nature Review Neuroscience, 4(1), 37–48. doi:10.1038/nrn1009.
Tognin, S., Viding, E., McCrory, E. J., Taylor, L., O’Donovan, M. C., McGuire, P., et al. (2011). Effects of DTNBP1 genotype on brain development in children. Journal of Child Psychology and Psychiatry, 52(12), 1287–1294. doi:10.1111/j.1469-7610.2011.02427.x.
Tramo, M. J., Loftus, W. C., Stukel, T. A., Green, R. L., Weaver, J. B., & Gazzaniga, M. S. (1998). Brain size, head size, and intelligence quotient in monozygotic twins. Neurology, 50(5), 1246–1252.
van Baal, G. C., Boomsma, D. I., & de Geus, E. J. (2001). Longitudinal genetic analysis of EEG coherence in young twins. Behavior Genetics, 31(6), 637–651.
Van Baal, G. C., De Geus, E. J., & Boomsma, D. I. (1996). Genetic architecture of EEG power spectra in early life. Electroencephalography and Clinical Neurophysiology, 98(6), 502–514.
van den Heuvel, M. P., van Soelen, I. L., Stam, C. J., Kahn, R. S., Boomsma, D. I., & Hulshoff Pol, H. E. (2013). Genetic control of functional brain network efficiency in children. European Neuropsychopharmacology, 23(1), 19–23. doi:10.1016/j.euroneuro.2012.06.007.
van Soelen, I. L., Brouwer, R. M., Peper, J. S., van Leeuwen, M., Koenis, M. M., van Beijsterveldt, T. C., et al. (2012a). Brain SCALE: brain structure and cognition: an adolescent longitudinal twin study into the genetic etiology of individual differences. Twin Research and Human Genetics, 15(3), 453–467. doi:10.1017/thg.2012.4.
van Soelen, I. L., Brouwer, R. M., van Baal, G. C., Schnack, H. G., Peper, J. S., Chen, L., et al. (2013). Heritability of volumetric brain changes and height in children entering puberty. Human Brain Mapping, 34(3), 713–725. doi:10.1002/hbm.21468.
van Soelen, I. L., Brouwer, R. M., van Baal, G. C., Schnack, H. G., Peper, J. S., Collins, D. L., et al. (2012b). Genetic influences on thinning of the cerebral cortex during development. NeuroImage, 59(4), 3871–3880. doi:10.1016/j.neuroimage.2011.11.044.
Volpe, J. J. (2000). Overview: normal and abnormal human brain development. Mental Retardation and Developmental Disabilities Research Reviews, 6(1), 1–5. doi:10.1002/(SICI)1098-2779(2000)6:1<1::AID-MRDD1>3.0.CO;2-J.
Wallace, G. L., Eric Schmitt, J., Lenroot, R., Viding, E., Ordaz, S., Rosenthal, M. A., et al. (2006). A pediatric twin study of brain morphometry. Journal of Child Psychology and Psychiatry, 47(10), 987–993. doi:10.1111/j.1469-7610.2006.01676.x.
Weaver, B. A., Feng, Q., Holmes, K. K., Kiviat, N., Lee, S. K., Meyer, C., et al. (2004). Evaluation of genital sites and sampling techniques for detection of human papillomavirus DNA in men. Journal of Infectious Diseases, 189(4), 677–685. doi:10.1086/381395.
Weiss, E., Siedentopf, C. M., Hofer, A., Deisenhammer, E. A., Hoptman, M. J., Kremser, C., et al. (2003). Sex differences in brain activation pattern during a visuospatial cognitive task: a functional magnetic resonance imaging study in healthy volunteers. Neuroscience Letters, 344(3), 169–172.
Westerhausen, R., Kreuder, F., Dos Santos Sequeira, S., Walter, C., Woerner, W., Wittling, R. A., et al. (2004). Effects of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: a combined high-resolution and diffusion-tensor MRI study. Brain Research Cognitive Brain Research, 21(3), 418–426. doi:10.1016/j.cogbrainres.2004.07.002.
Westlye, L. T., Walhovd, K. B., Dale, A. M., Bjornerud, A., Due-Tonnessen, P., Engvig, A., et al. (2010). Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cerebral Cortex, 20(9), 2055–2068. doi:10.1093/cercor/bhp280.
Wiggins, J. L., Bedoyan, J. K., Peltier, S. J., Ashinoff, S., Carrasco, M., Weng, S. J., et al. (2012). The impact of serotonin transporter (5-HTTLPR) genotype on the development of resting-state functional connectivity in children and adolescents: a preliminary report. NeuroImage, 59(3), 2760–2770. doi:10.1016/j.neuroimage.2011.10.030.
Wright, I. C., Sham, P., Murray, R. M., Weinberger, D. R., & Bullmore, E. T. (2002). Genetic contributions to regional variability in human brain structure: methods and preliminary results. NeuroImage, 17(1), 256–271.
Wu, K., Taki, Y., Sato, K., Hashizume, H., Sassa, Y., Takeuchi, H., et al. (2013). Topological organization of functional brain networks in healthy children: differences in relation to age, sex, and intelligence. PLoS One, 8(2), e55347. doi:10.1371/journal.pone.0055347.
Yakovlev, P. I., & Lecours, A. (1967). The myelogenetic cycles of regional maturation of the brain. (Regional Development of the Brain in Early Life).
Yang, Y., Joshi, A. A., Joshi, S. H., Baker, L. A., Narr, K. L., Raine, A., et al. (2012). Genetic and environmental influences on cortical thickness among 14-year-old twins. Neuroreport, 23(12), 702–706. doi:10.1097/WNR.0b013e328355a62a.
Yoon, U., Fahim, C., Perusse, D., & Evans, A. C. (2010). Lateralized genetic and environmental influences on human brain morphology of 8-year-old twins. NeuroImage, 53(3), 1117–1125. doi:10.1016/j.neuroimage.2010.01.007.
Zhang, Y. E., Landback, P., Vibranovski, M. D., & Long, M. (2011). Accelerated recruitment of new brain development genes into the human genome. PLoS Biology, 9(10), e1001179. doi:10.1371/journal.pbio.1001179.
Ziermans, T., Dumontheil, I., Roggeman, C., Peyrard-Janvid, M., Matsson, H., Kere, J., et al. (2012). Working memory brain activity and capacity link MAOA polymorphism to aggressive behavior during development. Translational Psychiatry, 2, e85. doi:10.1038/tp.2012.7.
Zuo, X. N., Kelly, C., Di Martino, A., Mennes, M., Margulies, D. S., Bangaru, S., et al. (2010). Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. Journal of Neuroscience, 30(45), 15034–15043. doi:10.1523/JNEUROSCI.2612-10.2010.
Acknowledgments
The authors would like to thank Jared Char, Youngsu Kwon and Dr Sody Munsaka for their assistance with literature searches and proof reading of the manuscript.
Grant Support
RC2-DA29475, 2 K24-DA016170 K01-DA021203; U54NS56883; G12-MD007601.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Douet, V., Chang, L., Cloak, C. et al. Genetic influences on brain developmental trajectories on neuroimaging studies: from infancy to young adulthood. Brain Imaging and Behavior 8, 234–250 (2014). https://doi.org/10.1007/s11682-013-9260-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-013-9260-1