Abstract
Children with autism spectrum disorder (ASD) are highly variable in their language abilities, but the neural bases of these individual differences are poorly understood. Structural magnetic resonance imaging (MRI) and magnetic resonance diffusion tensor imaging (DTI) tractography were used to examine asymmetries in language-related gray- and white-matter and their relationships to language ability in a sample of 20 children with ASD, aged 4–7 years, and a reference sample of 20 typically developing (TD) children, aged 6–11 years. Children with ASD did not differ significantly from TD children in gray matter asymmetries, but were significantly less left-lateralized than TD children in the volume and radial diffusivity (RD) of the arcuate fasciculus (AF). They did not differ in the fractional anisotropy (FA) or the mean or axial diffusivity of the AF. Within the ASD group, exploratory analyses revealed that decreased leftward/increased rightward asymmetry of pars opercularis was associated with higher language ability and bilaterally increased FA and decreased RD of the AF. In conclusion, children with ASD exhibited atypical asymmetry in language-related white-matter structure as well as an atypical pattern of brain-language relationships that suggest that they may meet language milestones and acquire normal language via a different neurodevelopmental trajectory from TD children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atypical language development is a common feature of autism spectrum disorder (ASD). Of the approximately 70 % of children with ASD who acquire functional language, most show developmental delays (CDCP 2007) and many exhibit structural language impairment (Kjelgaard and Tager-Flusberg 2001). However, whereas impairment in communication and appropriate language use is a defining feature of ASD, not all individuals with ASD exhibit language delay or impairment, which are more variable features of the disorder.
Behavioral and molecular genetics research have demonstrated that some of the genetic variants that lead to impairment of structural language may also confer increased susceptibility to ASD (e.g., Vernes et al. 2008; see Abrahams and Geschwind 2008, for a review). Accordingly, a clearer understanding of the neural bases of structural language function and dysfunction in ASD can help to shed light on its complex neurodevelopmental etiology. Yet, despite significant research efforts and progress toward this end, the neurodevelopmental correlates of language ability in ASD are not well understood.
In the present study, we investigated asymmetries of language-related gray and white matter in children with ASD aged 4 to 7 years using structural magnetic resonance imaging (MRI) and magnetic resonance diffusion tensor imaging (DTI). In addition, we explored how differences in language-related gray and white matter asymmetries might relate to differences in language competence in young children with ASD.
Functional lateralization of language cortex in ASD
Language is functionally lateralized to the left cerebral hemisphere in the large majority of typically developing (TD) individuals (Toga and Thompson 2003), and left-hemisphere language dominance is already well-established in children as young as 5 years (Szaflarski et al. 2006). In comparison to TD individuals, children and adults with ASD have been found to exhibit reduced or reversed (i.e., rightward) functional lateralization of language (Boddaert et al. 2003; Dawson et al. 1989; Eyler et al. 2012; Kleinhans et al. 2008; Knaus et al. 2010; Müller et al. 1999; Redcay and Courchesne 2008; Takeuchi et al. 2004; Wang et al. 2006). However, findings on the relationship between functional language lateralization and language ability in individuals with ASD have been inconsistent. Whereas Redcay and Courchesne (2008) reported a positive association between increased rightward functional activation of language cortex and language skills in 2-3-year olds, Dawson et al. (1989) reported a negative association between these variables in school-aged children, and Knaus et al. (2010) found no association in adolescents with ASD. Although these disparate findings might be attributed to sample differences, they also suggest the possibility of an altered developmental course in children with ASD who acquire language relatively early (Eyler et al. 2012; Redcay and Courchesne 2008).
Structural asymmetries of language-related cortex in ASD
Structural imaging studies of school-age children and adolescents with ASD have demonstrated atypical volumetric asymmetries of the inferior language cortices comprising Broca’s area, including increased rightward asymmetry of pars opercularis (POP; Herbert et al. 2002), decreased leftward asymmetry of pars triangularis (PTR; Herbert et al. 2005), and rightward asymmetry of PTR and POP combined, but only in language-impaired children with ASD and not in language-normal children with ASD (de Fossé et al. 2004). However, Knaus et al. (2009) found no evidence of atypical asymmetry in anterior language cortex in children and adolescents with ASD. Structural imaging studies of the posterior language cortices comprising Wernicke’s area have also produced evidence of abnormal volumetric asymmetries in ASD, most notably of the planum temporale (PT), for which increased leftward asymmetry was reported by Herbert et al. (2002) and by de Fossé et al. (2004), but which was again limited to language-impaired children. In contrast, neither Knaus et al. (2009) or Rojas and colleagues (Rojas et al. 2005) found evidence of increased leftward asymmetry of the PT in children and adolescents with ASD.
Consistent with their subgroup findings, de Fossé et al. (2004) reported a positive correlation between verbal IQ (but not nonverbal IQ) and the degree of leftward asymmetry of inferior frontal cortex in their total sample of children with ASD. Thus, in contrast to fMRI evidence linking rightward functional asymmetry of frontal language cortex to more advanced language abilities in toddler-age children with ASD (Redcay and Courchesne 2008), de Fossé et al.’s volumetric findings from school-age children suggest that rightward structural asymmetry of language-related frontal cortex is associated with poorer language skills.
Abnormalities of language-related white matter in ASD
DTI is a magnetic resonance imaging technique that measures the orientation of white matter fibers based on the direction and degree of microscopic water diffusion by estimating a diffusion tensor at each voxel of the MR image (Basser and Pierpaoli 1996). The diffusion tensor is represented by three eigenvectors and three corresponding eigenvalues that define an ellipsoid. These three vectors cross at the center point of the ellipsoid and are perpendicular to each other. The long principal vector is measured as λ 1, the primary direction of diffusion, presumably parallel to white matter fibers and referred to as axial diffusivity. The two smaller vectors are measured as λ 2 and λ 3, and represent the diffusion of water perpendicular to the primary vector, which is normally restricted by cellular membranes and other tissue. The diffusivities of these two vectors are averaged, (λ 2 + λ 3)/2, to produce a measure of radial diffusivity. The average of all three eigenvalues, (λ 1 + λ 2 + λ 3)/3, is known as mean diffusivity, which measures the degree of restriction of diffusion of water irrespective of direction, with higher values indicating decreased restriction. In contrast, fractional anisotropy (FA) quantifies the degree of directedness of water diffusion within the corresponding brain tissue by measuring the degree to which the second and third vectors are small relative to the principal vector of water diffusion. FA ranges from a value of 0, meaning isotropic, or completely unrestricted in direction, to 1, meaning anisotropic, or singularly directional. Although FA and MD are the most commonly reported DTI measures of restriction and structural coherence within white matter pathways, we quantified AD and RD in order to provide a more complete characterization of AF white matter microstructure in our ASD and TD samples (cf. Fletcher et al. 2010; Gao et al. 2009).
Diffusion tensor tractography uses the directionality information yielded by DTI to virtually reconstruct the brain’s white matter tracts (Conturo et al. 1999; Jones et al. 1999). Numerous studies of TD adults have used DTI tractography to track the arcuate fasciculus (AF), the fiber bundle that curves dorsally around the Sylvian fissure to connect inferior frontal and posterior temporal language cortex. The majority of these studies have reported leftward asymmetry in the volume and structural coherence (e.g., mean FA) of this tract (Barrick et al. 2007; Glaser and Rilling 2008; Parker et al. 2005; Powell et al. 2006; Vernooij et al. 2007).
Tractography studies of language-related white matter tracts in TD children have reported significant leftward asymmetry of the volume (Lebel and Beaulieu 2009; Qiu et al. 2011) and FA (Eluvathingal et al. 2007; Lebel and Beaulieu 2009) of the AF in children as young as age 5 or 6 years. Qiu et al. (2011) reported leftward lateralization of AF volume in 75 % of children 6–7 years olds and 73 % of children 9–11 years old. Similarly, Lebel and Beaulieu (2009) found leftward asymmetry of AF volume in 78 % of children aged 5 to 13 years, and that leftward lateralization of AF volume was positively associated with children’s language ability. These studies were consistent in finding no effect of age on the degree of leftward lateralization of the AF, whether measured in terms of volume or FA, suggesting that these asymmetries are already well-established by early childhood.
A number of studies have investigated the volume and microstructural properties of the AF in school-age and adolescent children with autism. Knaus et al. (2010) reported that the FA of the AF was similarly left-lateralized in 15 adolescents with ASD and a comparison group of TD children. In a sample of 10 adolescents with autism and age-matched TD controls, Fletcher et al. (2010) found no difference in the degree of leftward lateralization of AF volume between groups and, similar to Knaus et al. (2010), found no group differences in leftward lateralization of FA. However, whereas TD children exhibited significantly lower left than right MD and RD of the AF, ASD children exhibited no asymmetry in either of these AF measures. Neither study found associations between measures of AF microstructure and language skills, perhaps owing to the relatively high level and restricted range of language ability in both study samples. Nagae et al. (2012) investigated differences in the FA and MD of the AF in 17 language-impaired children with autism, 18 language-normal children with autism, and 25 TD children who were 6 to 18 years of age. They also found no group differences in the leftward lateralization of FA. However, consistent with Fletcher et al. (2010), MD was significantly increased in the left AF of language-impaired children with autism relative to TD children, and left AF MD values for the language-normal children with autism fell in between the other two groups. Although the right SLF showed the same pattern of MD values across groups, the group differences did not reach statistical significance. In analyses of all participants combined, Nagae et al. found that language ability was inversely associated with left AF MD and, to a lesser but still significant degree, right AF MD. These authors did not measure AD or RD.
Aims and rationale for the present study
We used volumetric MRI and DTI to assess both language-related gray- and white-matter asymmetries and their relationship to concurrent language ability in 20 4- to 7-year-old children with ASD. Children this age were old enough to be trained to complete a brief neuroimaging protocol, but were still young enough that we could expect significant variability in their language skills to be able to identify possible brain correlates of these differences in children with ASD who develop some fluent language. This age range also represents a gap in neuroimaging studies of language ability in ASD, which have either focused on toddlers (cf. Redcay and Courchesne 2008, using a sleep protocol) or school-aged children who are better able to comply with MRI procedures. To conduct this study, we implemented an intensive training protocol, requiring 3–4 sessions prior to brain imaging, designed to acclimate children to the imaging environment and to train them to a level of movement control sufficient to yield useable MRI data.
Because of the significant time commitment required for MRI training, we were unable to recruit an age-matched comparison sample of TD children. Therefore, we compared our ASD sample with a group of TD children aged 6 to 11 years, taking into account any possible age-related effects on our findings. The TD comparison group were the youngest participants from a larger sample of TD children for whom we had collected comparable brain and behavioral data in a separate study (e.g., Knaus et al. 2009) that used the same MRI/DTI acquisition parameters as in the present study and that we analyzed following the same procedures used for the ASD sample.
Based on the evidence reviewed above, we hypothesized that children with ASD, relative to prior findings from TD children of the same age and in direct comparisons with our TD reference group, would exhibit atypical lateralization of language-related cortical volumes, specifically PTR, POP, and PT, and decreased or reversed lateralization of the volumes and microstructural features, specifically MD and RD, of language-related white matter tracts. In addition, we conducted exploratory analyses to determine if asymmetry differences might account for variability in children’s functional language abilities.
Methods
Participants
Table 1 displays participant characteristics. Children were selected for having at least phrase speech to increase sample homogeneity. All participants met research classification criteria for autism or ASD based on the ADI-R (Rutter et al. 2003) and ADOS (Lord et al. 1999), administered by trained examiners who had established inter-rater reliability. Diagnoses were confirmed by an expert clinician (R.M.J.). Children were excluded if they had an autism-related medical condition (e.g., neurofibromatosis), frank neurological damage, history of head trauma or seizures, a major physical anomaly, or gestational age less than 35 weeks.
Measures
The DAS (Elliott 1990) was used to assess verbal and nonverbal IQ. Language level was measured with the OWLS (Carrow-Woolfolk 1995) oral expression and listening comprehension subscales, which together yielded a standardized composite score for oral language ability. The OWLS composite oral language and DAS verbal standard scores were highly correlated (r = .86, p < .001). To increase the reliability of language level estimates, and to reduce the number of study-wise statistical tests, a composite standard language score was calculated by averaging these two measures for each participant. Standardized, age-corrected IQ and language scores were used in all statistical analyses. Handedness was evaluated with a modified version of the Dean Laterality Preference Schedule (Dean 1988; Piro 1998) that consisted of 12 unimanual tasks.
Of 31 participants initially enrolled, 11 were excluded because of inability to attain sufficient movement control. These 11 children (8 males) did not differ from the final sample in age (M = 5;10; SD = 1:2), but had lower verbal (M = 77, SD = 32) and nonverbal (M = 84, SD = 32) IQ scores. Of the 20 children for whom gray matter data were obtained, 4 were unable to complete the 3 DTI sequences, which were conducted after the anatomical scan. The remaining 16 children were compared with the youngest 16 children from the TD reference sample on white matter measures.
MRI reference data were from 20 right-handed TD children (15 males) aged 6 to 11 years. TD children were assessed with the KBIT-2 (Kaufman and Kaufman 2004) for verbal and nonverbal IQ, and with the CELF-3 (Semel et al. 1995) for language ability. A composite language score was constructed by averaging the CELF-3 total language standard score and KBIT-2 verbal IQ score, which were significantly correlated (r = .66, p < .001).
MRI and DTI acquisition
Imaging data were collected using a 6-channel SENSE receiver coil on a 3 Tesla Philips Intera scanner. A high-resolution anatomical scan was acquired with the following parameters: 3D magnetization-prepared rapid-acquisition gradient-echo (MP-RAGE) imaging; TR/TE/TI = 7.2/3.4/885 ms; flip angle = 8°; FOV = 230 × 230 mm; 100–120 contiguous 1.5 mm axial slices; matrix = 256 × 256; image resolution = 0.9 × 0.9 × 1.4 mm. Three DTI sequences were acquired as follows: single-shot SE-EPI; TR/TE = 10,646/91 ms; FOV = 230 × 230 mm; 73 contiguous 2.0 mm axial slices, b-value = 1,000 sec/mm2; 15 gradient directions + 1 reference image (b = 0); matrix = 128 × 128; image resolution = 1.8 × 1.8 × 2.0 mm. Total scanning time was approximately 20 min.
MRI volumetric analyses of language-related cortices
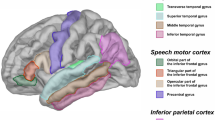
Cortical reconstruction and volumetric parcellations were conducted with Freesurfer 4.0.4 (Fischl et al. 2002). Data were checked throughout the processing stream to ensure that registration, removal of non-brain tissue, and segmentation were accurate. Gray matter labels for pars opercularis (POP), pars triangularis (PTR), and superior temporal gyrus (STG), including the planum temporale (PT), were extracted bilaterally from Freesurfer. Manual edits of the Freesurfer STG label, using 3D Slicer 3.4 (Pieper et al. 2006), were necessary to define the PT and posterior STG (pSTG). The anterior boundaries of the pSTG and PT were defined in the coronal plane as the most anterior image in which the Freesurfer parcellation of Heschl’s gyrus (HG) was present. PT was defined in the coronal plane as the gray matter on the superior surface of pSTG lateral to HG. The posterior boundary of the PT was the most posterior point at which the Sylvian fissure was visible in the coronal plane. The remaining label was defined as pSTG. These boundaries were similar to those used in previous studies (Knaus et al. 2004, 2007, 2009). Inter-rater reliability for the manually-edited labels was assessed for 5 brains bilaterally, yielding intra-class correlations of .91 for PT and .98 for pSTG. Labels are illustrated in Fig. 1.
To account for individual differences in total brain size, cortical volumes were calculated as a proportion of estimated total intracranial volume (Buckner et al. 2004). Asymmetry quotients for each region were calculated using the formula (Left volume–Right volume)/[(Left volume + Right volume)×. 50] (Galaburda et al. 1987), yielding positive values for leftward asymmetry and negative values for rightward asymmetry.
DTI analyses
Each participant’s three DTI acquisitions were corrected for motion, co-registered and averaged to enhance signal to noise. The resulting averaged DTI dataset was pre-processed (non-brain tissue removal, eddy current and motion correction) and then analyzed using FMRIB Diffusion Toolbox (FDT 2.0) from FSL 4.1.2 (Smith et al. 2004; Woolrich et al. 2009). To track long-range association fibers connecting anterior and posterior language cortices, a probabilistic diffusion model was applied (Behrens et al. 2007). First, Bayesian estimates of the fiber orientation distributions at each voxel in the DTI dataset were calculated using BEDPOSTX. For the tractography analyses, POP and PTR labels were combined to form one anterior ROI for each hemisphere, and pSTG and PT labels were combined to form one posterior ROI for each hemisphere. FDT’s probabilistic tractography algorithm was then used to model pathways connecting ipsilateral anterior and posterior language regions in native diffusion space. This algorithm selects a sample from the fiber orientation distribution at the current voxel and then selects the sample closest to that orientation in the next voxel, allowing for potential crossing of multiple fibers within a voxel. Five thousand streamlines were sent from each voxel in each of the anterior and posterior seeding ROIs, generating a connectivity distribution image that included only those streamlines that passed through both labels. The resulting connectivity distributions were further constrained by a waypoint ROI. This ROI was defined on the diffusion tensor color map in each hemisphere by identifying the most inferior axial slice in which the fornix was visible, and then moving in an anterior-to-posterior direction to identify the coronal slice in which the large, triangular anterior-posterior bundle of fibers (including the AF) was most intense. The ROI was then designated as an inclusion mask through which all streamlines had to pass. The FDT output was a connectivity distribution image in which the value for each voxel equaled the sum of the connectivity distributions from each seeding point. All tracts were visually inspected to ensure that they were anatomically plausible.
To calculate pathway volume, the connectivity distribution was first corrected to account for individual differences in seeding label size by dividing the value of each voxel in the connectivity distribution by the total number of voxels in the anterior and posterior labels multiplied by 5,000 (number of streamlines sent per voxel). The corrected tracts were then thresholded to include only voxels that received at least 1 × 10-6 % of the total streamlines sent out, creating a probabilistic connectivity distribution (see Rilling et al. 2008). See Fig. 2.
Prior to quantifying the microstructural properties of the pathways, we corrected the connectivity distributions for label size, and then divided the value of each voxel by the number of successful streamlines for the corresponding tract. This correction was made to adjust for possible individual differences in the trackability of diffusion data related to, for example, scan quality. Once corrected for trackability, the tracts were thresholded to include only voxels that received at least 0.1 % of the total streamlines sent out. To ensure that only white matter voxels were included in our calculations, a white matter mask was extracted from Freesurfer for each participant, and calculations of the mean FA, MD, AD, and RD of the left and right AF were limited to voxels within the confines of the mask. As control measures, mean FA, MD, AD, and RD of each participant’s total left- and right-hemisphere cerebral white matter was also calculated in FSL using Freesurfer masks.
The TD reference data (a high-resolution structural scan and 3 DTI scans) were acquired with the same scanner and head coil used for the ASD participants. Anatomical scanning parameters were similar (see Knaus et al. 2009), and DTI scanning parameters were the same. The same software packages were used to conduct automated gray matter volumetric analyses and editing and tractography, following the same technical procedures described above.
Because we did not have an age-matched control group, group comparisons were limited to patterns of asymmetries between left and right brain structures, and the association between these asymmetries and language abilities within each group. In addition, we conducted within-group correlations to examine the effects of age cross-sectionally on the development of language-related brain asymmetries. If reduced or reversed asymmetries in our ASD group (relative to our TD reference group and to findings for TD children reported in the literature) were simply the effect of their age, we would expect these to be reflected in our correlational analyses. We did not assess age effects on gray and white matter results in both groups combined because age and group were highly inter-correlated. Thus, any measure revealing a between-groups difference in brain metrics would be expected to correlate artifactually with age in a combined analysis.
In the analyses that follow, within-group paired-samples t-tests of left versus right gray and white matter values are reported for descriptive purposes only, in order to provide a basic characterization of the direction and magnitude of asymmetries within each group before comparing them between groups. In the between-group tests, we adopted a relatively conservative alpha of .01 in order to reduce the likelihood of a Type 1 error given that 4 gray matter and 5 white matter tests were conducted. To assess the within-group associations between gray and white matter asymmetries and language function, we used an alpha of .05, as these analyses were mainly exploratory, and as a consequence we have interpreted the findings from these analyses with the caution that they are preliminary.
Results
Asymmetries of language-related gray matter
Mean asymmetry quotients (AQs) are presented in Table 2, and left and right volume data for individual participants are plotted in Fig. 3. Paired-samples t-tests showed significantly larger left than right volume for POP, t (19) = 3.0, p < .01, and for PT, t (19) = 4.4, p < .001, but no difference between left and right for PTR and pSTG, in children with ASD. Similarly, the TD reference group evidenced larger left than right volumes for POP, t (19) = 3.3, p < .01, and for PT, t (19) = 4.6, p < .001, but not for PTR or pSTG.
Group comparisons of mean AQ values for each of the 4 cortical ROIs revealed no group differences. Although the ASD group exhibited increased leftward asymmetry of the PT relative to the TD reference group, F (1, 38) = 4.8, p < .05, this difference was not significant at our critical value of .01. POP asymmetry was negatively correlated with age in the ASD group, r = −.54, p < .02, reflecting greater rightward asymmetry at older ages. There were no other associations between age and cortical AQs in either group.
Asymmetries of language-related white matter
Mean AQs for all white matter measures are presented in Table 3, and individual participant data are plotted in Fig. 4. Paired-samples t-tests showed larger left than right AF volume, t (15) = 6.2, p < .001, in the TD group, but no difference between left and right AF volumes in the ASD group. Between-group comparisons showed that ASD children were significantly less leftward than TD children in AF volume asymmetry, F (1, 30) = 9.4, p < .01. There was no association between age and AF volumetric asymmetry in the ASD group, r (14) = .08, n.s., or the TD group, ASD, r (14) = .07, n.s.
Within-group comparisons revealed higher left than right FA of the AF in both children with ASD, t (15) = 2.1, p < .05, and TD children, t (15) = 3.3, p < .01. Neither group showed significant left-right differences in the MD or the AD of the AF. However, whereas TD children exhibited significantly higher right than left RD, t (15) = 4.0, p < .001, children with autism showed virtually no difference in RD between the left and right AF, t (15) = 0.1, n.s. As can be seen in Table 3, in between-group comparisons, there was significantly increased rightward asymmetry of AF RD in the TD relative to the ASD group, F (1, 30) = 7.6, p < .01. In contrast, there was no group difference in total cerebral hemispheric asymmetry of RD, F (1, 30) = .04, n.s., which tended rightward in both groups. To confirm the specificity of this finding to the AF, we conducted a second ANOVA that included the total left-versus-right-hemisphere AQ for RD as a covariate. With the inclusion of this covariate, F (1, 29) = 3.0, p < .09, the group effect on AF RD asymmetry remained virtually the same, F (1, 29) = 8.5, p < .01. There were no associations between age and FA, MD, AD, or RD asymmetry in the ASD or TD group.
Gray matter asymmetries and language ability
Exploratory correlational analyses were conducted to assess associations between cortical asymmetries and language ability. Pearson product–moment correlations (r) were used for variables that were normally distributed. Spearman rank-order correlations (r s ) were used when variables showed significant (p < .01) skewness or kurtosis, and in cases in which significant Pearson correlations appeared to be due to the effects of outliers.
There was a significant negative correlation between POP AQ and language ability, r (18) = −.47, p < .05, such that increased rightward asymmetry of POP was associated with more advanced language abilities. This correlation is plotted in Fig. 5. To determine if the association between POP AQ and language level was mediated by other factors, such as handedness or age, we compared the 14 children with positive (leftward) AQs to the 6 children with negative (rightward) AQs for POP. As can be seen in Table 4, there was no evidence that rightward POP asymmetry was associated with left-handedness; however, children with rightward asymmetry were older.
In the TD reference sample, leftward PT asymmetry was associated with more advanced language ability, r (18) = .60 p < .01. None of the cortical AQs were associated with nonverbal IQ in either the ASD or TD group.
White matter relationships to language ability
AQs for AF volume and FA, MD, AD, and RD were not correlated with concurrent language ability.
Relationships between gray- and white-matter asymmetries
Correlational analyses revealed no relationships between gray matter asymmetries and asymmetries of the volume or microstructural features of the AF in ASD or TD children, suggesting that structural asymmetries of gray and white matter were not developing in tandem. We also considered the possibility that inter-relationships between gray and white matter development might explain our findings linking rightward asymmetry of POP to more advanced language. It seemed plausible that increased myelin proliferation into the cortical neuropil of the left POP could reduce the MRI gray matter signal for left relative to right volumes (Sowell et al. 2004). Accordingly, seemingly aberrant gray matter development could be explained, at least in part, by developmentally normal changes in white matter. However, correlational analyses revealed no inverse relationships between POP asymmetry and AF asymmetry measures. Rather, in the ASD group, POP asymmetry was inversely correlated with mean FA of both the left AF, r (14) = −.83, p < .001, and right AF, r (14) = −.80, p < .001, and positively correlated with mean RD of the left AF, r (14) = .59, p < .02, and right AF, r (14) = .63, p < .01, indicating that rightward POP asymmetry was accompanied by bilaterally increased FA and decreased RD of the AF. These associations were equally significant when the effect of age was partialled from the correlations.
Discussion
This is the first study to investigate gray- and white-matter correlates of language ability in preschool and young school-age children with ASD. Children in this age range have rarely been studied in MRI research because they are too old for a sleep protocol, but have not yet gained the movement-control abilities of older children. This study yielded four main findings. First, children with ASD did not differ from the TD reference group in the pattern and degree of gray matter asymmetries. Second, children with ASD were significantly less left-lateralized than TD children in the volume of arcuate fasciculus. Third, children with ASD were significantly less right-lateralized than TD children in the radial diffusivity of arcuate fasciculus, consistent with the findings of Fletcher et al. (2010) in a sample of adolescent children with ASD. Fourth, although both groups showed a tendency toward leftward pars opercularis asymmetry, within the ASD group, decreased leftward/increased rightward asymmetry of pars opercularis was associated with earlier language onset and higher language ability. We discuss each of these findings below.
Gray matter asymmetries
In contrast to studies of older school-age children (de Fossé et al. 2004; Herbert et al. 2002, 2005), we found no difference between our ASD sample and a reference sample of TD children aged 6 to 10 years in gray matter asymmetries of language-related cortices (see also Knaus et al. 2009). Our null between-groups findings were not explained by the age difference between the ASD and TD samples. Gray matter asymmetry quotients were not associated with age in either group, with the one exception that rightward POP asymmetry was positively correlated with age in children with ASD. Based on evidence from TD children, we would have expected any age-related effects to have manifested in decreased leftward asymmetries among younger children (Amunts et al. 2003; Knaus et al. 2009), which we did not find.
White matter asymmetries
Children with ASD exhibited leftward volumetric asymmetry of the AF significantly less often (44 %) than children in the TD reference sample (88 %). AF volumetric asymmetry was not associated with age in either the ASD or TD group, consistent with prior studies of TD children in the same age range (Lebel and Beaulieu 2009; Qiu et al. 2011). In addition, the low rate of leftward lateralization of the AF among ASD participants in the current study was in striking contrast to the predominance of leftward AF lateralization among 5-to-7-year-old TD children reported by Lebel and Beaulieu (2009), who also used DTI tractography to estimate AF volume. The markedly lower frequency of leftward AF asymmetry that we found in similarly young children with ASD therefore seems unlikely to be a simple effect of their age. Unlike Lebel and Beaulieu (2009), we did not find that reduced volumetric asymmetry of the AF was associated with poorer language skills in either or ASD or TD children, perhaps owing to the much smaller sample sizes in the present study.
In the only prior study that examined volumetric asymmetries of the AF in ASD, Fletcher et al. (2010) found that ASD and TD adolescents were equally left lateralized in AF volume. This difference may reflect the more advanced language abilities of their older, high-functioning sample, and suggests the possibility that young children with ASD, who are usually language delayed, may show increased lateralization of the AF as they develop more fluent language. However, the inconsistencies in volumetric findings may also have resulted from use of anatomic segmentation methods to identify the AF in Fletcher et al., in contrast to the use of tractography in the present study.
As for the microstructural properties of the AF, children with ASD showed significant leftward asymmetry of the FA of the AF that was on par with that found in the TD reference group, consistent with prior findings (Fletcher et al. 2010; Knaus et al. 2010; Nagae et al. 2012). In contrast, children with ASD evidenced a complete absence of RD asymmetry of the AF, whereas TD children showed significant rightward lateralization. RD asymmetry was significantly different between groups and was independent of overall hemispheric asymmetry in RD, which was roughly equal between groups. Fletcher et al. (2010) similarly reported a complete absence of AF RD asymmetry in 11- to 16-year-old adolescents with ASD, which they described as one of the most striking differences between their ASD and TD groups (and of substantially greater magnitude than group differences in the RD of the left AF alone).
Our microstructural white matter asymmetry findings are compelling in that they closely parallel those of Fletcher et al. (2010), which is the only prior study to our knowledge that examined not only the FA and MD of the AF in ASD, but also the axial and radial components of these measures. In discussing their findings, Fletcher et al. posed the critical question of whether such differences in white matter microstructure of the AF reflect a primary neurobiological abnormality in ASD, or if they are more the result than the cause of abnormal language acquisition and function in ASD. Our finding of a similar pattern of microstructural white matter differences in a much younger sample of children with ASD who were selected for not yet having attained fluent language tends to support the former conclusion.
DTI studies that have examined AD and RD in an attempt to characterize the underlying cytoarchitecture of white matter development and degeneration have used these measures as markers of axonal integrity and myelination, respectively (Gao et al. 2009; Song et al. 2002). According to this division, our findings of a lack of RD asymmetry, and of bilateral associations between increased RD and poorer language function, point to abnormalities in the axonal myelination of the AF in young children with ASD. Future DTI research affording direct comparisons of both the volumetric and microstructural features of the left and right AF, and their asymmetries, with age-matched TD controls will allow stronger inferences about the white matter components of language delay and atypical language acquisition in young children with ASD.
Gray matter asymmetries and language ability
Whereas children with ASD and TD children exhibited similar patterns of gray matter asymmetries, the relationships between these asymmetries and language differed between groups. Most notably, rightward asymmetry of POP was positively associated with language level in children with ASD, independently of handedness and nonverbal IQ. This association was inconsistent with prior findings (de Fossé et al. 2004; Herbert et al. 2002) linking rightward volumetric asymmetries of the language-related frontal cortices to poorer language functioning in school-age children with autism. The factor most obviously differentiating the children in the present study, aged 4–7, from those in the prior studies, aged 7–11, was their younger age. However, this difference provides no plausible explanation for the completely opposite pattern of structure-function relations between groups that seem irreconcilable simply as a function of chronological age. We propose, rather, that the difference in structure-function relationships reflects a cohort effect. Children in the prior studies were born approximately two to three decades ago, when ASD was diagnosed at significantly later ages than in recent years, and were far less likely to have had access to intensive early intervention. In contrast, the current sample was drawn from a cohort diagnosed early and enrolled in intensive early interventions targeting language and related communicative skills, which may have fostered a functionally productive right-lateralization of frontal language cortex. This would be consistent with the earlier age of language onset in children with rightward POP asymmetry as well as recent findings linking rightward functional lateralization in 3-year-olds with ASD with more advanced language skills (Redcay and Courchesne 2008).
Finally, we also found that rightward asymmetry of POP was associated with increased mean FA of both the left and right AF in children with ASD. The link between structural characteristics of the AF specifically to POP in children with ASD was interesting in light of recent findings from TD adults demonstrating that the arcuate fasciculus, at least in the typically language-dominant left hemisphere, connects posterior language cortex primarily to POP, whereas PTR and the frontal operculum appear to track principally via ventral pathways to posterior language cortex (Anwander et al. 2007; Frey et al. 2008). The association of bilaterally increased FA of the AF with rightward POP asymmetry provides further anatomical support to our proposal that children with ASD who acquire language early, despite atypical asymmetry of language cortex, can progress in their language development by other means.
The association we found between rightward asymmetry of POP and higher language ability in children with ASD was based on exploratory analyses and requires replication in both structural and functional imaging studies of young children with ASD. Several questions remain, all of which may inform future research. First, is language functionally lateralized to the right frontal cortex in the children we examined, or is rightward volumetric asymmetry a vestige of earlier developments, with language function now lateralized in a more typical pattern? Second, what are the causes of atypical language lateralization? Some researchers (Kleinhans et al. 2008; Redcay and Courchesne 2008) have suggested that atypical language lateralization may result from differences in the timing and nature of right and left cerebral hemisphere maturation, which make them differentially susceptible to the effects of abnormally accelerated brain development beginning sometime in middle-to-later infancy in children with ASD (Redcay and Courchesne 2005; Schumann et al. 2010; Wolff et al. 2012). More specifically, these authors have suggested that because the right-hemisphere homologues of Broca’s area mature earlier, faster, and with greater innate genetic specification (e.g., Chiron et al. 1997; Geschwind et al. 2002), their development may be less disrupted by an abnormal brain growth trajectory than their left hemisphere counterparts, in which development is more protracted and experience-dependent and, consequently, more vulnerable. Finally, do children with right-lateralization and advanced language skills continue to progress normally or do they plateau in their language development? Alternatively, might children with right-lateralized language function fare better than other children, particularly those with bilaterally-organized language (Kleinhans et al. 2008; Knaus et al. 2009), as a result of increased processing efficiency, which has been theorized to drive, from an evolutionary perspective, the lateralization of complex, distributed brain functions, such as language (Corballis 2009).
Finally, PT asymmetry was strongly leftward and did not differ significantly between the ASD and TD groups. Leftward asymmetry of the PT is the most consistently observed volumetric asymmetry of language-related cortex (Shapleske et al. 1999), and the PT is widely recognized as playing a key role in auditory perception, an integral component of both language comprehension and production (Hickok and Poeppel 2007). Our findings from younger children than those previously studied are perhaps most interesting in that PT asymmetry, although strongly leftward, was not associated with language level in children with ASD, but was in the somewhat older TD reference sample, consistent with findings from prior studies of non-ASD children with and without language impairment (e.g., Eckert et al. 2001). It is possible that the lack of a structure-function correlation reflects a more diffuse organization of language in the posterior perisylvian cortices, akin to that which has been observed in functional neuroimaging studies of language organization in ASD (e.g., Eyler et al. 2012; Harris et al. 2006; Just et al. 2004; Müller et al. 1999). Direct examination of the relationship between the degree of structural asymmetry, for example of PT, and the focalization or diffusivity of cortical activation during on-line language processing with functional neuroimaging would be necessary to evaluate this possibility.
Limitations of current study
There are several limitations to the present study. First, the study lacked an age-matched control group. Although we have carefully considered and ruled out the possibility that group differences were attributable to age by demonstrating an absence of age-related effects within our participant groups and in similar studies reported in the literature, the inclusion of an age-matched TD control group would have strengthened our findings and conclusions. Second, the requirement of attaining sufficient motion control for participation had the effect of excluding children with poorer language skills. Investigation of young children with the broadest possible range of language skills, from phrase to fluent speech, is likely to be most informative. Third, our correlational findings of structural asymmetries and language function, mostly notably the link between rightward POP asymmetry and higher language ability in children with ASD, were not corrected for multiple comparisons. Although these findings were consistent with the results of a recent study (Redcay and Courchesne 2008) suggesting altered development of language in ASD, they must be considered preliminary. Finally, we have speculated broadly on both the possible antecedents and sequelae of the brain-language relationships in children with ASD we have begun to identify here. A clearer, more definitive account of these relationships will require longitudinal study from infancy through the early-to-middle school-age years.
References
Abrahams, B. S., & Geschwind, D. H. (2008). Advances in autism genetics: on the threshold of a new neurobiology. Nature Review Genetics, 9, 341–355.
Amunts, K., Schleicher, A., Ditterich, A., & Zilles, K. (2003). Broca’s region: cytoarchitectonic asymmetry and developmental changes. The Journal of Comparative Neurology, 465, 72–89.
Anwander, A., Tittgemeyer, M., von Cramon, D. Y., Friederici, A. D., & Knosche, T. R. (2007). Connectivity-based parcellation of Broca’s area. Cerebral Cortex, 17, 816–825.
Barrick, T. R., Lawes, I. N., Mackay, C. E., & Clark, C. A. (2007). White matter pathway asymmetry underlies functional lateralization. Cerebral Cortex, 17, 591–598.
Basser, P. J., & Pierpaoli, C. (1996). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. Series B, 11, 209–219.
Behrens, T. E., Johansen Berg, H., Jbabdi, S., Rushworth, M. F., & Woolrich, M. W. (2007). Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage, 34, 144–155.
Boddaert, N., Belin, P., Chabane, N., Poline, J. B., Barthélemy, C., Mouren-Simeoni, M. C., et al. (2003). Perception of complex sounds: abnormal pattern of cortical activation in autism. The American Journal of Psychiatry, 160, 2057–2060.
Buckner, R. L., Head, D., Parker, J., Fotenos, A. F., Marcus, D., Morris, J. C., et al. (2004). A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage, 23, 724–738.
Carrow-Woolfolk, E. (1995). Oral and written language scales. Bloomington: Pearson Assessment.
Center for Disease Control and Prevention (CDCP). (2007). Prevalence of autism spectrum disorders--autism and developmental disabilities monitoring network, 14 sites, United States, 2002. Surveillance Summaries, Feb. 9, 2007. Morbidity and Mortality Weekly Report, 56, 12–28.
Chiron, C., Jambaque, I., Nabbout, R., Lounes, R., Syrota, A., & Dulac, O. (1997). The right brain hemisphere is dominant in human infants. Brain, 120, 1057–1065.
Conturo, T. E., Lori, N. F., Cull, T. S., Akbudak, E., Snyder, A. Z., Shimony, J. S., et al. (1999). Tracking neuronal fiber pathways in the living human brain. Proceedings of the National Academy of Sciences of the United States of America, 96, 10422–10427.
Corballis, M. C. (2009). The evolution and genetics of cerebral asymmetry. Philosophical Transactions of the Royal Society of London B, 364, 867–879.
Dawson, G., Finley, C., Phillips, S., & Lewy, A. (1989). A comparison of hemispheric asymmetries in speech-related brain potentials of autistic and dysphasic children. Brain and Language, 37, 26–41.
de Fossé, L., Hodge, S. M., Makris, N., Kennedy, D. N., Caviness, V. S., Jr., McGrath, L., et al. (2004). Language-association cortex asymmetry in autism and specific language impairment. Annals of Neurology, 56, 757–766.
Dean, R. S. (1988). Lateral preference schedule. Odessa: Psychological Assessment Resources, Inc.
Eckert, M. A., Lombardino, L. J., & Leonard, C. M. (2001). Planar asymmetry tips the phonological playground and environment raises the bar. Child Development, 72, 988–1002.
Elliott, C. D. (1990). Differential ability scales: Introductory and technical handbook. New York: The Psychological Corporation.
Eluvathingal, T. J., Hasan, K. M., Kramer, L., Fletcher, J. M., & Ewing-Cobbs, L. (2007). Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cerebral Cortex, 17, 2760–2768.
Eyler, L. T., Pierce, K., & Courchesne, E. (2012). A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain, 135, 949–960.
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33, 341–355.
Fletcher, P. T., Whitaker, R. T., Tao, R., DuBray, M. B., Froehlich, A., Ravichandran, C., et al. (2010). Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. NeuroImage, 51, 1117–1125.
Frey, S., Campbell, J. S., Pike, G. B., & Petrides, M. (2008). Dissociating the human language pathways with high angular resolution diffusion fiber tractography. Journal of Neuroscience, 28, 11435–11444.
Galaburda, A., Corsiglia, J., Rosen, G., & Sherman, G. (1987). Planum temporale asymmetry, reappraisal since Geschwind and Levitsky. Neuropsychologia, 25, 853–868.
Gao, W., Lin, W., Chen, Y., Gerig, G., Smith, J. K., Jewells, V., et al. (2009). Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. American Journal of Neuroradiology, 30, 290–296.
Geschwind, D. H., Miller, B. L., DeCarli, C., & Carmelli, D. (2002). Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proceedings of the National Academy of Sciences, 99(5), 3176–3181.
Glaser, M. F., & Rilling, J. K. (2008). DTI tractography of the human brain’s language pathways. Cerebral Cortex, 18, 2471–2482.
Harris, G. J., Chabris, C. F., Clark, J., Urban, T., Aharon, I., Steele, S., et al. (2006). Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain and Cognition, 61, 54–68.
Herbert, M. R., Harris, G. J., Adrien, K. T., Ziegler, D. A., Makris, N., Kennedy, D. N., et al. (2002). Abnormal asymmetry in language association cortex in autism. Annals of Neurology, 52, 588–596.
Herbert, M. R., Ziegler, D. A., Deutsch, C. K., O’Brien, L. M., Kennedy, D. N., Filipek, A. I., et al. (2005). Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain, 128, 213–226.
Hickok, G., & Poeppel, D. (2007). The cortical organization of speech processing. Nature Neuroscience, 8, 393–402.
Jones, D. K., Simmons, A., Williams, S. C., & Horsfield, M. A. (1999). Non-invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magnetic Resonance in Medicine, 42, 37–41.
Just, M. A., Cherkassky, V. L., Keller, T. A., & Minshew, N. J. (2004). Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain, 127, 1811–1821.
Kaufman, A. S., & Kaufman, N. L. (2004). Kaufman brief intelligence test (2nd ed.). Circle Pines: American Guidance Service.
Kjelgaard, M., & Tager-Flusberg, H. (2001). An investigation of language impairment in autism: implications for genetic subgroups. Language & Cognitive Processes, 16, 287–308.
Kleinhans, N. M., Muller, R.-A., Cohen, D. N., & Courchesne, E. (2008). Atypical functional lateralization of language in autism spectrum disorders. Brain Research, 1221, 115–125.
Knaus, T. A., Bollich, A. M., Corey, D. M., Lemen, L. C., & Foundas, A. L. (2004). Sex-linked differences in the anatomy of perisylvian language cortex: a volumetric MRI study of gray-matter volumes. Neuropsychology, 18, 738–747.
Knaus, T. A., Corey, D. M., Bollich, A. M., Lemen, L. C., & Foundas, A. L. (2007). Anatomical asymmetries of anterior perisylvian speech-language regions. Cortex, 43, 499–510.
Knaus, T. A., Silver, A. M., Dominick, K. C., Schuring, M. D., Shaffer, N., Lindgren, K. A., et al. (2009). Age-related changes in the anatomy of language regions in autism spectrum disorder. Brain Imaging and Behavior, 1, 51–63.
Knaus, T. A., Silver, A. M., Kennedy, M., Lindgren, K. A., Dominick, K. C., Siegel, J., et al. (2010). Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain and Language, 112, 113–120.
Lebel, C., & Beaulieu, C. (2009). Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Human Brain Mapping, 30, 3563–3573.
Lord, C., Rutter, M., DiLavore, P. C., & Risi, S. (1999). Autism diagnostic observation schedule - WPS (ADOS-WPS). Los Angeles: Western Psychological Services.
Müller, R.-A., Behen, M. E., Rothermel, R. D., Chugani, D. C., Muzik, O., Mangner, T. J., et al. (1999). Brain mapping of language and auditory perception in high-functioning autistic adults: a PET study. Journal of Autism and Developmental Disorders, 29, 19–31.
Nagae, L. M., Zarnow, D. M., Blaskey, L., Dell, J., Khan, S. Y., Qasmieh, S., et al. (2012). Elevated mean diffusivity in the left hemisphere superior longitudinal fasciculus in autism spectrum disorders increases with more profound language impairment. American Journal of Radiology, 33, 1720–1725.
Parker, G. J., Luzzi, S., Alexander, D. C., Wheeler-Kingshott, C. A., Ciccarelli, O., & Lambon Ralph, M. A. (2005). Lateralization of ventral and dorsal auditory-language pathways in the human brain. NeuroImage, 24, 656–666.
Pieper, S., Lorensen, B., Schroeder, W., & Kikinis, R. (2006). The NA-MIC Kit: ITK, VTK, pipelines, grids and 3D slicer as an open platform for the medical image computing community. Proceedings of the 3rd IEEE International Symposium on Biomedical Imaging: From Nano to Macro, 1, 698–701.
Piro, J. M. (1998). Handedness and intelligence: patterns of hand preference in gifted and nongifted children. Developmental Neuropsychology, 14, 619–630.
Powell, H. W., Parker, G. J., Alexander, D. C., Symms, M. R., Boulby, P. A., Wheeler-Kingshott, C. A., et al. (2006). Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. NeuroImage, 32, 388–399.
Qiu, D., Tan, L., Siok, W., Zhou, K., & Khong, P. (2011). Lateralization of the arcuate fasciculus and its differential correlation with reading ability between young learners and experienced readers: A diffusion tensor tractography study in a Chinese cohort. Human Brain Mapping, 32, 2054–2063.
Redcay, E., & Courchesne, E. (2005). When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biological Psychiatry, 58, 1–9.
Redcay, E., & Courchesne, E. (2008). Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2-3-year-old children autism spectrum disorder. Biological Psychiatry, 64, 589–598.
Rilling, J. K., Glasser, M. F., Preuss, T. M., Ma, X., Zhao, T., Hu, X., et al. (2008). The evolution of the arcuate fasciculus revealed with comparative DTI. Nature Neuroscience, 11, 426–428.
Rojas, D. C., Camou, S. L., Reite, M. L., & Rogers, S. J. (2005). Planum temporale volume in children and adolescents with autism. Journal of Autism and Developmental Disorders, 35, 479–486.
Rutter, M., Le Couteur, A., & Lord, C. (2003). Autism diagnostic interview – revised. Los Angeles: Western Psychological Services.
Schumann, C. M., Bloss, C. S., Barnes, C. C., Wideman, G. M., Carper, R. A., Akshoomoff, N., et al. (2010). Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. Journal of Neuroscience, 30, 4419–4427.
Semel, E., Wiig, E. H., & Secord, W. A. (1995). Clinical evaluation of language fundamental (3rd ed.). San Antonio: The Psychological Corporation, Harcourt Brace & Co.
Shapleske, J., Rossell, S. L., Woodruff, P. W., & David, A. S. (1999). The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Research Reviews, 29, 26–49.
Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E., Johansen-Berg, H., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, S208–S219.
Song, S.-K., Sun, S.-W., Ramsbottom, M. J., Chang, C., Russell, J., & Cross, A. H. (2002). Dysmelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage, 17, 1429–1436.
Sowell, E. R., Thompson, P. M., Leonard, C. M., Welcome, S. E., Kan, E., & Toga, A. W. (2004). Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience, 24, 8223–8231.
Szaflarski, J. P., Holland, S. K., Schmithorst, V. J., & Byars, A. W. (2006). fMRI study of language lateralization in children and adults. Human Brain Mapping, 27, 202–212.
Takeuchi, M., Harada, M., Matsuzaki, K., Nishitani, H., & Mori, K. (2004). Difference of signal change by a language task on autistic patients using functional MRI. The Journal of Medical Investigation, 51, 59–62.
Toga, A. W., & Thompson, P. M. (2003). Mapping brain asymmetry. Nature Reviews Neuroscience, 4, 37–48.
Vernes, S. C., Newbury, D., Abrahams, B., Winchester, L., Nicod, J., Groszer, M., et al. (2008). A functional genetic link between distinct developmental language disorders. The New England Journal of Medicine, 359, 2337–2345.
Vernooij, M. W., Smits, M., Wielopolski, P. A., Houston, G. C., Krestin, G. P., & van der Lugt, A. (2007). Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. NeuroImage, 35, 1064–1076.
Wang, T., Lee, S. S., Sigman, M., & Dapretto, M. (2006). Neural basis of irony comprehension in children with autism: the role of prosody and context. Brain, 129, 932–943.
Wolff, J. J., Gu, H., Gerig, G., Elison, J. T., Styner, M., Gouttard, S., et al. (2012). Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. The American Journal of Psychiatry, 169, 589–600.
Woolrich, M. W., Jbabdi, S., Patenaude, B., Chappell, M., Makni, S., Behrens, T., et al. (2009). Bayesian analysis of neuroimaging data in FSL. NeuroImage, 45, S173–S186.
Acknowledgments
This research was funded by a pilot study grant from the National Association of Autism Research/Autism Speaks to R.M.J. and by NIDCD grant U19 DC 03610 to H.T-F. We thank the children and families who generously gave their time to participate.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joseph, R.M., Fricker, Z., Fenoglio, A. et al. Structural asymmetries of language-related gray and white matter and their relationship to language function in young children with ASD. Brain Imaging and Behavior 8, 60–72 (2014). https://doi.org/10.1007/s11682-013-9245-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-013-9245-0