Abstract
White matter (WM) fiber tract differences are present in autism spectrum disorder (ASD) and could be important markers of behavior. One of the earliest phenotypic differences in ASD are language atypicalities. Although language has been linked to WM in typical development, no work has evaluated this association in early ASD. Participants came from the Infant Brain Imaging Study and included 321 infant siblings of children with ASD at high likelihood (HL) for developing ASD; 70 HL infants were later diagnosed with ASD (HL-ASD), and 251 HL infants were not diagnosed with ASD (HL-Neg). A control sample of 140 low likelihood infants not diagnosed with ASD (LL-Neg) were also included. Infants contributed expressive language, receptive language, and diffusion tensor imaging data at 6-, 12-, and 24 months. Mixed effects regression models were conducted to evaluate associations between WM and language trajectories. Trajectories of microstructural changes in the right arcuate fasciculus were associated with expressive language development. HL-ASD infants demonstrated a different developmental pattern compared to the HL-Neg and LL-Neg groups, wherein the HL-ASD group exhibited a positive association between WM fractional anisotropy and language whereas HL-Neg and LL-Neg groups showed weak or no association. No other fiber tracts demonstrated significant associations with language. In conclusion, results indicated arcuate fasciculus WM is linked to language in early toddlerhood for autistic toddlers, with the strongest associations emerging around 24 months. To our knowledge, this is the first study to evaluate associations between language and WM development during the pre-symptomatic period in ASD.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) is characterized by differences in social-emotional reciprocity and the presence of restricted/repetitive behaviors and interests [1], and is estimated to impact approximately one in 36 children in the USA [2]. Although language delays are no longer diagnostic criteria for ASD, atypical speech is often one of the primary parental concerns when first pursuing a diagnosis [3]. Furthermore, speech and language outcomes are tightly related to other metrics of well-being, including social and educational success for autistic individuals with and without flexible speech [4]. Autism is highly heritable [5], and younger siblings of children with ASD are at high familial likelihood for ASD (HLFootnote 1), with ~20% of high likelihood (HL) infants siblings going on to receive an ASD diagnosis themselves [6]. Furthermore, an additional 30% of the infant siblings who are not diagnosed with ASD display other developmental concerns, including language delays [7], which are apparent by 12 months of age [8, 9]. A recent meta-analysis and empirical investigation suggested that HL siblings without ASD were three to four times more likely to exhibit language delay versus siblings of non-autistic youth (low likelihood siblings; LL) without ASD [10]. Indeed, language atypicalities can be considered an endophenotype of ASD that emerges in higher frequency in first-degree relatives [11], and thus studying language may provide more power to uncover underlying mechanisms than relying on the categorical diagnosis of ASD alone [11,12,13].
Autism is increasingly considered a disorder characterized by differences in neural circuitry that emerge as early as the first postnatal year [14, 15]. One of the earliest differences in brain development observed in ASD is the atypical organization of White matter (WM), which is evident by 6 months of age in fiber tracts spanning the brain [16]. WM is composed of fiber bundles containing millions of axons that connect different regions of the brain to form functional circuits [17]. Axons are wrapped in myelin comprised of fatty lipid proteins that gives WM its characteristic white appearance. Myelin sheaths surrounding axons serve as electrical insulators, augmenting the integrity of signal transduction within the brain [18]. Diffusion tensor imaging (DTI) can be used to assess WM growth in vivo by measuring the organizational properties of WM, including diffusion anisotropy indexed by fractional anisotropy (FA). FA can provide an index of the rapid maturation of fiber pathways in the brain during the first two postnatal years [18], with higher levels of FA typically found to associate with increased postnatal age [19, 20] and thought to reflect an increase in myelination and other fiber maturation processes, as well as axon density and axon caliber [18].
Longitudinal studies have demonstrated that neural differences during infancy are developmental in nature, with distinct trajectories in autism unfolding over time [16], and emerging evidence suggests that differences in developmental patterns of FA in ASD have implications for behavior during infancy and early childhood. For example, social cognition and behavioral control have been linked to the inter- and intra-frontal WM pathways [21] in toddlers with ASD, and repetitive behaviors and sensory interests at two years of age have been associated with WM FA in the genu and cerebellar pathways at 6 months of age [22]. Furthermore, WM FA in the superior longitudinal fasciculus and splenium of the corpus callosum have been linked to autism trait severity from ages 2.5 to 7 years [23]. Interestingly, in the study by Wolff et al. [22], disordinal WM-behavior associations were observed between infants later diagnosed with ASD and controls, such that at 6 months of age, higher FA was related to increased repetitive behaviors at age 2 among infants who later developed ASD, while the opposite was true for controls (higher FA, less repetitive behaviors). Similarly, Andrews et al. [23] reported differential trajectories of FA development among individuals with increasing autism severity over time (slower development of FA) compared those with stable or decreasing trajectories of symptom severity. While Solso et al. [21] did not test for differential brain-behavior associations among infants based on later diagnostic outcome, they did find that associations between WM and behavior in the ASD group differed at different time points in development (higher FA early, poorer outcomes at toddler evaluation; higher FA later, better outcomes). This suggests that the maturation of WM may have an age-specific relationship to emerging behavior in ASD that is different from that observed in typical development.
In typically developing groups, WM microstructure has been shown to relate to general cognitive ability, motor, language, and visual reception skills in the first two years of life, suggesting that WM serves an important role in early cognition [20]. Specific to language, expressive and receptive language in non-autistic toddlers has been associated with development of the arcuate fasciculus [20, 24] and corpus callosum [24], wherein higher FA [20, 24] at a single time point in development during infancy has been related to better language performance. Longitudinal studies during this same period have reported somewhat mixed results. One study from 6 to 24 months of age reported faster rates of FA development in the splenium were related to greater expressive language abilities at age 2 [25] whereas another study reported that slower, protracted development of another diffusion metric (radial diffusivity) across many fiber tracts (arcuate segments, corticothalamic tracts, inferior and superior longitudinal fasciculi, and inferior fronto-occipital fasciculus), from 1 to 2 years of age was associated with better receptive language abilities [20]. Another study using a different technique [26] to assess WM development has also reported that slower, prolonged development of myelin may confer increased cognitive abilities in the first year of life. Apparent discrepancies in findings could be due to several factors including the age range studied, methodological variability (e.g., use of different diffusion metrics, measures of myelination) and the modeling of developmental change (e.g., calculation of change rates versus modeling of repeated measures). Despite these reported links between WM and language in typical development, and the well documented differences in WM development in ASD, no studies to date have evaluated associations between WM microstructure and language development in ASD. Systematically evaluating the relationship between WM microstructure and language in ASD is imperative for understanding the neurobiology of language development in ASD and informing and monitoring language interventions [27, 28].
Current study
The goal of the current study was to evaluate the longitudinal association between WM microstructure and language abilities in HL infants with an ASD diagnosis (HL-ASD) compared to those without an ASD diagnosis (HL-Neg) and LL infants without ASD (LL-Neg) at 6-, 12-, and 24 months. We specifically sought to evaluate: (1) whether WM development is related to (a) expressive and (b) receptive language development in the first two postnatal years, and (2) whether any observed associations between WM tract organization and language ability varied as a function of group membership. We hypothesized that (1) better performance on expressive and receptive language measures would be associated with increased FA in WM tracts across this developmental period, and (2) the HL-ASD group would diverge from the HL-Neg and LL-Neg groups in terms of their language and WM associations. WM tracts of interest included the segments of the arcuate fasciculus and corpus callosum which have been linked to language in infants, and two other bilateral fiber tracts canonically involved in language, including the uncinate [29] and inferior longitudinal fasciculi [25].
Methods and materials
Participants
Participants were enrolled in the Infant Brain Imaging Study (IBIS), a prospective, longitudinal study of infants at high and low likelihood for ASD, defined by having an older sibling with a diagnosis of ASD (HL), or an older typically developing sibling (LL). Infants were enrolled at one of four sites: Children’s Hospital of Philadelphia, University of North Carolina, University of Washington, and Washington University. Exclusion criteria for IBIS participation were (1) evidence of a genetic condition, (2) vision or hearing impairment, (3) gestational age < 36 weeks and/or birth weight < 2000 g, (4) significant perinatal adversity of prenatal toxin exposure, (5), contraindication for magnetic resonance imaging (MRI), (6) primary home language other than English, (7) sibling statuses of adopted, half-sibling, or twins, and (8) first-degree relatives with diagnoses of psychosis, schizophrenia, or bipolar disorder.
The current sample included 461 infants who met the following criteria: (1) usable MRI scan at 6-, 12- or 24 months, (2) complete cognitive and behavioral assessment battery at age 24 months including a diagnostic evaluation of ASD, and (3) developmental assessment battery completed at 6-, 12-, or 24 months. Our primary sample of interest were HL infants who met diagnostic criteria for a clinical diagnosis of ASD at age 24 months (HL-ASD: n = 70). We also included HL infants who did not meet diagnostic criteria for ASD (HL-Neg; n = 251) and LL infants who did not meet diagnostic criteria for ASD (LL-Neg; n = 140; Table 1) as comparison samples. For complete information about the number of participants at each visit and group, see Supplementary Table 1 (ST1). Written, informed consent was obtained for all participants from their caregiver or guardian, and all study procedures were approved by the institutional review boards at each clinical site. The LORIS data management platform [30] served as the behavioral, clinical, and imaging hub for this study; data is available upon request.
Diagnostic and developmental evaluations
Clinical best estimate diagnoses were completed for the participants included in the study based on DSM-IV-TR criteria using all available assessment data including the Autism Diagnostic Observation Schedule (ADOS) [31], Autism Diagnostic Interview-Revised (ADI-R) [32], Mullen Scales of Early Learning (MSEL) [33] and Vineland Adaptive Behavior Scales II [34]. Reliability for the diagnostic instruments was established and maintained between sites through monthly case reviews. For a detailed evaluation protocol, see Estes et al. [9].
Expressive and receptive language was assessed using the MSEL at 6-, 12-, and 24 months. The MSEL is a standardized, direct assessment of development for ages 0–68 months [33]. MSEL subscales include expressive and receptive language in addition to visual reception, fine and gross motor skills. The MSEL has garnered evidence as a valid and reliable measure of language in early infancy and in autistic populations [35]. For the current project, the age equivalent scores for expressive and receptive language were used as dependent variables of interest to avoid T-score floor effects [36, 37].
MRI data acquisition and processing
Magnetic resonance imaging scans were acquired at all sites using 3T Siemens TIM Trio scanners via 12-channel head coils assessed during natural sleep. Intra- and inter-site reliability was established and maintained across sites and time [38]. Diffusion weighted images were collected using an ep2d_diff pulse sequence with a FOV of 190 mm (6 and 12 months) or 206 mm (24 months), 75–81 transversal slices, and voxel size of 2 mm3, TR = 12,800-13,300 ms, TE = 102 ms, with 26 DWI volumes with b values between 0 and 1000 s/mm2 in increments of 40, including a single b = 0 s/mm2, and 25 gradient directions. As described previously [22, 25], diffusion-weighted images were processed with DTI-prep, which detects common artifacts, corrects for motion and eddy deformations, and flags poor quality gradients for manual removal by expert raters [39, 40]. Datasets with fewer than 18 gradients were excluded from further processing to ensure consistent signal-to-noise ratio. In sum, ~10.5% of DTI datasets were excluded following quality control procedures with 25% of excluded cases due to subject movement; an additional 4% were excluded for incomplete acquisition. There were no differences between diagnostic groups in proportion of scans excluded (Supplementary Table 1). We tested for potential associations between the quality of the diffusion datasets included in the study (i.e., remaining gradients after quality control) and our primary language measures of interest by visit and by likelihood group. The estimated correlations ranged from –0.02 to 0.20, with 0.04 < r < 0.20 for the correlations with expressive language scores and –0.02 < r < 0.18 for the correlations with receptive language scores; none reached the level of statistical significance.
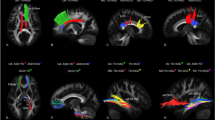
Group analysis of diffusion weighted data used an atlas-based processing pipeline providing consistent spatial parameterization within and between individual datasets across ages [19, 22, 41]. Deterministic fiber tractography was performed with average atlas space using 3D Slicer and refined via FiberViewerLight. Fiber tract definitions followed anatomically informed methods [42]. Tracts of interest included bilateral segments of the arcuate, uncinate, and inferior longitudinal fasciculi, as well as the genu, splenium, and body of the corpus callosum (Fig. 1). These tracts were selected based on prior publications [20, 25] to include fiber bundles previously associated with various aspects of language [43], and segments of the corpus callosum, given previous links to language in infants [24, 25]. Of note, due to limitations of our diffusion sequence at the time of data collection starting over a decade ago (e.g., low number of diffusion gradients compared to current state-of-the-art protocols) and the difficulty of tracking the arcuate in infants due to its curved structure and relatively low level of myelination [18], we were not able to generate homologous left and right arcuate fasciculi in our atlas (see Fig. 1). While the left arcuate in our atlas is defined as the direct fronto-temporal (arcuate-FT) segment connecting Broca’s to Wernicke’s areas, the right arcuate is defined as the anterior fronto-parietal (arcuate-FP) segment connecting Broca’s to Geschwind’s areas. FA values were obtained via DTIAtlasFiberAnalyzer [41]. FA represents the degree of diffusion along the primary fiber orientation relative to the transverse diffusion. All components of this processing pipeline are available as part of the UNC/Utah NAMIC DTI Fiber Analysis Framework [41].
a depicts the left hemisphere and b depicts the right hemisphere. Seafoam green, anterior portion of the corpus callosum (CC-Genu); light blue, corpus callosum body (CC-Body); dark blue, splenium (CC-Splenium); orange, uncinate; red, inferior longitudinal fasciculus; yellow, arcuate fasciculus (left = arcuate fronto-temporal, right = arcuate fronto-parietal).
Statistical analysis models
Correlations between FA and language scores were calculated at each visit to establish cross-sectional associations between the variables. To model the associations between WM microstructure and expressive/receptive language over the 6-month, 12-month, and 24 month visits, linear mixed-effect models with linear regression splines were fit to the data. Fixed effects included group (HL-Neg and LL-Neg compared to HL-ASD participants), WM (scaled such that each coefficient corresponded to a 0.1-unit increase in FA), sex (female as referent group), piecewise linear estimates for 6–12 months visit interval and 12–24 months visit interval. Additional covariates included mother’s education (less than college compared to college or more) and study site. We included interaction terms between group and visit, group and FA, and visit and FA to address differences in FA-language associations between groups and over time. Random effects included a random intercept for each participant and a random slope for the two piecewise linear time periods. The model used an autoregressive correlation structure to account for within-participant variance over time. Missing data were assumed missing at random with patterns of missingness not varying by likelihood group or covariates. Multiple imputations (k = 5) of the mixed-effects models were pooled and summarized to produce the fixed effects and random effects results. Mixed-effects models were estimated using the “nlme” package [44]; multiple imputations were estimated using both “mice” [45] and “amelia” [46] packages in R 4.1.0. Type S and Type M error rates were calculated for the linear regression results to account for multiple comparisons and estimate degree of inflation (Gelman & Carlin, 2014 [47]; Supplementary Table 6; Supplementary Fig. 2).
Results
Descriptive statistics and cross-sectional analyses
Descriptive statistics for the sample are available in Table 1. As reported in previous work [25], the sample is predominantly white-non-Hispanic, with college educated caregivers. Correlations (Supplementary Fig. 1) suggest small, positive associations, 0.21 <r < 0.42, between WM FA and language across select WM tracts at 6 months (LL-NEG: CC-genu, CC-splenium, left uncinate; HL-NEG: all tracts; HR-ASD: all but two tracts). In the HL-Neg and LL-Neg groups, WM FA correlations with language decreased in magnitude or became nonsignificant at the 12-month visit. By 24 months, only two significant correlations remained for the LL-Neg and HL-Neg groups (right uncinate with expressive language in the LL-Neg group and right arcuate-FP with expressive language in the HL-Neg group). However, at 24 months, the left arcuate-FT and right arcuate-FP fasciculus remained positively and significantly correlated with expressive and receptive language in the HL-ASD group. Correlations between expressive and receptive language domains ranged from r = 0.33 (6 months) to r = 0.74 (24 months). Correlations controlled for false discovery rate using Benjamini-Hochberg FDR correction method [48].
Longitudinal models
Results from the mixed-effects regression models indicated only one WM tract was significantly associated with expressive language: the right arcuate-FP. Importantly, the hypothesized interaction between the right arcuate-FP and group reached significance for both the HL-Neg group, β = −2.105, p = 0.017, and the LL-Neg group, β = −2.424, p = 0.033, indicating the HL-ASD group demonstrated a significantly different trajectory between WM microstructure in the right arcuate-FP and expressive language outcomes over time (Table 2). The mixed effects model predicting expressive language and WM FA in the arcuate-FP over time is depicted in Fig. 3, where different slopes of language development over time are apparent for the HL-ASD based on FA; HL-ASD infants with the least gains in FA values over time exhibit the weakest gains over time and overall lowest language scores by 24 months. All other WM tracts were non-significant in their associations with expressive language (Supplementary Table 3).
Regarding receptive language, no WM tracts were significantly associated with receptive language either via main effect or interactions (Supplementary Table 4). However, there were several receptive language findings that may merit further study. The left arcuate-FT was associated with receptive language both directly, β = 1.811, p = 0.089, and in an interaction with the LL-Neg group, β = −2.102, p = 0.057, despite not reaching statistical significance. The right arcuate-FP also demonstrated associations with receptive language both directly, β = 1.925, p = 0.054, and in an interaction with the LL-Neg group, β = −1.885, p = 0.070 (Table 3). The non-significant associations between arcuate segments and expressive/receptive language are depicted visually in Fig. 2.
Each data point reflects an individual’s WM arcuate fractional anisotropy and expressive (top) or receptive (bottom) language age equivalent score at each time point, colored by group status. Bold lines represent the linear relationship between WM FA and language age equivalent for each group at each visit. FT fronto-temporal, FP fronto-parietal. Red, HL-ASD; gray, HL-Neg; blue, LL-Neg.
Sensitivity analyses were conducted to determine the specificity of findings to language by comparing FA development to non-verbal developmental quotients (NVDQ; [49]). We found no evidence of group differences in the associations between FA and NVDQ scores for any tracts (Supplementary Table 5).
Discussion
In the present study, we sought to evaluate the associations between WM microstructure FA and expressive and receptive language abilities in the first two postnatal years in infants and toddlers at low and high familial likelihood for autism. Results suggested the right fronto-parietal segment of the arcuate fasciculus is significantly associated with expressive language development across this period in autism. The HL-ASD group demonstrated a significantly different FA-language relation compared to the other two groups, wherein the HL-ASD group showed a positive association across development between WM FA and expressive language, which was weaker or absent in the other two groups. Notably, our findings demonstrated that HL-ASD infants with the least gains in FA values exhibited the least gains in language over time (Fig. 3) and overall lowest language scores by 24 months (Figs. 2 and 3). No other fiber tracts emerged as significant predictors of expressive language. Although no WM tracts were significantly associated with receptive language, some interesting trends were observed that may warrant further study. Supplementary analyses provided evidence of the specificity of these findings to language, as replicating the analytic approach with nonverbal developmental quotients comprised of scores on fine motor and visual reception tasks resulted in no significant group by FA interactions across any tracts of interest. Taken together, this suggests that the arcuate may have a specific developmental relationship to expressive language that emerges over the course of the first years of life in infants who later receive a clinical ASD diagnosis at 24 months.
FA percentiles were calculated by taking the participant’s difference in 24-month and 6-month visit right arcuate-FP FA values and finding its kth percentile among the whole sample (i.e., lowest percentile represents those with the least gains in FA over this period). Lower third panel indicates percentile ≤ 33%, middle third panel indicates between percentile 33% and 66%, and upper third panel indicates percentile ≥ 66%. Differential trajectories of model-predicted language are observed for the HL-ASD group based on FA percentile group (e.g., difference in slope and predicted age-equivalent language score at 24 months in lower third vs. upper third), while similar trajectories are observed for the HL-Neg and LL-Neg samples across FA percentile groups. Breakdown by FA percentiles is for visualization purposes only; FA was treated as a continuous variable in longitudinal models.
The results connecting the arcuate-FP to expressive language align with prior literature suggesting an association between this WM tract and language in autistic individuals [50]. We did not, however, find associations between arcuate-FP development and language in our non-autistic groups, as would have been expected based on the prior reports [20, 22]. This could be due to a variety of factors, including the relatively small sample size of the LL-Neg group compared to other studies [20], or differences in the ages modeled [20, 24]. Further, while other studies have linked the development of FA in the splenium [20] and other segments of the corpus callosum [24] across this developmental period to language abilities in non-autistic samples, we found no associations between splenium FA and the development of language, nor did we find differences based on diagnostic outcome group. Again, this could be due to differences in modeling, as our approach included multiple subject groups (instead of a single typically developing group) and statistically predicted language development (e.g., change in language from 12 to 24 months), as opposed to predicting language at a single timepoint in development. Our results may suggest a level of specificity between language and arcuate WM in the HL-ASD group, which could reflect the early specialization of this fiber pathway in ASD [25]. It also suggests that the arcuate-FP should be further evaluated as a language biomarker in ASD [27].
Interestingly, the only significant results observed in the current study were related to expressive language. Although some potential trends of interest emerged with receptive language that mirrored our expressive language findings, these did not reach the threshold for statistical significance. Findings between WM and expressive language, but not receptive language, is surprising given receptive language often is used to differentiate ASD from other clinical groups in toddlerhood (e.g., ASD from language delay; [51]). The pattern observed in our study between FA and expressive language may underscore the unique role expressive language plays in early infancy as a gateway for further language opportunities. Indeed, if early, aberrant vocalizations [52] negatively impact future opportunities for language learning by reducing parent-child conversational turns [53], expressive language measurement at this age may reveal unique insights into subsequent receptive and expressive development.
Our finding that higher FA values were associated with better performance on expressive language measures in the HL-ASD group suggests that mechanisms underlying fiber organization, axon density or caliber, and/or myelination may play a role in contributing to heterogeneity in language growth trajectories in early ASD. Interestingly, the arcuate is one of the last white matter pathways in the brain to mature [20, 54], and this protracted maturation may reflect a notable role for experience-dependent myelination and plasticity on the development of the arcuate; processes that may be disrupted in ASD [15]. Though traditional diffusion tensor MRI methods like those utilized in this study limit our ability to speak to which mechanisms, specifically, may be driving the associations observed in this report. Newer methodologies, including HARDI and NODDI, that can resolve crossing fibers and providing estimates of neurite density and orientation within a single voxel will be critical to disentangling the neurobiology underlying the FA findings in this study [55, 56]. Our team is currently collecting a new cohort of HL infants to replicate and extend our work, and the updated imaging protocols include multi-shell diffusion MRI and the ability to conduct NODDI analyses; in future we will be able to address these questions more directly. Cross-species studies may also yield important mechanistic insight into observations made in-vivo, as mouse models of Angelman syndrome—a neurodevelopmental disorder sharing clinical features with ASD— have demonstrated that atypical FA patterns were linked specifically to axon caliber, while myelination and axon density were intact [57].
Correlation results indicated that the FA-language association in ASD is strongest between 12 and 24 months of age, when the rate of maturation in WM fibers has been reported to slow in infants who develop ASD, ultimately resulting in lower FA values in ASD compared to controls at 24 months of age [16]. When taken together with our findings, which shares an overlapping sample of HL infants to those reported by Wolff et al., this suggests that WM development during the second year of life may be an important period for ASD-related language development, with higher FA values in this group being protective and associated with better language outcomes. Future work will be needed to replicate these findings in a new sample. It should also be noted that other studies that overlap with this developmental window have shown that increased FA in ASD (compared to typical development) may still be present in the second year of life. Solso et al. [21] report a similar trajectory in 1–4-year-olds (e.g., higher FA earlier, followed by slower maturation and lower FA later), but the decrease in FA is not apparent in their sample until sometime after age 2. Andrews et al. [23] studied 2.5–7-year-olds and found that at the start of the trajectory (~2 yrs of age) the ASD group showed increased FA, with decreased FA becoming apparent later in childhood. Despite reporting increase in FA as a group effect, these previous studies did not explore behavioral heterogeneity related to FA changes in early development. The factors underlying heterogeneity in FA maturation in the HL-ASD group deserve further study and once identified, may be important targets for behavioral intervention to support language development. Future work may also consider whether this relation between increased FA and language development is evidenced in other clinical language-delayed groups, such as apraxia of speech or specific language impairments.
Further, the developmental timing of FA-language associations implicates mechanisms that pre-date or coincide with the emergence of autistic traits [14] and the emergence of burgeoning expressive/receptive language skills [25]. Future research may seek to evaluate denser developmental sampling within the second postnatal year to evaluate whether associations in autistic and non-autistic toddlers have clear divergence points during the second year of life. Lastly, although previously established in this sample in prior works [9, 25], the significant age by group interactions suggested the HL-ASD group had distinct developmental patterns of expressive and receptive language when compared to their HL-Neg and LL-Neg peers. Results suggested the HL-ASD group exhibited a slowed developmental trajectory of expressive and receptive language, wherein by 24 months, autistic toddlers demonstrated significantly lower expressive and receptive language abilities than 24-month-old HL-Neg and LL-Neg peers. These results are commensurate with previous literature suggesting some autistic toddlers demonstrate slowed language growth curves [58]. It is important to note the role of measurement here as well, as the divergence at 24 months may also reflect different types of language being measured, with canonical babbling emphasized at 6–12 months (with some evidence of hyper-vocal traits in infancy; [53]), followed by greater reliance on consonant-vowel-consonant early word forms from 12 to 24 months (wherein autistic toddlers may demonstrate slowed growth; [59]). Further parsing the HL-ASD group by language growth curve, and using more nuanced measures, may reveal more subtle differences in the language-WM phenotype, which represents an area for future research. Relatedly, our models in this study evaluated in a unidirectional hypothesis (e.g., role of FA predicting language); future research may also wish to consider the bidirectional development of WM FA and language, asking whether early changes in language abilities, perhaps on a finer grained timescale, may also predict later changes in FA.
Although results for receptive language suggested associations with the bilateral arcuate-FP warrant future study, the statistically significant results were unique to the right arcuate-FP. These findings are interesting given well documented left brain lateralization for language areas [60]. A few possible reasons may explain our right-dominant findings, including potential differences related to tractography or lateralization in ASD. When considering the FA measurements, the left and right arcuate-FP tracts were not homologous (Fig. 1); as described in the methods, we were unable to resolve the posterior segment of the right arcuate fasciculus terminating in the temporal lobe. This is partly due to a limitation of neuroimaging in young infants where myelination levels are relatively low, which can impact tractography, and is particularly true for the arcuate fasciculus given its highly curved structure [61]. Further, our imaging sequence was developed over a decade ago and includes a relatively low number of diffusion gradients; this lower number of gradients has implications for tractography, including the inability to resolve crossing fibers which may also have contributed to the failure to resolve the curved posterior segment of the arcuate in the right hemisphere. Another explanation is that lateralization does not occur to the same degree in ASD as in typical development, which has been reported in numerous previous studies [62] for many WM tracts [60], and specifically the arcuate in ASD [63]. In fact, right-laterality in ASD may be primarily driven by language delays [64], suggesting our right arcuate-FP and language findings may reflect right-lateralization differences unique to ASD. Given previous work suggesting laterality differences as early as 6-weeks of age [65], future research should continue to evaluate laterality in the arcuate and its association with language in ASD longitudinally. Lastly, a final explanation of the right-predominance finding could be that this reflects a right-dominant language process, such as prosody. These findings may support the hypothesis that right-lateralized prosodic speech processes, established to be atypical in ASD, may be foundational for the subsequent language acquisition process [66].
To our knowledge, this study is the first to examine and report longitudinal WM development in relation to language development during the presymptomatic period in ASD. Our results suggest an association between the maturation of the arcuate fasciculus and language development, particularly between the right fronto-parietal segment of the arcuate and expressive language, that is specific to infants who are later diagnosed with ASD. This association develops in the first two postnatal years with clear associations emerging by 24 months. Future work is needed to replicate these findings, further examine the developmental time course of WM-language associations, and identify factors (e.g., language exposure in the home) [67] that may contribute to heterogeneity in arcuate and language development in early ASD.
Data availability
Raw neuroimaging and behavioral that support the findings of this study are publicly available at the National Institutes of Mental Health Data Archive in collections 0019 and 2027. Any additional data may be made available by the corresponding author upon reasonable request.
Notes
The term “high likelihood” is used here in place of the term “high-risk” and signifies an elevated likelihood of receiving an autism diagnosis due to family history and shared genetics [6].
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Association; 2013. https://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596.
Maenner MJ, Warren ZW, Williams AR, Amoakohene E, Bakian AV, Bilder DA, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years- autism and developmental disabilities monitoring network, 11 sites, United States, 2020. MMWR Surveill Summ. 2023;72:1–14.
Kozlowski AM, Matson JL, Horovitz M, Worley JA, Neal D. Parents’ first concerns of their child’s development in toddlers with autism spectrum disorders. Dev Neurorehabilit. 2011;14:72–8.
Schaeffer J, Abd El-Raziq M, Castroviejo E, Durrleman S, Ferré S, Grama I, et al. Language in autism: domains, profiles and co-occurring conditions. J Neural Transm. 2023;130:433–57.
Sandin S, Lichtenstein P, Kuja-Halkola R, Hultman C, Larsson H, Reichenberg A. The heritability of autism spectrum disorder. JAMA. 2017;318:1182.
Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium Study. Pediatrics. 2011;128:e488–95.
Charman T, Young GS, Brian J, Carter A, Carver LJ, Chawarska K, et al. Non-ASD outcomes at 36 months in siblings at familial risk for autism spectrum disorder (ASD): a baby siblings research consortium (BSRC) study. Autism Res. 2017;10:169–78.
Swanson MR, Shen MD, Wolff JJ, Elison JT, Emerson RW, Styner MA, et al. Subcortical brain and behavior phenotypes differentiate infants with autism versus language delay. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:664–72.
Estes A, Zwaigenbaum L, Gu H, John TS, Paterson S, Elison JT, et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J Neurodev Disord. 2015;7:24.
Marrus N, Hall LP, Paterson SJ, Elison JT, Wolff JJ, Swanson MR, et al. Language delay aggregates in toddler siblings of children with autism spectrum disorder. J Neurodev Disord. 2018;10:636.
Geschwind DH. Autism: Many Genes, Common Pathways? Cell. 2008;135:391–5.
Constantino JN, Charman T, Jones EJH. Clinical and translational implications of an emerging developmental substructure for autism. Annu Rev Clin Psychol. 2021;17:365–89.
Viding E, Blakemore SJ. Endophenotype approach to developmental psychopathology: implications for autism research. Behav Genet. 2007;37:51–60.
Girault JB, Piven J. The Neurodevelopment of autism from infancy through toddlerhood. Neuroimaging Clin N Am. 2020;30:97–114.
Piven J, Elison JT, Zylka MJ. Toward a conceptual framework for early brain and behavior development in autism. Mol Psychiatry. 2017;22:1385–94.
Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169:589–600.
Fields RD. Change in the brain’s white matter. Science. 2010;330:768–9.
Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276:48–71.
Geng X, Gouttard S, Sharma A, Gu H, Styner M, Lin W, et al. Quantitative tract-based white matter development from birth to age 2 years. NeuroImage. 2012;61:542–57.
Girault JB, Cornea E, Goldman BD, Knickmeyer RC, Styner M, Gilmore JH. White matter microstructural development and cognitive ability in the first 2 years of life. Hum Brain Mapp. 2018;111:7456.
Solso S, Xu R, Proudfoot J, Hagler DJ, Campbell K, Venkatraman V, et al. Diffusion tensor imaging provides evidence of possible axonal overconnectivity in frontal lobes in autism spectrum disorder toddlers. Biol Psychiatry. 2016;79:676–84.
Wolff JJ, Swanson MR, Elison JT, Gerig G, Pruett JR, Styner MA, et al. Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Mol Autism. 2017;8:8.
Andrews DS, Lee JK, Harvey DJ, Waizbard-Bartov E, Solomon M, Rogers SJ, et al. A longitudinal study of white matter development in relation to changes in autism severity across early childhood. Biol Psychiatry. 2021;89:424–32.
Sket GM, Overfeld J, Styner M, Gilmore JH, Entringer S, Wadhwa PD, et al. Neonatal white matter maturation is associated with infant language development. Front Hum Neurosci. 2019;13:434.
Swanson MR, Wolff JJ, Elison JT, Gu H, Hazlett HC, Botteron K, et al. IBIS Network. Splenium development and early spoken language in human infants. Dev Sci. 2017;20. https://doi.org/10.1111/desc.12360.
Deoni SCL, O’Muircheartaigh J, Elison JT, Walker L, Doernberg E, Waskiewicz N, et al. White matter maturation profiles through early childhood predict general cognitive ability. Brain Struct Funct. 2016;221:1189–203.
Swanson MR, Hazlett HC. White matter as a monitoring biomarker for neurodevelopmental disorder intervention studies. J Neurodev Disord. 2019;11:33.
Swanson MR. The role of caregiver speech in supporting language development in infants and toddlers with autism spectrum disorder. Dev Psychopathol. 2020;32:1230–9.
Elison JT, Wolff JJ, Heimer DC, Paterson SJ, Gu H, Hazlett HC, et al. Frontolimbic neural circuitry at 6 months predicts individual differences in joint attention at 9 months. Dev Sci. 2013;16:186–97.
Das S, Glatard T, MacIntyre LC, Madjar C, Rogers C, Rousseau ME, et al. The MNI data-sharing and processing ecosystem. NeuroImage. 2016;124:1188–95.
Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23.
Rutter M, LeCouteur A, Lord C. The autism diagnostic interview-revised (ADI-R). Los Angeles: Western Psychological Services; 2003.
Mullen EM. Mullen Scales of Early Learning (AGS ed.). Circle Pines, MN: American Guidance Service Inc.; 1995.
Sparrow S, Balla D, Cicchetti D. Vineland adaptive behavior scales: 2nd ed. Shoreview: AGS Publishing; 2005.
Swineford LB, Guthrie W, Thurm A. Convergent and divergent validity of the Mullen scales of early learning in young children with and without autism spectrum disorder. Psychol Assess. 2015;27:1364–78.
Brian AJ, Roncadin C, Duku E, Bryson SE, Smith IM, Roberts W, et al. Emerging cognitive profiles in high-risk infants with and without autism spectrum disorder. Res Autism Spectr Disord. 2014;8:1557–66.
for the IBIS Network, Girault JB, Swanson MR, Meera SS, Grzadzinski RL, Shen MD, et al. Quantitative trait variation in ASD probands and toddler sibling outcomes at 24 months. J Neurodev Disord. 2020;12:5.
Gouttard S, Styner M, Prastawa M, Piven J, Gerig G. Assessment of reliability of multi-site neuroimaging via traveling phantom study. In: Metaxas D, Axel L, Fichtinger G, Székely G, editors. Medical image computing and computer-assisted intervention – MICCAI 2008. Berlin, Heidelberg: Springer; 2008. p. 263–70. http://springerlink.bibliotecabuap.elogim.com/10.1007/978-3-540-85990-1_32.
Liu Z, Wang Y, Gerig G, Gouttard S, Tao R, Fletcher T, et al. Quality control of diffusion weighted images. In: Liu BJ, Boonn WW, editors. San Diego, California, USA; 2010. p. 76280J. http://proceedings.spiedigitallibrary.org/proceeding.aspx?doi=10.1117/12.844748.
Oguz I, Farzinfar M, Matsui J, Budin F, Liu Z, Gerig G, et al. DTIPrep: quality control of diffusion-weighted images. Front Neuroinform. 2014 ;8. http://journal.frontiersin.org/article/10.3389/fninf.2014.00004/abstract.
Verde AR, Budin F, Berger JB, Gupta A, Farzinfar M, Kaiser A, et al. UNC-Utah NA-MIC framework for DTI fiber tract analysis. Front Neuroinform. 2014;7. http://journal.frontiersin.org/article/10.3389/fninf.2013.00051/abstract.
Oishi K, editor. MRI atlas of human white matter. 2nd ed. London: Academic Press; 2011. p. 257.
Dick AS, Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012;135:3529–50.
Pinheiro, J., Bates, D., & R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. Version R package version 3.1-164. 2023. https://cran.r-project.org/web/packages/nlme/nlme.pdf.
van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45. http://www.jstatsoft.org/v45/i03/.
Honaker J, King G, Blackwell M. Amelia II: a program for missing data. J Stat Softw. 2011;45:1–47.
Gelman, A., & Carlin, J. Beyond power calculations: Assessing type S (sign) and type M (magnitude) errors. Perspect Psychol Sci. 2014;9:641–51.
Benjamini YHY. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300.
Stephens RL, Langworthy B, Short SJ, Goldman BD, Girault JB, Fine JP, et al. Verbal and nonverbal predictors of executive function in early childhood. J Cogn Dev. 2018;19:182–200.
Cermak CA, Arshinoff S, Ribeiro de Oliveira L, Tendera A, Beal DS, Brian J, et al. Brain and language associations in autism spectrum disorder: a scoping review. J Autism Dev Disord. 2022;52:725–37.
Seol KI, Song SH, Kim KL, Oh ST, Kim YT, Im WY, et al. A comparison of receptive-expressive language profiles between toddlers with autism spectrum disorder and developmental language delay. Yonsei Med J. 2014;55:1721.
Patten E, Belardi K, Baranek GT, Watson LR, Labban JD, Oller DK. Vocal Patterns in Infants with Autism Spectrum Disorder: Canonical Babbling Status and Vocalization Frequency. J Autism Dev Disord. 2014;44:2413–28.
Swanson MR, Shen MD, Wolff JJ, Boyd B, Clements M, Rehg J, et al. Naturalistic language recordings reveal “hypervocal” infants at high familial risk for autism. Child Dev. 2018;89. https://srcd.onlinelibrary.wiley.com/doi/10.1111/cdev.12777.
Dubois J, Dehaene-Lambertz G, Perrin M, Mangin JF, Cointepas Y, Duchesnay E, et al. Asynchrony of the early maturation of white matter bundles in healthy infants: quantitative landmarks revealed noninvasively by diffusion tensor imaging. Hum Brain Mapp. 2008;29:14–27.
Tuch DS. Q-ball imaging. Magn Reson Med. 2004;52:1358–72.
Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. 2012;61:1000–16.
Judson MC, Burette AC, Thaxton CL, Pribisko AL, Shen MD, Rumple AM, et al. Decreased axon caliber underlies loss of fiber tract integrity, disproportional reductions in white matter volume, and microcephaly in Angelman syndrome model mice. J Neurosci. 2017;37:7347–61.
Tek S, Mesite L, Fein D, Naigles L. Longitudinal analyses of expressive language development reveal two distinct language profiles among young children with autism spectrum disorders. J Autism Dev Disord. 2014;44:75–89.
Brady NC, Kosirog C, Fleming K, Williams L. Predicting progress in word learning for children with autism and minimal verbal skills. J Neurodev Disord. 2021;13:36.
Flagg EJ, Cardy JEO, Roberts W, Roberts TPL. Language lateralization development in children with autism: Insights from the late field magnetoencephalogram. Neurosci Lett. 2005;386:82–7.
Dubois J, Hertz-Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene-Lambertz G. Structural asymmetries in the infant language and sensori-motor networks. Cereb Cortex. 2009;19:414–23.
Jouravlev O, Kell AJE, Mineroff Z, Haskins AJ, Ayyash D, Kanwisher N, et al. Reduced language lateralization in autism and the broader autism phenotype as assessed with robust individual‐subjects analyses. Autism Res. 2020;13:1746–61.
Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich A, Ravichandran C, et al. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. NeuroImage. 2010;51:1117–25.
Floris DL, Wolfers T, Zabihi M, Holz NE, Zwiers MP, Charman T, et al. Atypical brain asymmetry in autism—a candidate for clinically meaningful stratification. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:802–12.
Liu J, Tsang T, Jackson L, Ponting C, Jeste SS, Bookheimer SY, et al. Altered lateralization of dorsal language tracts in 6‐week‐old infants at risk for autism. Dev Sci. 2019;22. https://onlinelibrary.wiley.com/doi/10.1111/desc.12768.
Fusaroli R, Lambrechts A, Bang D, Bowler DM, Gaigg SB. Is voice a marker for Autism spectrum disorder? A systematic review and meta‐analysis. Autism Res. 2017;10:384–407.
Swanson MR, Donovan K, Paterson S, Wolff JJ, Parish-Morris J, Meera SS, et al. Early language exposure supports later language skills in infants with and without autism. Autism Res. 2019;113:12111.
Acknowledgements
We thank the children and their families for their ongoing participation in this study. We also thank the numerous research assistants and volunteers who have worked on this project over the years. This work was supported by grants through the National Institutes of Health (K01-MH122779, PI: JBG; R01-HD055741, PI: JP; R01-HD055741-S1, PI: JP; P30-HD003110, PI: JP; U54-EB005149, PI: Kikinis) and the Simons Foundation (SFARI Grant 140209). Author TM was supported by NICHD T32HD040127. The funders had no role in study design, data collection, analysis, data interpretation, or the writing of the report.
Funding
RCM serves on the advisory board of Nous Imaging, Inc. and receives funding for meals and travel from Siemens Healthineers and Philips Healthcare. All other authors report no biomedical financial interests or potential conflicts of interest.
Author information
Authors and Affiliations
Consortia
Contributions
1. Study concept & design: McFayden, Rutsohn, Cetin, Forsen, Swanson, Meera, Truong, Hazlett, Piven, Girault. 2. Data acquisition: Botteron, Dager, Estes, McKinstry, Pandey, Schultz, St. John, Zwaigenbaum, Hazlett, Piven. 3. Data analysis & Interpretation: McFayden, Rutsohn, Cetin, Forsen, Swanson, Wolff, Elison, Gerig, Styner, Truong, Hazlett, Piven, Girault. 4. Drafting of manuscript: McFayden, Rutsohn, Cetin, Forsen, Girault. 5. Critical revisions of the manuscript for important intellectual content: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McFayden, T.C., Rutsohn, J., Cetin, G. et al. White matter development and language abilities during infancy in autism spectrum disorder. Mol Psychiatry 29, 2095–2104 (2024). https://doi.org/10.1038/s41380-024-02470-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-024-02470-3
- Springer Nature Limited