Abstract
Traumatic brain injury (TBI) and orthopedic injury (OI) patients are prone to anxiety and mood disorders. In the present study, we integrated anatomical and diffusion tensor neuroimaging to investigate structural properties of the amygdala and hippocampus, gray matter regions implicated in anxiety and mood disorders. Children and adolescents were evaluated during the late sub-acute phase of recovery following trauma resulting from either moderate to severe TBI or OI. Mean diffusivity (MD) of the amygdala and hippocampus was elevated following TBI. An interaction of hemisphere, structure, and group revealed that MD of the right amygdala was elevated in females with TBI. Self-reported anxiety scores were not related to either volume or microstructure of the hippocampus, or to volume or fractional anisotropy of the amygdala. Left amygdala MD in the TBI group accounted for 17.5% of variance in anxiety scores. Anxiety symptoms may be mediated by different mechanisms in patients with TBI or OI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) in children and adolescents, with a prevalence of 500,000 per year between the ages of 0–14 in the United States (Langlois et al. 2005), continues to be a significant source of morbidity in survivors. Both prospective and retrospective studies have described a high occurrence of new-onset psychiatric disorders, particularly internalizing disorders, in up to 60% to 71% of children and adolescents within the first two years following TBI (Bloom et al. 2001; Brown et al. 1981; Max et al. 1998; Vasa et al. 2002). Particularly in pediatric studies, it can be quite challenging to differentiate between new-onset disorders and new-onset symptoms following TBI. Yet, the importance of evaluating elevated subclinical symptoms, which do not meet DSM-IV criteria for a specific disorder, is highlighted by a recent report on new-onset anxiety in children following TBI (Max et al. 2011) where both subclinical anxiety symptoms and clinically significant anxiety disorders were elevated 17% and 8%, respectively, within the first six months following childhood TBI. Whether elevated anxiety symptoms resolve through the passage of time or place the child at increased risk for developing a clinically significant anxiety disorder at a later time point is relatively unknown.
The impact of trauma on neuropsychological and quality of life outcomes has been explored in relatively few investigations designed to examine the incidence of psychological sequelae following traumatic orthopedic injury (OI) not involving the brain. In a study of 401 OI children and adolescents who were hospitalized and survived major physical trauma, Acute Stress Disorder was diagnosed in 40% before hospital discharge (Holbrook et al. 2005). In this study, no information about subclinical symptoms was reported for the remaining 60% who did not meet diagnostic criteria for Acute Stress Disorder. However, the high incidence of Acute Stress Disorder following traumatic OI underscores the importance of identifying children and adolescents at risk for developing clinically significant neuropsychological morbidity such as Acute Stress Disorder and Post Traumatic Stress Disorder since these disorders have been shown to strongly impact short- and long-term measures of quality of life in children and adolescents (Holbrook et al. 2005).
More recent studies of neuropsychological sequelae following OI in children and adolescents have evaluated post traumatic symptoms of stress and elevated anxiety levels (Jonovska et al. 2008). These sequelae were presumed to be related to the hospitalization and/or specific procedures involved, as symptoms were markedly reduced by 6 months post-trauma and correlated with duration of hospital stay. Additional research has indicated that subclinical levels (e.g. developmentally appropriate assessment of intensity and frequency) of anxiety and stress are responsible for substantial functional impairment and distress in children following OI (Carrion et al. 2002) or TBI (Max et al. 2011).
Post-traumatic stress symptoms (PTSS) have infrequently been compared in children and adolescents with TBI in relation to those sustaining OI. In a recent prospective study (Hajek et al. 2010), parent report of PTSS was examined in children with mild TBI and OI during the first year after injury. Although no consistent group differences in parental report of PTSS were observed, the OI group was more likely to meet symptom criteria for PTSD one year after injury than the mild TBI group. In a longitudinal study with assessments at 6 and 12 months after injury (Levi et al. 1999), analyses of parental report of PTSS indicated an increased number of stress symptoms in children with severe TBI relative to those with moderate TBI or OI at the 6 month time point. One year post-injury, the moderate and severe TBI groups did not differ from each other but had more PTSS than the OI group. In the same study, analyses of child self-report data indicated comparable intensity of PTSS at 6 months in all groups. At the one year follow-up, children with severe TBI reported significantly more intense PTSS than the moderate TBI group (Levi et al. 1999).
Relative to children with OI, rates of new onset anxiety disorders have been reported to be significantly higher in children with either mild or moderate/severe TBI when assessed 6 months after injury (Luis and Mittenberg 2002). Although OI patients are frequently utilized as a comparison group for pediatric TBI studies, examinations of self-reported anxiety have rarely been conducted between TBI and OI cohorts enrolled in the same research study. Furthermore, to our knowledge, the neural correlates of self-reported anxiety have not yet been explored with volumetric and microstructural data during the sub-acute recovery phase from OI and TBI in children.
Despite evidence that children with TBI are vulnerable to developing chronic anxiety and mood disorders (Bloom et al. 2001; Bombardier et al. 2010; Max et al. 1998, 1997, 2011), few studies have focused on identifying the underlying mechanisms of these behavioral and neuropsychiatric changes following pediatric TBI (Maller et al. 2010). Several studies have focused on evaluating post-traumatic lesion volume and/or regional volumetric changes in their pediatric populations (Grados et al. 2008; Vasa et al. 2004). Additional studies in pediatric TBI have related increased incidence of anxiety, depression, and other internalizing symptoms to focal injury to superior frontal gyrus and left temporal regions, as well as to diffuse brain lesions. In contrast, decreased symptoms have been related to focal lesions in the right medial frontal gyrus, right cingulum, and bilateral hippocampi (Vasa et al. 2002, 2004; Max et al. 1998, 1997, 2011).
In pediatric TBI, little information is currently available regarding quantitative analyses of limbic structures in the mesial temporal lobe (MTL) implicated in internalizing disorders. To date, only two studies (Beauchamp et al. 2011; Wilde et al. 2007) have investigated volumetric properties of both the amygdala and hippocampus in chronic pediatric TBI at 1 to 10 years post-injury. Both studies noted smaller hippocampal volumes. Other studies have reported a trend for decreased hippocampal volume in pediatric TBI at least 3 months post-injury (Di Stefano et al. 2000) and significant reduction in hippocampal volume in pediatric TBI assessed five years post-injury (Tasker et al. 2005). Amygdala volumes have been more variable, with reduction noted in some studies (Wilde et al. 2007) and enlargement reported in others (Beauchamp et al. 2011). Unfortunately, these investigations did not report measures of anxiety or depression. Furthermore, most studies have evaluated their pediatric study population during the chronic phase of TBI recovery (e.g. at least one year or more following injury). Currently, little information is available in the extant literature of pediatric TBI studies regarding volumetric measures of the amygdala and hippocampus during the sub-acute phase of recovery from TBI or OI.
Relative to studies of volumetric changes, diffusion tensor magnetic resonance imaging (DTI) studies have the potential to provide improved characterization of post-traumatic sequelae which are either poorly visualized or eventually appear as nonspecific cerebral volume loss during the chronic phase of recovery (Mukherjee and McKinstry 2006). DTI permits non-invasive in-vivo quantification of micro-structural changes in both gray matter (GM) and white matter (WM) through examination of the magnitude and directionality of the diffusion of water in tissues (Basser 1995; Pierpaoli et al. 1996). DTI provides indices to calculate metrics of diffusivity and anisotropy. Fractional anisotropy (FA) reflects the degree of directionality of water diffusion within a voxel and mean diffusivity (MD) reflects the magnitude of water diffusion, both of which are related to microstructural tissue characteristics (Pierpaoli et al. 1996) including myelin and cellular membranes (Le Bihan 2003). Although clarification of the microstructural correlates of these FA and MD in both WM and GM is ongoing, FA is believed to index the integrity and degree of fiber organization in WM and degree of complexity of apical dendrites in GM (Bock et al. 2010). Therefore, higher FA in WM corresponds to increased integrity while higher FA in GM corresponds to reduced complexity of dendritic arbors. Measures of MD have been related to expansion of extracellular space in both WM and GM, possibly attributed to neuronal or glial loss (Pierpaoli et al. 1996).
To date, only a single study has simultaneously investigated volumetric and microstructural measures of GM in patients recovering from TBI at two months and twelve months post injury (Bendlin et al. 2008). In this adult TBI study, the longitudinal component of the research design indicated that GM volumes were significantly reduced in the TBI group over the ten month assessment interval; the cross-sectional analysis indicated that MD was elevated in several GM regions, both cortical and subcortical, in the TBI group relative to the control group at two months post-injury (Bendlin et al. 2008). Thus far, microstructural measures of tissue integrity, such as MD and FA, have not been examined in the amygdala and hippocampus of children following TBI.
Since DTI studies of microstructure have been quite successful at detecting early stages of WM damage following TBI (Ewing-Cobbs et al. 2008; Kraus et al. 2007; Levin et al. 2008), we integrated two modalities of MR imaging (e.g. DTI and structural MRI) to determine if significant group differences in either macro- or micro-structural properties of MTL GM structures were evident in children and adolescents at approximately three months after hospitalization for either TBI or OI. As a secondary aim, we wanted to explore whether volume, MD, or FA values in the amygdala related to self-reported levels of anxiety in our TBI and/or OI study participants. To assess discriminant validity of this hypothesized brain-behavior relation, exploratory analyses of the hippocampus and self-reported anxiety were also conducted. Although anxiety and mood disorders are frequently comorbid in both adults and children following TBI, the published literature of adult studies targeting the MTL suggests a dissociation between the neural correlates of anxiety (e.g. amygdala) and the neural correlates of depression (e.g. hippocampus). Since we did not collect measures of depression from our TBI and OI cohorts, we could not directly test for such dissociation between the neural correlates of anxiety and depression following TBI or OI in children. However, we did indirectly test this dissociation favored by the “amygdalocentric” view of anxiety disorders (Rauch et al. 2006) by predicting that structural measures of the hippocampus should not be related to self-report anxiety measures.
Methods
Participants
Participants in the TBI and OI groups were recruited prospectively from consecutive inpatient admissions to the Children’s Memorial Hermann Hospital Level 1 Pediatric Trauma Center from 2006–2008 as part of a federally funded research program to prospectively investigate longitudinal academic outcomes in child survivors of moderate to severe TBI (R01-NS046308 to LEC). Inclusion criteria for the TBI group were: 1) hospitalization for injury resulting from acceleration-deceleration or blunt impact injuries caused by vehicular accidents, falls, or impact with a blunt object; 2) moderate and severe TBI, defined as the lowest post-resuscitation Glasgow Coma Scale (GCS; (Teasdale and Jennett 1974) score ranging between 3–12; 3) children living within a 125 mile radius of the hospital; and 4) no prior diagnosis of TBI or neurological disorder. Severe injury was defined as a GCS of 3–8; moderate injury was defined as a GCS of 9–12. Due to the requirement for hospitalization, the sample is skewed toward children with severe rather than moderate TBI. The OI group consisted of participants who were hospitalized with orthopedic injuries which excluded head injury. The OI group met inclusion criteria 1, 3, and 4 as noted above. Full written parental consent and child assent (as appropriate) were obtained according to study protocol approved by our local Institutional Review Board.
Recruitment process
For the TBI group, medical records of 239 candidates were screened and 50 met inclusion criteria. A total of 32 patients and their families were enrolled and consented to participate in the research study. Of those who were not enrolled or consented to participate in the study, 13 declined to participate and 5 were not able to be contacted. For the OI group, a total of 303 medical records were screened; 133 candidates were identified as having met inclusion criteria. A total of 33 patients were enrolled and consented to participate in the research study. Of those who were not enrolled or consented to participate in the research study, 62 declined to participate (frequently due to the longitudinal nature of the study design) and 38 were not able to be contacted.
All subjects included in the present analyses were required to have a good quality T1-weighted dataset as well as a good quality DTI dataset and a completed SCARED-R questionnaire. From the 32 enrolled TBI subjects, a total of 11 datasets were excluded due to the following reasons: 4 subjects refused the MRI procedure; 1 subject exhibited motion artifact on the T1-weighted sequence; 1 subject declined to complete the SCARED-R; 5 subjects did not have whole brain T1-weighted data. From the 33 enrolled OI subjects, a total of 13 datasets were excluded due to the following reasons: 4 subjects refused the MRI procedure; 3 subjects exhibited motion artifact on the T1-weighted sequence; 1 subject had an MRI which was clinically read as abnormal; 5 subjects did not have whole brain T1-weighted data. Due to these exclusions, the sample size of TBI subjects for the present study was 21 and the sample size of OI subjects was 20.
Procedure
Self-report measures of anxiety
We obtained both self-report and parent report of children’s anxiety symptoms. Questionnaires and interviews examining anxiety and internalizing disorders which are completed by different respondents yield different profiles. Indeed, there is often a low to moderate correlation of symptoms between child and parent report based on either interview or questionnaire format (Gerring et al. 2002; Langer et al. 2010; Manassis et al. 2009). Parents make judgments and inferences based on observation of the child’s behavior and verbalizations; self-report of internalizing symptoms relies on description of subjective experiences (Levi et al. 1999).
Anxiety level was self-assessed by each participant at the 3-month post-injury assessment using the revised version of the Screen for Child Anxiety Related Emotional Disorders (e.g. SCARED-R) (Birmaher et al. 1999, 1997). While a total of 41 items yield a composite score, five subscales target specific domains, including 1) thirteen items for panic disorder or significant somatic symptoms 2) nine items for generalized anxiety 3) eight items for separation anxiety 4) seven items for social anxiety and 5) four items for significant school avoidance. For each child, composite scores were summed across all items in all target domains, with each item having a rating of 0, 1, or 2. Composite scores in the present study ranged from 2 to 58. As a reference, developers of the instrument suggested a cutoff score of ≥25 as being a positive indicator of an anxiety disorder.
In addition to obtaining a self-report measure of anxiety, an experienced clinician conducted a semi-structured interview (KSADS-PL; (Kaufman et al. 1997)) with each study participant’s parent to obtain information about pre-injury, current and lifetime events. All diagnoses were assigned by a child psychiatrist (AS) with extensive experience diagnosing psychiatric disorders in children with TBI and OI. KSADS-PL diagnoses were examined to provide clinical information regarding the presence of pre-injury anxiety disorders and to identify the onset of post-injury anxiety disorders.
Neuroimaging acquisition and analysis
All MRIs were performed on a Philips 3T scanner with SENSE (Sensitivity Encoding) technology using an 8 channel phase array head coil. After conventional scout and T2-weighted sequences, a three-dimensional isotropic T1-weighted sequence (MPRAGE) was performed in the coronal plane. Acquisition parameters of the isotropic 3D MPRAGE sequence were as follows: repetition time/echo time = 8.5/4.0 ms; flip angle = 6˚; square field of view = 240 × 240 mm; matrix = 256 × 256; slice thickness = 0.94 mm; in-plane pixel dimensions (x,y) = 0.94,0.94 mm; number of excitations (NEX) = 1; scan time ~6 min. The DTI sequence consisted of a single-shot spin-echo diffusion sensitized echo-planar imaging sequence with the following parameters: 21 non-collinear equally distributed diffusion encoding directions (Hasan and Narayana 2005); repetition time/echo time = 6100/84 ms; b = 0, and 1000 sec/mm2; square field-of-view = 240 × 240 mm; matrix = 256 × 256; slice thickness = 3.0 mm; in-plane pixel dimensions (x,y) = 0.94,0.94; SENSE acceleration factor = 2; sequence repeated twice and averaged; number of b = 0 images collected and averaged = 8; scan time ~7 min.
All scans were analyzed blind to diagnosis, age, gender, and measures of anxiety. Structural MRIs (e.g. T1-weighted images) were reviewed for image quality prior to morphometric analyses using Freesurfer v4.0.5 software (www.surfer.nmr.mgh.harvard.edu). On a 64bit Linux computer, a fully-automated process (e.g. recon-all-all) was executed from the command line to skull-strip and segment each T1-weighted image of the brain into 3 classes of voxels: gray matter (GM), white matter (WM), and cerebrospinal fluid. Subsequently, within Freesurfer, a fully-automated routine determined delimiting boundaries of subcortical structures, including the hippocampus and amygdala. Results were visually inspected for accuracy using Freesurfer’s Tkmedit viewer before including in statistical analyses. As recently reported by Bigler and colleagues (Bigler et al. 2010), Freesurfer-based boundary delineation of the hippocampus and amygdala in TBI patients provides sensitive measures of atrophy of these structures as well as comparable volumetric results to manual delineation.
Among the output files generated by Freesurfer are image files containing masks of individually labeled neocortical and non-neocortical GM regions (Fischl et al. 2002). These image files were mapped back to native scanner space along with the T1-weighted image used for segmentation and resultant output files written to nifti format. Subsequent analyses requiring intra-subject co-registration of the GM masks, the T1-weighted images, and the DTI dataset collected from the same imaging session were performed using FSL v4.1.0 (Smith et al. 2004)(e.g. FMRIB’s Software Library, www.fmrib/ox.ac.uk/fsl). Minor head motion and eddy currents were corrected in the DTI series with the Eddy Current Correction tool included in FSL’s Diffusion Toolbox v2.0. Subsequently, skull-stripping and removal of non-brain tissue was performed using BET v2.1. FSL’s Linear Image Registration Tool v5.5 (FLIRT; (Jenkinson et al. 2002)) was used to perform a within subject inter-modal linear registration between each subject’s T1-weighted series (reference image) and corresponding non-diffusion-weighted images (b = 0) from the DTI dataset (see Fig. 1). The resultant transformation matrix, and calculation of its inverse, provided the basis for co-registering the GM masks obtained from Freesurfer’s analyses of the high-resolution T1-weighted images with each individual’s DTI dataset. The diffusion tensors were reconstructed using FSL’s DTIFIT tool within the Diffusion Toolbox. Quantitative measures of MD and FA were obtained from each study participant using regions circumscribed by the co-registered masks of the amygdala and hippocampus and command line utilities included with the FSL software package.
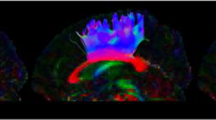
Co-registration of Freesurfer GM masks for the amygdala (red) and hippocampus (blue) with processed DTI dataset from one participant. The first row displays sagittal, coronal, and magnified axial views of the participant’s T1 image set with transparent overlays of GM masks for the amygdala and hippocampus in the left hemisphere
Statistical analysis
Distributions of all data were reviewed for normality prior to selecting parametric tests for evaluating statistical significance of findings.
Group comparisons of self-report anxiety measures
The composite score on the SCARED-R was used in a one-way analysis of variance (ANOVA) to determine differences between groups and gender, including group by gender interaction effects on self-reported anxiety.
Group comparisons of brain structural variables
A repeated measures ANOVA was used to evaluate structural properties of the amygdala and hippocampus, including interaction effects of group by gender and group by gender by hemisphere. The statistical model consisted of nominal predictor variables (e.g. group and gender) including one repeated factor (e.g. hemisphere). Each structure (e.g. amygdala and hippocampus) and its structural properties (e.g. corrected volume, FA, and MD) were evaluated separately as dependent variables for either significant interactions and simple main effects, or significant main effects. Follow-up analyses for simple main effects included the mean square error for group, gender, and hemisphere as well as interaction terms. Even though we have relatively small sample sizes for TBI and OI groups, we did check for age effects and observed that the results were unaffected. Therefore, we report our findings without age included in the model to limit the number of variables and protect against over-fitting of the model. Significance was determined at P-values <0.05.
Brain-behavior analysis
To test our hypothesis that structural properties of the amygdala are related to anxiety, an ANOVA was used with anxiety as the dependent variable; group (TBI or OI) and hemisphere (left or right) and metric (e.g. MD, FA, corrected volume) of the amygdala were the independent variables. Due to the small sample size, a separate ANOVA was used for each metric. The brain-behavior analysis described above was repeated for the hippocampus to evaluate the specificity of the proposed brain-behavior relation between anxiety and the amygdala. All analyses were carried out using JMP Statistical Discovery Software (SAS Institute, Cary, NC).
Results
The TBI group consisted of 15 males and 6 females ranging from 6.5 to 16.4 years of age (mean = 12.08; SD = 3.58); the OI group consisted of 13 males and 7 females ranging from 8 to 15.9 years of age (mean = 12.25; SD = 2.79). The TBI and OI groups did not significantly differ in gender [X2(1) = 0.19; p > 0.66] or handedness [X2(1) = 1.22; p > 0.27]. No significant age differences existed between groups at time of assessment [F(1,39) = −0.16; p = 0.87].Since five subjects in the OI group (3M: 2F) and 5 subjects in the TBI group (4M:1F) were excluded from MRI data analyses due to incomplete imaging data, we compared demographic and clinical information of our two group study samples with and without these 10 subjects. Inclusion of these subjects resulted in a total sample size of 51 subjects (TBI = 26; OI = 25). Consistent with our findings for 41 subjects (TBI = 21; OI = 20) reported above, there were no significant group differences in gender [X2 (1) = 0.49; p > 0.48], age [F(1,49) = 0; p = 0.99], or SCARED composite scores [F(1,49) = 0.24; p = 0.63]. None of the OI cases had pre- or post-injury clinical diagnoses; 1/5 of the TBI cases had pre-injury diagnosis of GAD while 4/5 of the TBI cases had post-injury diagnoses of anxiety-NOS (n = 2), PTSD (n = 1), and the remaining case had a post-injury dual diagnosis of GAD and PTSD. The mechanism of injury for the 5 OI cases included fall (n = 3), motor vehicle accident (n = 1), and struck pedestrian (n = 1). The mechanism of injury for the 5 TBI cases included struck pedestrian (n = 3) and motor vehicle accident (n = 2). These data demonstrate that there are no significant differences in the representativeness of our two group samples of OI and TBI participants when participants without whole brain imaging data are included or excluded.
As expected, the Injury Severity Score (Baker et al. 1974) indicated that the TBI group sustained more severe injuries than the OI group, F(1, 40) = 68.4, p < .001. However, when injury to body regions excluding the head was examined, the groups had comparable Injury Severity Scores [F(1,40) = 6.25, p = 0.66]. Both groups were imaged on average three months from date of injury (OI females: mean = 96.43 days, SD = 48.45; OI males: mean = 95.08 days, SD = 59.88; TBI females: mean = 95.67 days, SD = 42.34; TBI males: mean = 84.47 days, SD = 39.73).
Clinical MRI and anxiety findings
Based on clinical readings of MRIs by a Board-certified Radiologist (LAK), 2/21 children in the TBI group had scans that were read as normal. Given our interest in neural correlates of internalizing symptoms, we examined frontal and temporal regions for evidence of shear injury and encephalomalacia. Of the 15/21 MRIs with clear evidence of shear injury, four cases had shear injury on the right, three cases had shear injury on the left, and eight cases had bilateral frontal and/or temporal lesions. Encephalomalacia in frontal and/or temporal regions was visualized bilaterally in 4/21 cases, on the left in four cases, and lateralized to the right in two cases. Table 1 contains clinical characteristics and radiological findings for the TBI group.
The average and standard deviation of the composite score on the SCARED-R was 25.2 ±11.3 for the OI group and 19.5 ±14.1 for the TBI group. An ANOVA of anxiety scores did not demonstrate a significant interaction of group by gender [F(1,37) = 0.0002; p = 0.99] nor significant main effects of either group [F(1,37) = 1.58; p = 0.22] or gender [F(1,37) = 0.59, p = 0.45]. The composite SCARED-R score for each TBI and OI participant is included in Tables 1 and 2, respectively.
Based on the KSADS-PL parent interviews examining pre-injury and concurrent anxiety symptoms (Kaufman et al. 1997), a total of 3 participants in the OI group and 0 participants in the TBI group met criteria for an anxiety disorder at the time of injury (2 generalized anxiety, 1 anxiety disorder not otherwise specified). In terms of post-injury disorders, new onset anxiety diagnoses were assigned in 6 participants in the OI group and 6 participants in the TBI group. Across groups, post-traumatic stress disorder (two cases in each group) and anxiety disorder, not otherwise specified (3 in TBI and 2 in OI groups) were the most frequent post-injury diagnoses. A few participants exhibited new-onset dual diagnoses post-injury (3 in TBI and 1 in OI groups). Individual participants’ pre-and post-injury anxiety diagnoses are provided in Table 1 (TBI group) and Table 2 (OI group).
Microstructure of the amygdala and hippocampus
Group comparisons
For relative volume (e.g. absolute volume corrected for estimated intracranial volume), repeated measures ANOVA indicated no significant interactions or main effects of group, gender, or hemisphere for the amygdala (all p > 0.05) or the hippocampus (all p > 0.05). Table 3 reports descriptive statistics for all metrics, including relative volume, FA, and MD for each gender within each group for the amygdala and hippocampus in each hemisphere.
For FA, no significant interactions or main effects of group, gender, or hemisphere were observed for the amygdala (all p > 0.05). Although no significant interactions were evident in the hippocampus (all p > 0.05), a main effect of group did reach statistical significance [F(1,37) = 4.416, p = 0.043] such that FA of the hippocampus was significantly lower in the TBI group relative to the OI group.
A significant three-way interaction between group, gender, and hemisphere was observed for MD values in the amygdala [F(1,37) = 5.356, p = 0.026]. Analysis of simple main effects using standard least squares means indicated a significant effect of group in the left amygdala (p = 0.038), with MD values of the left amygdala being larger in the TBI group relative to the OI group. In the right amygdala, a significant interaction of group by gender was observed (p = 0.034; R2 = 0.11). As shown in Fig. 2, females with TBI demonstrated higher MD values than females with OI in the right amygdala. For the hippocampus, interactions of gender and hemisphere were not significant (p > 0.05). However, a significant main effect of group was evident [F(1,37) = 7.177, p = 0.011] as MD values of the hippocampus were larger in the TBI than in the OI group.
Brain-behavior analysis
ANOVAs were used to explore brain-behavior relations between different structural properties of the amygdala and self-report anxiety. A significant interaction between group, hemisphere, and MD of the amygdala on anxiety [F(4,40) = 2.83; p = 0.038) was observed. While a positive relation between MD of the left amygdala and anxiety was significant for the TBI group [F(1,40) = 8.38; p = 0.006), no significant relation was observed in the OI group. The interaction term accounted for 17.5% of the variance, representing nearly all of the variance accounted for by the whole model. Even when the TBI participants with the highest and lowest SCARED-R scores were dropped from the analyses, the findings remained significant for the whole model [F(3,38) = 3.708; p = 0.02] and for the positive relation between MD of the left amygdala and anxiety for the TBI group [F(1,38) = 2.64; p = 0.012]. ANOVAs repeated for FA and corrected volume of the amygdala failed to demonstrate a significant association with anxiety in either group. As predicted, none of the structural metrics from the hippocampus was related to anxiety in either group.
A regression analysis was used to display the relation between SCARED scores and MD of the left amygdala in each group (see Fig. 3). A significant positive linear association between SCARED scores and MD is observed in the TBI group (dark circles; R2 = 0.19; F(1,20) = 5.90; p = 0.025), but not in the OI group (R2 = 0.08; F(1,19) = 2.77; p = 0.113).
Regression plot of SCARED scores and MD of the left amygdala by group. A significant positive linear association between SCARED scores and MD is observed in the TBI group (dark circles; R2 = 0.19; F(1,20) = 5.90; p = 0.025). Confidence intervals of the linear fit for the TBI group are bounded by the shaded region. Open triangles represent data for OI group (R2 = 0.08; F(1,19) = 2.77; p = 0.113). MD values are expressed as ×10−3 mm2/s
Discussion
In the present study, we investigated potential neural correlates of increased anxiety which frequently occurs following pediatric TBI (Bloom et al. 2001; Brown et al. 1981; Max et al. 1998, 1997, 2011; Vasa et al. 2002). We integrated structural MRI and DTI modalities for quantifying both macro- and micro-structural properties of two MTL GM structures implicated in anxiety and mood disorders, the amygdala and hippocampus. Although anxiety or mood symptoms may reflect alteration in other GM regions or the disruption of white matter pathways connecting MTL GM with prefrontal cortical regions (Campbell-Sills et al. 2011; Johansen-Berg et al. 2008; Kim and Whalen 2009), the present study limited its investigation to the amygdala and hippocampus since these GM structures have been previously reported to 1) exhibit atrophy over time following TBI and 2) relate to neuropsychological measures of anxiety and depression. Volumetric, MD, and FA values of the amygdala and hippocampus were examined in relation to composite scores on a self-report anxiety measure, the SCARED-R, in children and adolescents approximately three months following TBI or OI. Both TBI and OI groups were well-matched in age and gender. Furthermore, OI and TBI groups did not significantly differ in self-reported composite anxiety scores. Based on parent interview, new-onset anxiety disorders were present in 6 children in each group.
In our group comparisons, we found no evidence of strong differences between groups in relative volumes of the amygdala. However, analyses of microstructural properties (e.g. MD) of the amygdala did vary by group. Specifically, a three-way interaction of group, gender, and hemisphere was observed for MD of the amygdala. Follow-up analyses indicated that females in the TBI group had significantly higher MD values in the right amygdala than females in the OI group. In contrast, for the left amygdala, only a main effect of group was evident, with the TBI group exhibiting significantly higher MD values than the OI group. Thus, while MD was significantly higher in the amygdala bilaterally of the TBI group, a gender effect was only evident in analyses of the right amygdala.
In our brain-behavior analyses of the amygdala in relation to anxiety, neither corrected volumetric measures nor FA were significantly related to self-reported anxiety scores in either group. However, MD of the left amygdala significantly related to anxiety in the TBI group, but not in the OI group. Furthermore, none of the structural measures of the hippocampus was related to anxiety in either group. This finding suggests a role for the amygdala in mediating self-report anxiety in TBI children during the sub-acute recovery phase.
As previously noted, the majority of studies investigating internalizing disorders following OI have specifically examined post-traumatic stress but not anxiety in general. Therefore, there is little information on the incidence and course of anxiety disorders after OI. In the OI group, elevated anxiety symptoms may be related to situational stresses associated with the injury, including sustaining an injury and receiving medical care, which are likely to diminish across the first year after injury (Winston et al. 2005). In the TBI group, the relation of increased diffusivity of the amygdala with elevated anxiety is likely to reflect the additional consequences of combined primary and secondary mechanisms of neuronal injury.
The main findings of this study contribute to the emerging literature relating idiopathic neuropsychiatric symptoms to structural changes in limbic structures. Previous studies have primarily utilized DTI to probe microstructural properties of WM in relation to various cognitive and neuropsychiatric outcomes following TBI. However, evaluating microstructural properties of GM regions interconnected by WM pathways is equally important. Probing microstructural properties of GM in addition to WM regions has the potential to differentiate pathophysiological mechanisms disrupting functional neural circuits and assist with the development of targeted treatment options designed to improve outcome.
Although the underlying mechanism or interpretation of elevated MD values in GM is not yet well-understood, the metric does indicate relative ease of water movement (Pierpaoli et al. 1996). In well-organized GM, barriers to diffusion include cellular membranes, both neuronal and glial. In disorganized GM, structurally-specific increased diffusion has been associated with cellular breakdown (e.g. necrosis) and excitotoxicity, ultimately leading to atrophy (Kantarci et al. 2005). Compelling evidence in the published literature suggests key roles for post-traumatic up-regulation of inflammatory response systems (Cederberg and Siesjö 2010; Ziebell and Morganti-Kossmann 2010) and apoptotic signal transduction pathways (Slemmer et al. 2008; Yakovlev et al. 1997) in mediating a protracted process of accelerated cellular death secondary to the primary injury (Zhang et al. 2005). Any and/or all of these processes could have contributed to the elevated MD values we observed in the TBI group and simultaneously provide an explanation as to why the group without head injuries was not represented by the same relation, despite high levels of self-reported anxiety. As described by others, mesial temporal GM structures are particularly vulnerable to shear-related injuries which may explain the increased incidence of neuropsychiatric disorders involving the amygdala and hippocampus in survivors of moderate-to-severe TBI (Jorge et al. 2007, 2004; Tateno et al. 2004). Future studies designed to elucidate underlying mechanisms of elevated MD in mesial temporal GM following TBI hold significant potential for developing targeted therapeutic interventions designed to minimize secondary tissue damage and improve outcomes.
Limitations
Several limitations of the present study are worth noting. Since demographic data was not collected on non-participants, direct comparisons between participants and non-participants to ascertain representativeness of our study samples are not possible. Since the sample size is small, findings require replication in larger samples so that relations of DTI metrics, gender, and anxiety can be validated and extended. Although the results indicate a lateralization for MD in the left amygdala being linearly associated with self-reported anxiety in the TBI group, the clinical MRI scans of the TBI group revealed mainly bilateral (8/21) or left hemisphere (7/21) frontal and/or temporal injuries. Whether the presence of multifocal left hemisphere or bilateral frontal/temporal injury partially explains the lateralization to the left amygdala in our findings requires a larger sample with more right hemisphere injuries represented.
Secondly, in our study population, the traumatic incident ranged from sports injuries, falls, pedestrians being struck by a vehicle, and motor vehicle collisions. Whether different external causes of trauma precipitating TBI differentially contribute differentially to the development of anxiety or mood disorders has not yet been widely addressed in the literature (Saatman et al. 2008).
Future directions
In the present study, the three month assessment time interval was possibly too early for significant volumetric changes to become apparent. However, a trend was evident for the TBI group to have smaller amygdala volumes than the OI group. Whether a two year assessment might demonstrate significantly decreased amygdala volumes in the TBI group is an experimental question currently being investigated as we continue to follow these same participants through 24 months post-injury.
The inclusion of community comparison children without injuries would enhance future studies of the relation between microstructure metrics and anxiety symptoms. Comparing metrics and outcomes in children with TBI and OI and community children would help clarify the impact of traumatic injury to the brain and other body regions on neuropsychiatric outcomes. Future studies should dissociate how different injury characteristics, including OI, focal brain injuries, and traumatic axonal injury, impact microstructure of GM and WM pathways implicated in developmental and acquired anxiety disorders. Microstructure of MTL structures, core regions in dorsal and ventral prefrontal cortices, and WM pathways that have been implicated in developmental (Monk 2008; Pine et al. 2008) and acquired anxiety disorders (Max et al. 2011; Vasa et al. 2002) should be examined during both sub-acute and chronic stages of recovery.
Few studies have investigated gender differences in structural or functional properties of the amygdala. Although based on a small sample size, the present study found that MD of the right amygdala was specifically elevated in females in the TBI group. Clearly, future studies should address gender differences in the neural bases of emotion responsivity and regulation, including modulation of their function, as a consequence of TBI.
Conclusions
The present study demonstrates an association between elevated MD of the left amygdala and increased self-reported anxiety in TBI evaluated at three months post-injury. Whether elevated MD in the amygdala or hippocampus later predicts GM atrophy in these regions is an important question for future research. Although speculative, one interpretation of the present findings is that a neuropsychiatric diagnosis of an anxiety disorder is not explained by a single etiological basis. Thus, children with TBI may have reduced microstructural integrity in the amygdala (e.g. ventral stream of anxiety) due to shear injury, diffuse axonal injury, or inflammatory processes triggered by the head injury. In contrast, children with OI do not seem to exhibit the same changes in microstructural integrity or a relation between MD of the amygdala and self-report anxiety. Perhaps the OI group has evidence of altered macro- or micro-structure in other brain regions or white matter connections between regions in dorsal streams of anxiety. Further research is required to identify the potential pathophysiological mechanisms underlying anxiety in these populations. This knowledge will inform different intervention strategies to reduce the morbidity associated with post-traumatic anxiety in children and adolescents hospitalized for injury to the brain or other body regions.
References
Baker, S. P., O’Neill, B., Haddon, W., & Long, W. B. (1974). The Injury Severity Score: a method for desccribing patients with multiple injuries and evaluation of emergency care. Journal of Trauma, 14, 187–196.
Basser, P. J. (1995). Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR in Medicine, 8(7–8), 333–344.
Beauchamp, M. H., Ditchfield, M., Maller, J. J., Catroppa, C., Godfrey, C., Rosenfeld, J. V., et al. (2011). Hippocampus, amygdala and global brain changes 10 years after childhood traumatic brain injury. International Journal of Developmental Neuroscience, 29(2), 137–143.
Bendlin, B. B., Ries, M. L., Lazar, M., Alexander, A. L., Dempsey, R. J., Rowley, H. A., et al. (2008). Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. NeuroImage, 42(2), 503–514.
Bigler, E. D., Abildskov, T. J., Wilde, E. A., McCauley, S. R., Li, X., Merkley, T. L., et al. (2010). Diffuse damage in pediatric traumatic brain injury: a comparison of automated versus operator-controlled quantification methods. NeuroImage, 50(3), 1017–1026.
Birmaher, B., Khetarpal, S., Brent, D., Cully, M., Balach, L., Kaufman, J., et al. (1997). The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry, 36(4), 545–553.
Birmaher, B., Brent, D., Chiappetta, L., Bridge, J., Monga, S., & Baugher, M. (1999). Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. Journal of the American Academy of Child and Adolescent Psychiatry, 38(10), 1230–1236.
Bloom, D. R., Levin, H. S., Ewing-Cobbs, L., Saunders, A. E., Song, J., Fletcher, J. M., et al. (2001). Lifetime and novel psychiatric disorders after pediatric traumatic brain injury. Journal of the American Academy of Child and Adolescent Psychiatry, 40(5), 572–579.
Bock, A. S., Olavarria, J. F., Leigland, L. A., Taber, E. N., Jespersen, S. N., & Kroenke, C. D. (2010). Diffusion tensor imaging detects early cerebral cortex abnormalities in neuronal architecture induced by bilateral neonatal enucleation: an experimental model in the ferret. Frontiers in Systems Neuroscience, 4. doi:10.3389/fnsys.2010.00149.
Bombardier, C. H., Fann, J. R., Temkin, N. R., Esselman, P. C., Barber, J., & Dikmen, S. S. (2010). Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA, 303(19), 1938–1945.
Brown, G., Chadwick, O., Shaffer, D., Rutter, M., & Traub, M. (1981). A prospective study of children with head injuries, III: psychiatric sequelae. Psychological Medicine, 11(1), 63–78.
Campbell-Sills, L., Simmons, A. N., Lovero, K. L., Rochlin, A. A., Paulus, M. P., & Stein, M. B. (2011). Functioning of neural systems supporting emotion regulation in anxiety-prone individuals. NeuroImage, 54(1), 689–696.
Carrion, V. G., Weems, C. F., Ray, R., & Reiss, A. L. (2002). Toward an empirical definition of pediatric PTSD: the phenomenology of PTSD symptoms in youth. Journal of the American Academy of Child and Adolescent Psychiatry, 41(2), 166–173.
Cederberg, D., & Siesjö, P. (2010). What has inflammation to do with traumatic brain injury? Child’s Nervous System, 26(2), 221–226.
Di Stefano, G., Bachevalier, J., Levin, H. S., Song, J. X., Scheibel, R. S., & Fletcher, J. M. (2000). Volume of focal brain lesions and hippocampal formation in relation to memory function after closed head injury in children. Journal of Neurology, Neurosurgery & Psychiatry, 69(2), 210–216.
Ewing-Cobbs, L., Prasad, M. R., Swank, P., Kramer, L., Cox, C. S., Jr., Fletcher, J. M., et al. (2008). Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. NeuroImage, 42(4), 1305–1315.
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355.
Gerring, J. P., Slomine, B., Vasa, R. A., Grados, M., Chen, A., Rising, W., et al. (2002). Clinical predictors of posttraumatic stress disorder after closed head injury in children. Journal of the American Academy of Child and Adolescent Psychiatry, 41(2), 157–165.
Grados, M. A., Vasa, R. A., Riddle, M. A., Slomine, B. S., Salorio, C., Christensen, J., et al. (2008). New onset obsessive-compulsive symptoms in children and adolescents with severe traumatic brain injury. Depression and Anxiety, 25(5), 398–407.
Hajek, C. A., Yeates, K. O., Gerry Taylor, H., Bangert, B., Dietrich, A., Nuss, K. E., et al. (2010). Relationships among post-concussive symptoms and symptoms of PTSD in children following mild traumatic brain injury. Brain Injury, 24(2), 100–109.
Hasan, K., & Narayana, P. (2005). DTI parameter optimization at 3.0 T: potential application in entire normal human brain mapping and multiple sclerosis research. MedicaMundi, 49.
Holbrook, T., Hoyt, D., Coimbra, R., Potenza, B., Sise, M., & Anderson, J. (2005). High rates of acute stress disorder impact quality-of-life outcomes in injured adolescents: mechanism and gender predict acute stress disorder risk. Journal of Trauma Injury, Infection and Critical Care, 59(5), 1126–1130.
Jenkinson, M., Bannister, P., Brady, M., & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841.
Johansen-Berg, H., Gutman, D. A., Behrens, T. E. J., Matthews, P. M., Rushworth, M. F. S., Katz, E., et al. (2008). Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cerebral Cortex, 18(6), 1374–1383.
Jonovska, S., Jengic, V., Kvesic, A., Pavlovic, E., Zupancic, B., Galic, G., et al. (2008). The quality of life during the treatment of long bone fractures in children and adolescents. Collegium Antropologicum, 32(4), 1121–1127.
Jorge, R. E., Robinson, R. G., Moser, D., Tateno, A., Crespo-Facorro, B., & Arndt, S. (2004). Major depression following traumatic brain injury. Archives of General Psychiatry, 61(1), 42–50.
Jorge, R. E., Acion, L., Starkstein, S. E., & Magnotta, V. (2007). Hippocampal volume and mood disorders after traumatic brain injury. Biological Psychiatry, 62(4), 332–338.
Kantarci, K., Petersen, R. C., Boeve, B. F., Knopman, D. S., Weigand, S. D., O’Brien, P. C., et al. (2005). DWI predicts future progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology, 64(5), 902–904.
Kaufman, J., Birmaher, B., Brent, D., Rao, U. M. A., Flynn, C., Moreci, P., et al. (1997). Schedule for affective disorders and schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–988.
Kim, M. J., & Whalen, P. J. (2009). The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. Journal of Neuroscience, 29(37), 11614–11618.
Kraus, M. F., Susmaras, T., Caughlin, B. P., Walker, C. J., Sweeney, J. A., & Little, D. M. (2007). White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain, 130(10), 2508–2519.
Langer, D. A., Wood, J. J., Bergman, R. L., & Piacentini, J. C. (2010). A multitrait-multimethod analysis of the construct validity of child anxiety disorders in a clinical sample. Child Psychiatry and Human Development, 41(5), 549–561.
Langlois, J., Rutland-Brown, W., & Thomas, K. (2005). The incidence of traumatic brain injury among children in the United States: differences by race. The Journal of Head Trauma Rehabilitation, 20(3), 229–238.
Le Bihan, D. (2003). Looking into the functional architecture of the brain with diffusion MRI. Nature Reviews Neuroscience, 4(6), 469–480.
Levi, R. B., Drotar, D., Yeates, K. O., & Taylor, H. G. (1999). Posttraumatic stress symptoms in children following orthopedic or traumatic brain injury. Journal of Clinical Child Psychology, 28(2), 232–243.
Levin, H., Wilde, E., Chu, Z., Yallampalli, R., Hanten, G., Li, X., et al. (2008). Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. The Journal of Head Trauma Rehabilitation, 23(4), 197–208.
Luis, C. A., & Mittenberg, W. (2002). Mood and anxiety disorders following pediatric traumatic brain injury: a prospective study. Journal of Clinical and Experimental Neuropsychology, 24(3), 270–279.
Maller, J. J., Thomson, R. H. S., Lewis, P. M., Rose, S. E., Pannek, K., & Fitzgerald, P. B. (2010). Traumatic brain injury, major depression, and diffusion tensor imaging: making connections. Brain Research Reviews, 64(1), 213–240.
Manassis, K., Tannock, R., & Monga, S. (2009). Anxious by maternal—versus self-report: are they the same children? Journal of the Canadian Academy of Child and Adolescent Psychiatry=Journal de l’Academie canadienne de psychiatrie de l’enfant et de l’adolescent, 18(2), 103–109.
Max, J., Robin, D., Lindgren, S., Smith, W., Sato, Y., Mattheis, P., et al. (1997). Traumatic brain injury in children and adolescents: psychiatric disorders at two years. Journal of the American Academy of Child and Adolescent Psychiatry, 36(9), 1278–1285.
Max, J., Koele, S., Smith, W., Sato, Y., Lindgren, S., Robin, D., et al. (1998). Psychiatric disorders in children and adolescents after severe traumatic brain injury: a controlled study. Journal of the American Academy of Child and Adolescent Psychiatry, 37(8), 832–840.
Max, J., Keatley, E., Wilde, E. A., Bigler, E. D., Levin, H. S., Schachar, R. J., et al. (2011). Anxiety disorders in children and adolescents in the first six months after traumatic brain injury. The Journal of Neuropsychiatry and Clinical Neurosciences, 23(1), 29–39.
Monk, C. S. (2008). The development of emotion-related neural circuitry in health and psychopathology. Development and Psychopathology, 20, 1231–1250.
Mukherjee, P., & McKinstry, R. C. (2006). Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clinics of North America, 16(1), 19–43.
Pierpaoli, C., Jezzard, P., Basser, P. J., Barnett, A., & Di Chiro, G. (1996). Diffusion tensor MR imaging of the human brain. Radiology, 201(3), 637–648.
Pine, D. S., Guyer, A. E., & Leibenluft, E. (2008). Functional magnetic resonance imaging and pediatric anxiety. Journal of the American Academy of Child and Adolescent Psychiatry, 47(11), 1217–1221.
Rauch, S., Shin, L., & Phelps, E. (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biological Psychiatry, 60(4), 376–382.
Saatman, K. E., Duhaime, A.-C., Bullock, R., Maas, A. I. R., Valadka, A., & Manley, G. T. (2008). Classification of traumatic brain injury for targeted therapies. Journal of Neurotrauma, 25(7), 719–738.
Slemmer, J. E., Zhu, C., Landshamer, S., Trabold, R., Grohm, J., Ardeshiri, A., et al. (2008). Causal role of apoptosis-inducing factor for neuronal cell death following traumatic brain injury. American Journal of Pathology, 173(6), 1795–1805.
Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E., Johansen-Berg, H., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl 1), S208–S219.
Tasker, R., Salmond, C., Westland, A., Pena, A., Gillard, J., Sahakian, B., et al. (2005). Head circumference and brain and hippocampal volume after severe traumatic brain injury in childhood. Pediatric Research, 58(2), 302–308.
Tateno, A., Jorge, R. E., & Robinson, R. G. (2004). Pathological laughing and crying following traumatic brain injury. The Journal of Neuropsychiatry and Clinical Neurosciences, 16(4), 426–434.
Teasdale, G., & Jennett, B. (1974). Assessment of coma and impaired consciousness: a practical scale. Lancet, 2, 81–84.
Vasa, R. A., Gerring, J. P., Grados, M., Slomine, B., Christensen, J. R., Rising, W., et al. (2002). Anxiety after severe pediatric closed head injury. Journal of the American Academy of Child and Adolescent Psychiatry, 41(2), 148–156.
Vasa, R. A., Grados, M., Slomine, B., Herskovits, E. H., Thompson, R. E., Salorio, C., et al. (2004). Neuroimaging correlates of anxiety after pediatric traumatic brain injury. Biological Psychiatry, 55(3), 208–216.
Wilde, E., Bigler, E., Hunter, J., Fearing, M., Scheibel, R., Newsome, M., et al. (2007). Hippocampus, amygdala, and basal ganglia morphometrics in children after moderate-to-severe traumatic brain injury. Developmental Medicine and Child Neurology, 49(4), 294–299.
Winston, F. K., Baxt, C., Kassam-Adams, N. L., Elliott, M. R., & Kallan, M. J. (2005). Acute traumatic stress symptoms in child occupants and their parent drivers after crash involvement. Archives of Pediatrics & Adolescent Medicine, 159(11), 1074–1079.
Yakovlev, A. G., Knoblach, S. M., Fan, L., Fox, G. B., Goodnight, R., & Faden, A. I. (1997). Activation of CPP32-like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. Journal of Neuroscience, 17(19), 7415–7424.
Zhang, X., Chen, Y. F., Jenkins, L. W., Kochanek, P. M., & Clark, R. S. B. (2005). Bench-to-bedside review: apoptosis/programmed cell death triggered by traumatic brain injury. Critical Care, 9(1), 66–75.
Ziebell, J. M., & Morganti-Kossmann, M. C. (2010). Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics, 7(1), 22–30.
Acknowledgments
This work was funded by NIH-NINDS-R01-NS046308 awarded to LEC. Additional intramural funding from the Department of Pediatrics awarded to JJ.
Author Disclosure Statement
No competing financial interests exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Juranek, J., Johnson, C.P., Prasad, M.R. et al. Mean diffusivity in the amygdala correlates with anxiety in pediatric TBI. Brain Imaging and Behavior 6, 36–48 (2012). https://doi.org/10.1007/s11682-011-9140-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-011-9140-5