Abstract
Background and aim
Diffusion tensor imaging (DTI) parameters in the corpus callosum have been suggested to be a biomarker for prognostic outcomes in individuals with diffuse axonal injury (DAI). However, differences between the DTI parameters on moderate and severe trauma in DAI over time are still unclear. A secondary goal was to study the association between the changes in the DTI parameters, anxiety, and depressive scores in DAI over time.
Methods
Twenty subjects were recruited from a neurological outpatient clinic and evaluated at 2, 6, and 12 months after the brain injury and compared to matched age and sex healthy controls regarding the DTI parameters in the corpus callosum. State-Trace Anxiety Inventory and Beck Depression Inventory were used to assess psychiatric outcomes in the TBI group over time.
Results
Differences were observed in the fractional anisotropy and mean diffusivity of the genu, body, and splenium of the corpus callosum between DAI and controls (p < 0.02). Differences in both parameters in the genu of the corpus callosum were also detected between patients with moderate and severe DAI (p < 0.05). There was an increase in the mean diffusivity values and the fractional anisotropy decrease in the DAI group over time (p < 0.02). There was no significant correlation between changes in the fractional anisotropy and mean diffusivity across the study and psychiatric outcomes in DAI.
Conclusion
DTI parameters, specifically the mean diffusivity in the corpus callosum, may provide reliable characterization and quantification of differences determined by the brain injury severity. No correlation was observed with DAI parameters and the psychiatric outcome scores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse axonal injury (DAI) following traumatic brain injury (TBI) leads to white matter pathology [1, 2], cognitive and psychiatric sequela [3,4,5,6], and brain atrophy over time [1, 3]. The anatomical sites commonly affected are the corticomedullary junctions, the corpus callosum (CC) [7, 8], and association white matter tracts. The CC is one of the most affected white matter regions in DAI [9,10,11]. The decreased integrity of the CC tracts was correlated with Wallerian degeneration [12], which is usually observed as a post-traumatic condition in patients with DAI [3].
Studies suggest that lesions in specific areas of the CC are associated with the duration of unconsciousness and might be a biomarker for clinical prognosis after the brain injury [13, 14]. Rutgers et al. [13] investigated the differences in the fractional anisotropy (FA) of the CC in participants with mild, moderate, and severe TBI and controls; however, they found no differences in DTI measures between moderate and severe TBI on DTI-derived parameters. The lack of significant differences between the moderate and severe TBI subgroups may be associated with the small sample size, leading to loss of statistical power [13]. Additionally, the authors selected patients with heterogeneous traumatic brain lesions and at variable intervals from subacute to the chronic stages of the TBI.
The quantitative analysis of the CC using the DTI-derived parameters allows the correlation with several clinical outcomes, such as depressive and anxiety symptoms, the most common psychiatric disturbances after the TBI. Evidence points out that depressive symptoms are associated with reduced fractional anisotropy (FA) in the CC genu in the non-lesioned brain [15]. Ware et al. [16] identified that chronic concussion–related to boxing exerted higher mean diffusivity (MD) in the CC and reduced FA in the CC splenium in comparison to the control group. The authors also found a positive correlation between FA, anxiety, and depression symptoms in this population. The CC is frequently injured in TBI, but the contribution of the integrity of the CC and neuropsychiatric symptoms in persons with DAI over time is inconclusive.
In the present study, we aim to (1) investigate the differences of the DTI-parameters (FA and MD) of the CC between healthy controls and participants with moderate and severe DAI; (2) compare differences of the DTI-derived parameters (FA and MD) in the CC between moderate and severe DAI and the FA and MD changes over the time; (3) to correlate the DTI-parameters (FA and MD) in the CC with anxiety and depression scores assessed 2, 6, and 12 months after the trauma.
Methods
This work is part of a larger prospective study analyzing clinical characteristics, brain magnetic resonance imaging (MRI), and cognitive outcomes in patients with DAI [3, 17]. For this study, we analyzed the DTI data, depression, and anxiety scores at three timepoints: 2, 6, and 12 months after the brain injury. The control group was assessed once. The project was approved by the Institutional Ethics Committee for Research (CAPPESQ) of the University of São Paulo under the number 0097/11.
Subjects
Twenty outpatients with DAI and 20 controls signed the consent form to participate in this study. A brain CT (computed tomography) scan or MRI interpreted by a certified neuroradiologist confirmed the diagnosis of traumatic DAI. According to the Marshall scale, DAI and possible associated injuries observed on CT scans were classified into grades III and IV. All participants were screened for MRI safety guidelines.
DAI group
Inclusion criteria: ages between 18 and 55 years; less than 6 months after the brain injury in the first visit; post-traumatic amnesia (PTA) < 30 min; Glasgow Coma Scale (GCS) ≤ 12; at least 4 years of formal education; and ability to follow verbal instructions.
Exclusion criteria: evidence of other abnormalities than DAI shown by neuroimaging (e.g., contusions larger than 10 cc, epidural hematoma, subdural hematoma); neurological diseases (e.g., epilepsy, stroke, tumor); previous TBI; current use of illicit drugs; and participation on any rehabilitation program during the period of the data collection.
Control group
Inclusion criteria: ages between 18 and 55 years and at least 4 years of formal education. Exclusion criteria: a history of neurological disease and current use of illicit drugs.
Instruments
Beck Depression Inventory [18], Brazilian version by [19]
Beck Depression Inventory (BDI) is a 21-question tool, measuring symptoms of depression (e.g., sadness, pessimism, failure, guilt, suicidal ideation).
State scale from State-Trait Anxiety Inventory [20], Brazilian version by [21]
State scale from State-Trait Anxiety Inventory (STAI-S) consists of 20 questions that assess state anxiety, in which all items range from 0 to 4 (from “almost never” to “almost always”). Higher scores indicate a higher anxiety level.
Imaging
Conventional MR images acquisition
MRI data was acquired on a 3 T scanner (Phillips Achieva, Best, The Netherlands) using a phased eight-channel head coil. Anatomical imaging protocol was acquired in the sagittal plane using a 3D T1-weighted Fast Field Echo (3DT1-FFE) sequence covering the entire brain (180 slices), with the following parameters: inversion time (IT) = 700 ms; TR/TE = 6.2 ms/2.7 ms; flip angle = 8°; acquisition matrix = 240 × 240; field of view (FOV) = 240 × 240 × 180 mm; voxel resolution = 1 mm3 (isotropic); slice thickness = 1.0 mm; and completion time = 4 min.
DTI data acquisition
DTI images were collected in the axial plane with gradients applied in 32 non-collinear directions. The entire brain was covered with 70 slices, 2 mm-thick each, with no gaps in between. Other parameters used were TR/TE = 8.500 /61 ms; b value = 1000 s/mm2; matrix = 128 × 128; FOV = 256 × 256 mm; 2mm3 isotropic voxel; NEX (number of excitations) = 2; and completion time = 14 min.
Pre-processing
All data were pre-processed using the functional MRI brain (FMRIB) software library (FSL), version 5.0 (available at http://www.fmrib.ox.ac.uk/fsl/), applying brain extraction tool (BET), FMRIB’s linear image registration tool (FLIRT), and correction of Eddy current–induced distortions [22, 23]. Motion correction was completed using ExploreDTI (A. Leemans, University Medical Center, Utrecht, The Netherlands) by rotating the B-matrix to keep the orientation input accurate. Investigation for residuals and outliers of the diffusion tensor fit was done with the same software, ending on residual maps similar on all groups [24, 25].
Tractography
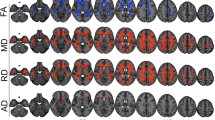
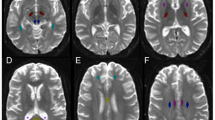
Tensor calculation and fiber tracking were performed with ExploreDTI (Fig. 1), [24, 25]. First, a whole-brain tractography was automatically obtained in the native space using a brute-force approach of every pixel. Deterministic tractography technique was then achieved following a predesigned combination of specific procedures, which included the positioning of multiple regions-of-interest (ROIs) on different planes. The corpus callosum was segmented into three parts: genu, body, and splenium, following the most standard segmentation used in the literature [11, 26, 27]. The FACT (fiber assignment by continuous tracking) algorithm was calculated with a FA threshold of 0.25 and a maximum angle of 30°, equally applied to all subjects [28]. The average quantitative values of FA and MD were extracted from all voxels inside each CC segment.
Statistical analyses
All the data was analyzed using IBM SPSS Statistics version 24.0 (SPSS Inc., IBM Company, Chicago, IL, USA) with two-tailed tests and a 5% level of significance. Analysis of variance (ANOVA) with Bonferroni’s post hoc analysis, or unpaired t-test, the χ2 test, and the nonparametric Kruskal–Wallis test were used to analyze differences in the baseline characteristics between patient groups. The mixed-effect model (REML) regression analyses were used. For the first analysis, the control group was considered the reference and the patient’s group as the comparison one. Each participant was added to the model as a random effect, and the severity (control DTI parameters’ single score, moderate TBI, and severe TBI), the time (2, 6, and 12 months) as fixed factors. In the first model, we have described the differences between controls and DAI (moderate and severe) and the effect of time. The second analysis evaluated the differences between patients when separated into groups according to the severity of the trauma (moderate vs. severe). Severity and time were considered as a fixed effect and participants as a random effect to the model. We evaluated parallelism of the lines correspondent to scores of DTI-derived parameters in both moderate and severe TBI over time. If parallelism was detected, no interaction analysis between DTI measures and time was performed. The results were presented accordingly to the region of the CC (CCgenu, CCboby, CCsplenium).
For the complementary analysis of the association between the DTI-derived parameters and the psychiatric symptoms in the patients’ group, the Pearson linear correlation was performed in each timepoint (2, 6, and 12 months after the brain injury).
Results
Forty individuals, 20 patients with DAI and 20 controls, participated in the study. DAI severity was estimated using the Glasgow Coma Scale (GCS) and the Posttraumatic Amnesia (PTA) scores during the hospital admission. Table 1 shows the summary of the demographic data of both DAI and the control group. Except for the expected GCS score differences between the DAI groups, no statistical differences were found in the groups’ demographic characteristics.
Genu of the corpus callosum
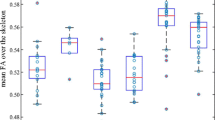
The severe DAI group has lower FA values when compared to the control group (z = − 5.85, p = 0.0001), but no significant differences were found between the controls and the moderate DAI group (z = − 1.30, p = 0.19). Patients with severe DAI also showed lower FA values compared to patients with moderate DAI (z = − 2.28, p = 0.02). No significant effect of the time between the three timepoints has been shown (p > 0.06). For the MD analysis of the CCgenu, moderate DAI had higher MD than the healthy controls group (z = 1.82, p = 0.007). Similar results were found between the severe and the control group (z = 5.04, p = 0.0001). Differences were also found between moderate and severe DAI groups (z = 2.02, p = 0.044), where the severe group had higher MD than the moderate DAI in the CCgenu. There was a significant increase in the MD values observed from the baseline to 12 months after the trauma (z = 2.29, p = 0.022) (Fig. 2).
Body of the corpus callosum
The moderate and severe DAI groups have lower FA values when compared to the controls (z = − 3.59, p = 0.0001 and z = − 8.33, p = 0.0001, respectively). There were no differences in the FA values between the moderate and the severe DAI group (z = − 1.94, p = 0.052). There was a significant decrease in the FA values between 2 and 6 months after the brain injury (z = − 2.27, p = 0.002). For the MD analysis of the CCbody, moderate and severe DAI groups have higher MD values than the controls (z = 2.16, p = 0.031 and z = 4.59, p = 0.0001, respectively). No significant differences between the moderate and severe DAI groups were found (z = 1.02, p = 0.31). A significant increase of MD values between the baseline and 6 months (z = 2.42, p = 0.02) and 12 months after the trauma (z = 3.13, p = 0.002) (Fig. 3).
Splenium of the corpus callosum
Healthy controls have higher FA values compared to both the moderate (z = − 3.16, p = 0.002) and severe DAI groups (z = − 6.93, p = 0.0001). There were no differences between FA values between moderate and severe DAI groups (z = − 1.45, p = 0.15). There were no significant changes in the FA values over time (p > 0.9). For the MD analysis of the splenium, the moderate and severe DAI group had higher MD values than the controls (z = 2.38, p = 0.02 and z = 5.52, p = 0.0001, respectively). There were significant differences between the moderate and severe DAI groups (z = 1.81, p = 0.007). There was a significant increase in MD values between the baseline and 12 months after the trauma (z = 4.10, p = 0.0001).
Correlation between white matter integrity of the corpus callosum and depressive and anxiety scores over time
The Pearson correlation test found no significant correlation between the DTI measures on depressive and anxiety symptoms scores in our DAI group in any of the three timepoints (Table 2).
Discussion
To our knowledge, this study was the first one to identify progressive differences after the trauma in the DTI-derived parameters in the CC between patients with moderate and severe DAI. FA value is the most widely used DTI parameter and represents the degree of directionality of microstructures (e.g., axons, myelin, and microtubules) that may indicate the integrity of the white matter tracts. Our results pointed to significant differences in the FA and MD measurements between controls and patients, especially in the severe group. The control group had significantly higher FA values than observed in groups with patients with moderate and severe DAI in the CC body and CC splenium. MD values were able to discriminate controls from patients with moderate and severe DAI in all CC regions (genu, body, and splenium). It was also able to discriminate between patients with moderate and severe DAI. FA values in the CC genu were lower, whereas MD values were higher in the CC genu and CC splenium in the severe DAI group than in the moderate. The results point out that DTI-derived parameters (FA and MD) in the CC can assist in the characterization of the injury severity, as proposed by others [29]. In the DAI group, the effect of time was observed, especially on the MD measurements. The increase of the MD values over time may be associate with volume loss in the white matter structures, as shown previously [3]. This increase can be explained by expanding the extracellular space associated with neuronal or glial loss in the chronic stage after the trauma.
The CC is the primary fiber pathway linking the bilateral hemispheres and is divided into C genu, CCbody, and CCsplenium. The CCgenu is the anterior bend of the CC and projects fibers to connect with medial and lateral frontal lobes, particularly the prefrontal cortex. Previous studies indicated that the alterations in the CC could be heterogeneous and depend on several factors. As pointed out previously, most of the callosal lesions post-TBI occur in the posterior body and splenium [30, 31], possibly due to the cue contra-cue effect at the moment of the brain injury. Those radiological measures were used to consider the DAI stage (or severity), such as suggested by Adams et al. [8] and Gennarelli et al. [32]. Differing from the previous studies that evidenced lesions in the posterior portion of the CC, our study pointed for lesions on the anterior (genu) and in the middle (body) of the CC in DAI.
DAI often results in a disruption of the white fibers in the parasagittal white matter of the cortex, CC, pontine-mesencephalic junction, frontotemporal regions, amygdala, hippocampus, basal ganglia, and thalamus [33, 34]. These fiber disruptions result in impairment of mood regulation [35] as well as neurochemical and neuroendocrine changes after the trauma affecting the hypothalamic–pituitary–adrenal axis, potentially causing depression [36]. The CC has a major role in interhemispheric functional connectivity [37] and connections to multimodal associative areas in the frontal and parietal lobes that affect mood control [37, 38]. It is still unclear whether specific DTI-derived parameters in the CC are related to psychiatric prognosis or can be claimed as a neural signature for depressive symptoms.
Unlike we expected, none of the DTI-parameters used in this study correlated to depressive or anxiety symptoms at any time point. Even minor lesions may have overwhelming functional implications, despite the severity of the brain injury, and increased risk of developing psychiatric disorders [39]. On the other hand, some authors argue that severe injury may decline cognitive function, leading to a reduction of the self-perception and detection of psychiatric disorders [35].
The GCS is a clinical tool designed to assess coma and impaired consciousness and is one of the most commonly used TBI severity scoring systems. Although the GCS score has been used as the gold standard of neurologic assessment of trauma patients since its development by Teasdale and Jennett in 1974 [40], other TBI severity classification systems have evolved, mostly based on single grade indicators, such as the loss of consciousness and the duration of posttraumatic amnesia within 24 h of the brain injury [41]. One limitation of these classifications is that the initial TBI score severity does not necessarily predict the extent of disability arising from TBI. In this way, researchers in the field propose that approaches to determining severity after injury should also include neuroimaging, in addition to clinical evaluation of altered consciousness, the presence of posttraumatic amnesia, and the GCS score [41]. In the present study, we have also considered the GCS score to classify the severity of the injury and the posttraumatic duration of coma as the predictive variable that could interfere with the DTI-derived metrics results (see supplementary materials). Besides the evidence that the DTI metrics between moderate and severe DAI differed, the generalization of the predictive features of the duration of coma for the clinical outcome between the two groups was not established. In this way, future studies are needed to understand the difference in DTI metrics derived from brain tracts and its association to clinical outcomes in persons with sustained moderate and severe TBI.
Our study had limitations. One limitation is the self-reported questionnaire Beck Depression Inventory (BDI) and State-Trace Anxiety Inventory (STAI). Although BDI is suggested by the Common Data Element [42] to assess depressive symptoms in persons with TBI, patients with severe injury may be not aware of his/her real impairments, resulting in unreliable estimating of their symptoms. Another limitation of the study is that we did not exclude the participants with hypointensities, indicative of hemorrhage, as did other studies [13]. During the acute stage after the TBI, usually, when the patients are in the intensive care unit, there is a relatively high incidence of post-traumatic seizures. However, this incidence drops dramatically in the subacute and chronic stages after the TBI. Due to the lack of seizures during the period of data collection in our sample, seizures were not considered a variable that could have interfered in the interpretation of our results.
In conclusion, our study suggests that DTI-derived parameters, specifically the MD of the genu and splenium of the corpus callosum may provide reliable characterization and quantification of differences determined by the brain injury severity. DTI-derived parameters in the CC vary within the same type of intracranial lesion (DAI), according to the severity of the trauma, as well as progressively across time, which might suggest that DTI is an interesting method for patient follow-up.
References
Ubukata S, Oishi N, Sugihara G, Aso T, Fukuyama H, Murai T, Ueda K (2019) Transcallosal fiber disruption and its relationship with corresponding gray matter alteration in patients with diffuse axonal injury. J Neurotrauma 36(7):1106–1114. https://doi.org/10.1089/neu.2018.5823
Johnson VE, Stewart W, Smith DH (2013) Axonal pathology in traumatic brain injury. Exp Neurol 246:35–43. https://doi.org/10.1016/j.expneurol.2012.01.013
Feltrin FS et al (2018) Longitudinal changes in brain volumetry and cognitive functions after moderate and severe diffuse axonal injury. Brain Inj 32(10):1208–1217. https://doi.org/10.1080/02699052.2018.1494852
Rabinowitz AR, Hart T, Whyte J, Kim J (2018) Neuropsychological recovery trajectories in moderate to severe traumatic brain injury: influence of patient characteristics and diffuse axonal injury. J Int Neuropsychol Soc 24(3):237–246. https://doi.org/10.1017/s1355617717000996
Scheid R, Walther KR, Guthke T, Preul C, von Cramon DY (2006) Cognitive sequelae of diffuse axonal injury. Arc Neurol 63(3):418–424. https://doi.org/10.1001/archneur.63.3.418
Ponsford J, Draper K, Schonberger M (2008) Functional outcome 10 years after traumatic brain injury: its relationship with demographic, injury severity, and cognitive and emotional status. J Int Neuropsychol Soc 14(2):233–242. https://doi.org/10.1017/s1355617708080272
Adams JH, Graham DI, Murray LS, Scott G (1982) Diffuse axonal injury due to nonmissile head-injury in humans - an analysis of 45 cases. Ann Neur 12(6):557–563. https://doi.org/10.1002/ana.410120610
Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR (1989) Diffuse axonal injury in head-injury - definition, diagnosis and grading. Histopathology 15(1):49–59
Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML (2013) A decade of DTI in traumatic brain injury: 10 years and 100 articles later. Am J Neuroradiol 34(11):2064–2074. https://doi.org/10.3174/ajnr.A3395
Sidaros A et al (2008) Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain 131:559–572. https://doi.org/10.1093/brain/awm294
Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM (2007) White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 130:2508–2519. https://doi.org/10.1093/brain/awm216
de Lacoste MC, Kirkpatrick JB, Ross ED (1985) Topography of the human corpus callosum. J Neuropathol Exp Neurol 44(6):578–591. https://doi.org/10.1097/00005072-198511000-00004
Rutgers DR, Fillard P, Paradot G, Tadie M, Lasjaunias P, Ducreux D (2008) Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. AJNR Am J Neuroradiol 29(9):1730–1735. https://doi.org/10.3174/ajnr.A1213
Takaoka M et al (2002) Semiquantitative analysis of corpus callosum injury using magnetic resonance imaging indicates clinical severity in patients with diffuse axonal injury. J Neurol Neurosurg Psychiatry 73(3):289–293. https://doi.org/10.1136/jnnp.73.3.289
Dillon DG, Gonenc A, Belleau E, Pizzagalli DA (2018) Depression is associated with dimensional and categorical effects on white matter pathways. Depress Anxiety 35(5):440–447. https://doi.org/10.1002/da.22734
Ware AL et al (2018) A preliminary investigation of corpus callosum subregion white matter vulnerability and relation to chronic outcome in boxers. Brain Imaging Behav 14(3):772–786. https://doi.org/10.1007/s11682-018-0018-7
Grassi DC, Zaninotto AL, Feltrin FS, Macruz FBC, Otaduy MCG, Leite CC, Guirado VMP, Paiva WS, Andrade CS (2021) Dynamic changes in white matter following traumatic brain injury and how diffuse axonal injury relates to cognitive domain. Brain Inj. https://doi.org/10.1080/02699052.2020.1859615
Beck AT, Erbaugh J, Ward CH, Mock J, Mendelsohn M (1961) An inventory for measuring depression. Arch Gen Psychiatry 4(6):561
Gorenstein C, Andrade L (1998) Inventário de Depressão de Beck: propriedades psicométricas da versão em português. Revista de Psiquiatria Clínica 25(5):245–250
Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983) Manual for the state-trait anxiety inventory. Consulting Psychologists Press, Palo Alto, CA
Gorenstein C, Andrade L (1996) Validation of a Portuguese version of the Beck depression inventory and the state-trait anxiety inventory in Brazilian subjects. Braz J Med Biol Res 29(4):453–457
Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5(2):143–156. https://doi.org/10.1016/s1361-8415(01)00036-6
Smith SM et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208–S219. https://doi.org/10.1016/j.neuroimage.2004.07.051
Leemans A, Jones DK (2009) The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61(6):1336–1349. https://doi.org/10.1002/mrm.21890
Leemans A, Jeurissen B, Sijbers J, Jones D (2009) ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proc Int Soc Magn Reson Med 17(2):3537
Kumar R et al (2009) Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: its correlation with neuropsychometric tests. Brain Inj 23(7):675–685. https://doi.org/10.1080/02699050903014915
Arenth PM, Russell KC, Scanlon JM, Kessler LJ, Ricker JH (2014) Corpus callosum integrity and neuropsychological performance after traumatic brain injury: a diffusion tensor imaging study. J Head Trauma Rehabil 29(2):E1-e10. https://doi.org/10.1097/HTR.0b013e318289ede5
Mori S, Crain BJ, Chacko VP, van Zijl PC (1999) Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45(2):265–269. https://doi.org/10.1002/1531-8249(199902)45:2%3c265::aid-ana21%3e3.0.co;2-3
Yiannakkaras C et al (2019) Whole brain and corpus callosum diffusion tensor metrics: How do they correlate with visual and verbal memory performance in chronic traumatic brain injury. J Integr Neurosci 18(2):95–105. https://doi.org/10.31083/j.jin.2019.02.144
Gentry LR, Godersky JC, Thompson B, Dunn VD (1988) Prospective comparative study of intermediate-field MR and CT in the evaluation of closed head trauma. AJR Am J Roentgenol 150(3):673–682. https://doi.org/10.2214/ajr.150.3.673
Gentry LR (Apr 1994) Imaging of closed head injury. Radiology 191(1):1–17. https://doi.org/10.1148/radiology.191.1.8134551
Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP (1982) Diffuse axonal injury and traumatic coma in the primate. Ann Neurol 12(6):564–574. https://doi.org/10.1002/ana.410120611
Meythaler JM, Peduzzi JD, Eleftheriou E, Novack TA (2001) Current concepts: diffuse axonal injury-associated traumatic brain injury. Arch Phys Med Rehabil 82(10):1461–1471. https://doi.org/10.1053/apmr.2001.25137
Silver JM, McAllister TW, Arciniegas DB (2009) Depression and cognitive complaints following mild traumatic brain injury. Am J Psychiatry 166(6):653–661. https://doi.org/10.1176/appi.ajp.2009.08111676
Rogers JM, Read CA (2007) Psychiatric comorbidity following traumatic brain injury. Brain Inj 21(13–14):1321–1333. https://doi.org/10.1080/02699050701765700
Jorge RE, Starkstein SE (2005) Pathophysiologic aspects of major depression following traumatic brain injury. J Head Trauma Rehabil 2(6):475–487. https://doi.org/10.1097/00001199-200511000-00001
Roland JL et al (2017) On the role of the corpus callosum in interhemispheric functional connectivity in humans. Proc Natl Acad Sci U S A 114(50):13278–13283. https://doi.org/10.1073/pnas.1707050114
Cyprien F et al (2014) Corpus callosum size may predict late-life depression in women: a 10-year follow-up study. J Affect Disord 165:16–23. https://doi.org/10.1016/j.jad.2014.04.040
van Reekum R, Cohen T, Wong J (2000) Can traumatic brain injury cause psychiatric disorders? J Neuropsychiatry Clin Neurosci 12(3):316–327. https://doi.org/10.1176/appi.neuropsych.12.3.316
Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness - practical scale. Lancet 2(7872):81–84
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on the Review of the Department of Veterans Affairs Examinations for Traumatic Brain Injury (2019) Evaluation of the disability determination process for traumatic brain injury in veterans. Washington (DC): National Academies Press (US); 2, Diagnosis and assessment of traumatic brain injury. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542595/
Maas AI et al (2010) Common data elements for traumatic brain injury: recommendations from the Interagency Working Group on Demographics and Clinical Assessment. Arch Phys Med Rehabil 91(11):1641–1649. https://doi.org/10.1016/j.apmr.2010.07.232
Acknowledgements
We are also thankful to patients and volunteers who agreed to join this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
None.
Conflict of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wellingson Silva Paiva and Celi Andrade share last authorship
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zaninotto, A., Grassi, D.C., Duarte, D. et al. DTI-derived parameters differ between moderate and severe traumatic brain injury and its association with psychiatric scores. Neurol Sci 43, 1343–1350 (2022). https://doi.org/10.1007/s10072-021-05455-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05455-0