Abstract
The solution culture method was used to study the effect of increasing nitrogen on the growth and photosynthesis of poplar seedlings under 100 mmol L−1 NaCl stress. I Increase in nitrogen reduced stomatal limitation of leaves under NaCl stress, improved utilization of CO2 by mesophyll cells, enhanced photosynthetic carbon assimilation capacity, significantly alleviated saline damage of NaCl, and promoted the accumulation of aboveground and root biomass. I Increased nitrogen enhanced photochemical efficiency (ФPSII) and electron transport rates, relieved the reduction of maximum photochemical efficiency (Fv/Fm) under NaCl, and reduced the degree of photoinhibition caused by NaCl stress. Increased nitrogen applications reduced the proportion of energy dissipating in the form of ineffective heat energy and hence a greater proportion of light energy absorbed by leaves was allocated to photochemical reactions. Under treatment with increased nitrogen, the synergistic effect of heat dissipation and the xanthophyll cycle in the leaves effectively protected photosynthetic PSII and enhanced light energy utilization of leaves under NaCl stress. The increased nitrogen promoted photosynthetic electron supply and transport ability under NaCl stress evident in enhanced functioning of the oxygen-evolving complex on the electron donor side of PS II. It increased the ability of the receptor pool to accept electrons on the PSII electron acceptor side and improved the stability of thylakoid membranes under NaCl stress. Therefore, increasing nitrogen applications under NaCl stress can promote poplar growth by improving the efficiency of light energy utilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
About 20% of the world’s arable land has been salinized which limits the production of plants (Zhu 2001; Bhatnagar et al. 2008). Nitrogen is a necessary macronutrient required for growth and development and an important component of plant proteins and nucleic acids (Mi et al. 2007). However, soil salinization limits nitrogen mineralization and reduces the availability of nitrogen (Calderon et al. 2000; Wang et al. 2004). Nitrogen deficiency is an important limiting factor on the growth of plants. Rational application of nitrogen fertilizer can help promote plant growth and restore vegetation in vulnerable ecological areas and improve plants resistance under stress (Yuan et al. 2009). The application of nitrogen fertilizer not only promotes plant growth, it ameliorates the saline-alkaline soil environment (Yuan et al. 2009), improves photosynthetic and protein synthesis abilities (Wang et al. 2015), and plays an important role in enhancing plant resistance (Pompeiano et al. 2014; Skeffington and Jeffrey 1988). For example, increasing nitrogen to improve resistance to abiotic stressors of plants such as Indian mustard (Brassica juncea (L.) Czem.; Nathawat et al. 2007), barley (Hordeum L.; Ali et al. 2001), and rape (Brassica campestris L.; Yang et al. 2012), has been confirmed.
Photosynthesis is the foundation of plant production and plants continue to grow only by maintaining a relatively high photosynthetic capacity in adverse environments. Increased nitrogen promotes nitrogen metabolism and enhances photosynthetic carbon metabolism (Li and Gao 2007). Chloroplasts contain up to 75% of the nitrogen in plant leaves, and nitrogen metabolism is closely related to both the light dependent and light independent reactions of photosynthesis (Shangguan et al. 2000). Except for driving photochemical reactions, a large portion of the photosynthetic electron flow in photosynthesis is consumed in nitrogen metabolism. Therefore, there is a relationship of interdependence and competition between nitrogen metabolism and photosynthetic carbon metabolism. In adverse conditions, carbon metabolism is inhibited and the light energy absorbed by leaves cannot be fully used for electron transfer; the result is an excess of light energy. Nitrogen metabolism can then consume part of this energy and reduce the damage caused by excess light energy, and hence protect the photosynthetic apparatus (Foyer et al. 2001).
Some salt ions in the soil are indispensable nutrient elements for normal growth (Wang et al. 2001), but excess salt causes osmotic stress and ion toxicity as well as interferes with nutrient ion equilibrium affecting plant growth and physiological functions (Munns et al. 2006; Yan et al. 2006; Ali et al. 2017). Salt stress affects chlorophyll synthesis and photosynthetic capability of plants (Gong et al. 2013; Dąbrowski et al. 2016), often leading to the reduction of PSII(Photosystem II)reaction center activity, inhibition of electron transport, limitation of carbon assimilation capacity and may even lead to peroxidation or dissociation of thylakoid membranes (Mitsuya et al. 2000; Takahashi et al. 2017).
In long-term evolution, plants generate a series of salt-tolerant mechanisms such as the salt exclusion effect, the accumulation of osmotic adjustment substance, and/or the start-up of anti-oxidation systems in the root systems (Song et al. 2006; Askari et al. 2006; Zhao et al. 2016). Plant root systems control the passing in and out of Na+ using ion channels (such as a voltage-dependent non-selective cation channel NSCC, and the glutamate activation channel GLR) or transporters (such as the Na+/H+ antiporter and high affinity K+ transporter HKT), as well as adapting to salt stress by adjusting intracellular ion equilibrium via an ion compartmentation effect (Wu et al. 2012; Cheng et al. 2009; Apse and Blumwald 2007). The adaptation mechanisms of different root systems to salt stress are obviously different. Poplar is a common species for afforestation and greening in northern China (Song et al. 2013). It is a pioneer species for vegetation restoration projects in degraded ecosystems. In areas with high salt and alkali contents, soil nitrogen deficiency has been an important limiting factor for plant restoration (Yuan et al. 2009; Wang et al. 2004). However, there are few studies on the effects of nitrogen application on the growth and photosynthesis of poplar under NaCl or sodium chloride stress. The solution culture method was adopted to study the effects of increasing nitrogen on the growth and photosynthesis of poplar seedlings under NaCl stress. I In addition, we investigated the effects on photosynthetic electron transport and energy allocation of poplar leaves under NaCl stress. This study also aimed to clarify the relationship between nitrogen metabolism and photosynthetic carbon metabolism in leaves under increasing nitrogen treatments. This will provide theoretical grounds for rational nitrogen management for vegetation restoration in vulnerable ecological areas dominated by saline and alkaline soils.
Materials and methods
Materials
This work was conducted at the plant physiological laboratory of the Northeast Forestry University, Harbin, between March and June of 2014. Black poplar hybrid (Populus simonii × P. nigra) seeds were sown at the beginning of March in vermiculite. They were regularly irrigated with 1/2 Hoagland nutrient solution and the water content was maintained at 80%. Water content was determined using the TZS-1K instrument (TOP, China). When seedlings had five leaves, they were carefully removed from the vermiculite, the roots washed, and seedlings fixed on a black foam board with punched holes using a sponge. The foam board was then floated in an opaque box filled with 1/2 Hoagland solution for hydroponics. Each hydroponic box was filled with a 10-L nutrient solution with two poplar seedlings. The seedlings were continuously ventilated by an electric air pump and the nutrient solution replaced every 3 days. The treated seedlings were incubated under greenhouse culture lamps at temperatures of 25 °C ± 3 °C, light intensity of 400 μmol m−2 s−1, photoperiods of 12 h/12 h (light/dark), and a relative humidity of 75%. After 15 days of hydroponics, the experiment was conducted when new white roots emerged on the existing seedling roots.
Experimental design
The 18 seedlings under relatively consistent growth conditions were randomly assigned to three treatment groups with six seedlings in each. The NaCl stress treatment was initiated by adding NaCl to the 1/2 Hoagland solution and the NaCl concentration was allowed to reach 100 mmol L−1. The increased nitrogen treatment (T2) started by adding Ca (NO3)2 into the 1/2 Hoagland solution containing 100 mmol L−1 of NaCl. (The NaCl concentration was according to Wang et al. 2004), which made the concentration of NO3−–N reach 17.5 mmol L−1 (NO3−–N in a 1/2 Hoagland solution was 7.5 mmol L−1) (Xu et al. 2017). Hydroponics in a 1/2 Hoagland solution was used as the control (CK). To eliminate the effect of introducing Ca2+ in the T2 treatment, an appropriate amount of CaCl2 was added in the CK and NaCl stress treatments to make the Ca2+ concentration consistent with the T2 treatment. After 20 days of incubation, the indices were determined when the phenotypic differences between treatments became obvious.
Methods
Leaf photosynthesis measurements
For each treatment, three pieces of the last third or fourth fully expanded leaf from the bottom of the plant were used to determine the net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci). The Li-6400 photosynthesis system was utilized to maintain a CO2 concentration of 450 uL L−1, light intensity of 800 μmol m−2 s−1, environment temperature of 23 °C, and a relative humidity of 75%.
Determination of the fluorescence index of chlorophyll
A 0.5 h dark adaptation treatment was conducted on poplar seedling leaves with dark adaptation clamps. Using a portable pulse modulated fluorometer FMS-2 (Hansatch Co., UK) and following the method of Hu et al. (2007), initial fluorescence (Fo), maximal fluorescence (Fm), variable fluorescence (Fv), the maximum fluorescence under light adaptation (\(F_{\text{m}}^{\prime }\)), minimal fluorescence under light adaptation (\(F_{\text{o}}^{{\prime }}\)), and steady-state fluorescence (Fs) were determined. The maximal photochemical efficiency of PSII (Fv/Fm), actual photochemical efficiency (ФPSII), electron transport rate (ETR), and excess light energy, (1 − qP)/NPQ, were calculated as follows:
where PFD is the light intensity (μmol m−2 s−1). Each sample was replicated three times. The direction of the light energy absorbed by the PSII reaction center was determined (Hendrickson et al. 2004; Zhou et al. 2007), i.e., the quantum yield used for the photochemical reaction (YPSII), quantum yield depending on the proton gradient on both sides of the thylakoid membrane and xanthophyll cycle (YNPQ), basic fluorescence quantum yield and quantum yield of heat dissipation (Yf,D), and quantum yield of heat dissipation in inactivated the PSII reaction center (YNF). The calculation of parameters are as follows:
where the sum of parameters equals one, namely \(\varPhi_{\text{NF}} + \varPhi_{\text{PSII}} + \varPhi_{\text{NPQ}} + \varPhi_{\text{f,D}} = \, 1 \cdot F_{\text{v}} /F_{\text{mM}}\) is the maximal photochemical efficiency without photoinhibition. Each treatment was measured three times.
Determination of the chlorophyll fluorescence kinetic curve
The second fully-expanded leaf from the top of the seedlings was selected for measurement. The OJIP curve of the leaves after a 30-min dark adaptation treatment was measured using the Mini modulated chlorophyll fluorometer (FluorPen FP 100 max, Czech). The measurement of each treatment was repeated five times. The OJIP curve was induced by a pulsed red light of 3000 μmol m−2 s−1. The relative fluorescence intensity of the OJIP curve at points O, J, I, and P (Fo, FJ, FI, and Fm, respectively) corresponded to the times of 0, 2, 30, and 1000 ms, while the points of L and K (FL and FK, respectively) corresponded to the times of 0.15 and 0.30 ms, respectively. To analyze the changes of relative variable fluorescence at points J, I, L, and K (VJ, VI, VL, and VK, respectively), the curves of O–P, O–J, and O–K were normalized based on the method of Strasser et al. (1995). Three replications were measured for each treatment.
Determination of growth parameters
Growth parameters of all treated poplar seedlings were measured after the photosynthetic and chlorophyll fluorescence parameters were determined. After measuring height and root length for each seedling, the root surface was dried with absorbent papers and the leaves, stems, and roots of seedlings in each treatment were separately placed in aluminum boxes at a fixed temperature of 105 °C for 30 min and then dried at 60 °C for 30 h. The quality was measured until it was constant, and then leaf, stem, and root biomass were obtained. Each measurement was repeated six times.
Statistical analysis
Excel and SPSS (22.0) software were used for the statistical analysis. The data in figures and tables are the average of three replicates ± standard deviation (SE). The differences between different groups were compared using a one-way analysis of variance (ANOVA) and the least significant difference (LSD) method.
Results
Effects of increased nitrogen on growth of poplar seedlings under NaCl stress
NaCl significantly inhibited the growth of poplar seedlings. Compared with the CK, there was no significant change in plant heights but root lengths significantly decreased by 37.8% (P < 0.05) (Fig. 1). In addition, the biomass of each part of poplar seedling leaves under NaCl stress was significantly lower than that of the CK. However, an increase of nitrogen significantly promoted the growth of seedlings under NaCl stress. The difference in heights and root lengths between NaCl + N and NaCl treatments was not significant but the biomass of roots, stems, and leaves increased by 48.1% (P < 0.05) and 37.5% (P < 0.05), respectively, compared with NaCl treatment.
Effects of increased nitrogen on photosynthetic gas exchange parameters of leaves under NaCl stress
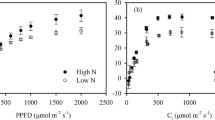
Compared with the CK, Pn, Tr, and Gs of leaves decreased by 31.0% (P < 0.05), 40.4% (P < 0.05), and 36.4% (P < 0.05) under NaCl stress, respectively, but Ci significantly increased by 24.6% (P < 0.05; Fig. 2). Increased nitrogen increased the size of stomatal openings of leaves under NaCl stress, and higher stomatal conductance promoted gas exchange in the leaves; Pn and Tr of leaves also significantly increased and the utilization of CO2 by the mesophyll cells was enhanced, which showed that the Ci of leaves in the NaCl + N treatment decreased by 9.1% compared with the NaCl treatment (P > 0.05).
Effects of increased nitrogen fertilizer on chlorophyll fluorescence of leaves under NaCl stress
NaCl significantly reduced Fv/Fm, ФPSII, and ETR of poplar leaves and (1 − qP)/NPQ increased 2.4 times compared with the CK (Fig. 3). Greater nitrogen levels increased the electron transport rate and photochemical efficiency of seedlings under NaCl stress, (1 − qP)/NPQ significantly decreased, photoinhibition was reduced, among which the Fv/Fm, ФPSII, and ETR of leaves in the NaCl + N treatment increased by 3.8% (P < 0.05), 96.1% (P > 0.05) and 98.2% (P > 0.05)respectively, compared with NaCl stress treatment.
Effects of increasing nitrogen fertilizer on light energy parameters in the PSII reaction center of leaves under NaCl stress
The YPSII and YNPQ of leaves under NaCl stress noticeably decreased, while the Yf,D and YNF increased, particularly YNF, which increased 3.5 times (Fig. 4). Increased nitrogen increased the quantum yield for the photochemical reaction absorbed by the PSII reaction center. The YPSII of leaves in the NaCl + N treatment increased by 108.9% compared with the NaCl treatment, while the Yf,D and YNF declined by 44.9 and 83.5%, respectively.
Effects of increased nitrogen on the standardized O–P curve of leaves under NaCl stress
The relative fluorescence intensity of point O was defined as zero while points P, J, and K were defined as 1. After the OJIP curves of leaves in different treatments were normalized, the relative variable fluorescence (VJ and VI) of points J and I on the standardized O–P curve increased more than those of the controls (CK) under NaCl stress (Fig. 5). The increasing rate of VJ was obviously greater than VI. The relative variable fluorescence (VK and VL) of points K and L on the standardized O–J and O–K curves also showed an increasing trend compared with CK (Fig. 5). However, increased nitrogen significantly alleviated the increase of relative variable fluorescence at each characteristic point of leaves under NaCl stress.
Effects of increased nitrogen fertilizer on V J, V I, V K, and V L of leaves under NaCl stress
Quantitative analysis of changes in VJ, VI, VK, and VL of poplar seedling leaves under NaCl stress showed that they increased by 20.7% (P < 0.05), 22.6% (P < 0.05), 20.7% (P < 0.05) and 22.6% (P < 0.05) compared with the CK, respectively (Fig. 6). However, increased nitrogen fertilizer alleviated the increasing rate of relative variable fluorescence of each characteristic point of leaves under NaCl stress, but the differences of each parameter between NaCl + N and NaCl treatments were insignificant.
Discussion
The damage of NaCl to plants not only included ion toxicity but the secondary action of saline ions also resulted in the inhibition of photosynthesis. NaCl reduced the stomatal conductance of poplar seedling leaves, and blocked the photosynthetic gas exchange which reduced Pn and Tr. However, the Ci showed an increasing trend, indicating that the decrease of Pn caused by NaCl was the result of the combined effects of stomatal and non-stomatal factors. In an adverse environment, due to the enhancement of starch hydrolysis and the accumulation of carbohydrates in mesophyll cells and a slowdown of output of photosynthetic products, photosynthesis was inhibited (Huppe and Turpin 1994). In this study, NaCl inhibited the photosynthetic carbon assimilation capacity of seedlings but increased nitrogen significantly increased photosynthetic capacity under NaCl stress. Since NO3−–N uses organic carbon formed by photosynthetic carbon assimilation (Huppe and Turpin 1994), and the light reduction of NO3− requires carbohydrates provided by photosynthesis. In NO3− reduction, a large amount of substances and energy were consumed and therefore there was a competitive relationship with carbon metabolism (Chen and Li 1984). Under NaCl stress, increased nitrogen may accelerate the transport and output of photosynthates and promote normal photosynthetic functioning in poplar seedling leaves.
With high levels of NaCl, the photochemical capacity of leaves declined and electron transport was blocked. This not only induced an insufficient assimilation power supply, it also accumulated an excessive number of electrons in the electron transport chain. If excessive electrons are not removed in time, they will attack the active oxygen species of the monovalent oxygen molecules in the thylakoid membrane, thus inhibiting photosynthesis (Reddy et al. 2004; Yamori et al. 2010; Zhang et al. 2017). This study demonstrated that under NaCl stress, the ETR (electron transport rate) of poplar seedlings decreased, excess light energy (1 − qP)/NPQ accumulated in leaves, and the Fv/Fm (maximum photochemical efficiency of PS II) declined, indicating that photoinhibition occurred in leaves under NaCl stress. Research has found that during nitrogen metabolism, the reduction of nitrite (NO2−) to ammonium had a similar effect to NADP+ (Nicotinamide adenine dinucleotide phosphate) which also uses the electrons transported by reduced ferredoxin (Fdred) (Robinson 1986). The energy allocated to nitrogen metabolism from Fdred can reach 20–25% and can be as high as 55% in certain plants (Tian et al. 2010). In this study, increased nitrogen increased the electron transport rate of leaves under NaCl stress and reduced the extent of photoinhibition. This may be that, under NaCl stress, greater nitrogen increased the receptive ability of photosynthetic electrons in the electron transport chain, reduced excess electrons, and promoted the normal functioning of electrons in the chain. As the light-independent reaction of leaves was inhibited under NaCl (Fig. 2), the ability to use assimilation power (ATP and NADPH) produced by photoreaction declined, resulting in an excess of assimilation power that not only produces feedback inhibition on the transport of photosynthetic electrons but is the main reason for the production of excess excitation energy in plant cells. Yang et al. (2012) found that NO3− will compete with CO2 for assimilation power in the reduction process, although the NO3− reduction process cannot directly use the NADPH produced by the light-dependent reaction, but rather the NADPH produced through the path of the “malic acid—oxaloacetic acid shuttle pathway” or “phosphoglycerate-dihydroxyacetone phosphate shuttle pathway”, the dicarboxylate shuttle was converted into the NADH specifically required by nitrate reductase, which can be used indirectly (Klepper et al. 1971). Therefore, under NaCl stress, increased nitrogen partially allocated the assimilation power of poplar leaves to the nitrogen metabolism process, increased the utilization ability of assimilation power, and reduced the potential pressure generated by excess excitation energy. The degree of photoinhibition of poplar seedlings under NaCl stress was thereby reduced and normal photosynthetic functioning and growth were promoted.
NaCl significantly changed the light energy allocation parameters absorbed by the PS II reaction center of poplar leaves; the YPSII (the quantum yield of the photochemical reaction) decreased significantly and the YNF increased noticeably, indicating that NaCl inhibited the activity of the PS II reaction center. To reduce the pressure on the center, the proportion of light energy absorbed by poplar leaves for non-photochemical reactions increased. The light energy allocation parameter Yf,D, includes two parts: quantum yield of fluorescence and quantum yield of thermal dissipation. Because the proportion of fluorescence emitted by plants is very small (Maxwell and Johnson 2000), Yf,D mainly reflects the proportion of heat dissipated in light energy allocation during the non-radiative energy dissipation. This study shows that the Yf,D (quantum yield of heat dissipation) of poplar leaves under NaCl stress increased while the YNPQ (quantum yield depending on the proton gradient on both sides of the thylakoid membrane and xanthophyll cycle) slightly decreased. Other studies have demonstrated that NPQ has a positive correlation with heat dissipation dependent on the xanthophyll cycle (Goss et al. 1998; Li et al. 2000). The results of this study indicate that poplar seedlings under NaCl stress reduce the generation of excess excitation energy by increasing the proportion of heat dissipation, while the mechanism of photosynthetic protection based on the xanthophyll cycle is limited. Increased nitrogen increased the proportion of light energy absorbed by leaves used for photochemical reactions under NaCl stress. This would increase the photosynthetic electron transport and maintain the supply of power required for carbon assimilation and thus improve light energy utilization. Increased nitrogen also reduced the Yf,D while YNPQ increased, indicating that increased nitrogen reduced the proportion of dissipation in the form of ineffective heat. The synergistic effect of heat dissipation and the xanthophyll cycle protect the normal physiological functioning of PS II so that the proportion of the inactive reaction center decreased under NaCl, providing a guarantee of normal functioning of photosynthesis.
To understand how increased nitrogen helped alleviate the extent of damage to the PS II reaction center in leaves under NaCl stress, OJIP curves were standardized by O–P, O–J, and O–K to specifically analyze the changes of VJ, VI, VK, and VL. The increase of VJ is a sign of blocked transport from QA to QB (Govindjee 1995; Zhang et al. 2016). The appearance of point I was related to the transport process from Q −A to QB (Strasser et al. 1995). The increase of VK was related to the damage of an oxygen-evolving complex (OEC) and the increase of VL was an important sign of thylakoid membrane dissociation (Strasser 1997). Under NaCl stress, VJ, VI, VK, and VL of poplar seedling leaves increased compared with the controls (CK), indicating that the stress not only inhibited the normal water splitting function of OEC on the PS II donor side but also reduced electron transport on the receptor side. In addition, NaCl resulted in changes to the thylakoid membranes of poplar leaves which might be related to the blocked electron transmission caused by the leakage of electrons in the electron transport chain, the leakage electron attacked the free oxygen molecules, which could produce superoxide anion and reactive oxygen. The increase of those reactive oxygen molecules led to the peroxidation of thylakoid membrane. (Reddy et al. 2004). Increased nitrogen reduced the variable fluorescence of each point in leaves, suggesting that nitrogen can promote photosynthetic electron transport of poplar leaves under stress, and play an important role in protecting the oxygen-evolving complex and alleviating peroxidation damage of thylakoid membranes.
Conclusions
Under NaCl stress, photosynthesis declined; the photochemical activity of the PS II reaction center decreased and the electron donor and receptor side of PS II were affected to different degrees. Increased nitrogen alleviated saline damage to poplar seedlings under NaCl stress, improved photosynthesis, reduced stomatal limitations, and increased CO2 utilization by mesophyll cells. In addition, under NaCl stress, increased nitrogen enhanced the functioning of the oxygen-evolving complex on the PS II electron donor side and the ability to accept electrons on the receptor side. Nitrogen increased the stability of the thylakoid membrane structure and alleviated the degree of damage to the PS II reaction center of poplar leaves.
References
Ali A, Tucker TC, Thompson TL, Salim M (2001) Effects of salinity and mixed ammonium and nitrate nutrition on the growth and nitrogen utilization of barley. J Agron Crop Sci 186:223–228
Ali S, Rizwan M, Qayyum MF, Ok YS, Ibrmahim M, Riaz M, Arif MS, Hafeez F, Al-webel MI, Shahzad AN (2017) Biochar soil amendment on alleviation of drought and salt stress in plants: a critical review. Environ Sci Pollut Res Int 24(14):12700–12712
Apse MP, Blumwald E (2007) Na+ transport in plants. FEBS Lett 581(12):2247–2254
Askari H, Edqvist J, Hajheidari M, Kafi M, Salekdeh GH (2006) Effects of salinity levels on proteome of Suaeda aegyptiaca leaves. Proteomics 6(8):2542–2554
Bhatnagar MP, Vadez V, Sharma KK (2008) Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep 27:411–424
Calderon JF, Louise EJ, Scow KM, Rolston DE (2000) Microbial response to simulated tillage in cultivated and uncultivated soils. Soil Biol Biochem 32(11):1547–1559
Chen JQ, Li MQ (1984) The relationship between nitrogen metabolism and photosynthesis in the leaves of higher plants. Plant Physiol Commun 1:1–8
Cheng YW, Qi YC, Zhu Q, Chen X, Wang N, Zhao X, Chen HY, Cui XJ, Xu LL, Zhang W (2009) New changes in the plasma-membrane-associated proteome of rice roots under salt stress. Proteomics 9(11):3100–3114
Dąbrowski P, Baczewska AH, Pawluśkiewicz B, Paunov M, Aleksandrov V, Goltsev V, Kalaji MH (2016) Prompt chlorophyll a, fluorescence as a rapid tool for diagnostic changes in PSII structure inhibited by salt stress in Perennial ryegrass. J Photochem Photobiol Biol 157(7):22–31
Foyer C, Ferrario-Mery S, Noctor G (2001) Interactions between carbon and nitrogen metabolism. In: Lea P, Morot-Gaudry JF (eds) Plant nitrogen. Springer, Berlin, pp 237–254
Gong B, Wen D, Vandenlangenberg K, Wei M, Wei M, Yang FJ, Shi QH, Wang XF (2013) Comparative effects of NaCl and NaHCO3, stress on photosynthetic parameters, nutrient metabolism, and the antioxidant system in tomato leaves. Sci Hortic 157(3):1–12
Goss R, Bǒhme K, Wihelm C (1998) The xanthophyll cycle of Mantoniella squamata converts violaxathin into antheraxanthin but not to zeaxanthin: consequences for the mechanism of enhanced non-photochemical energy dissipation. Planta 205:613–621
Govindjee (1995) Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol 22:131–160
Hendrickson L, Furbank RT, Chow WS (2004) A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth Res 82:73–81
Hu YB, Sun GY, Wang XC (2007) Induction characteristics and response of photosynthetic quantum conversion to changes in irradiance in mulberry plants. J Plant Physiol 164:959–968
Huppe HC, Turpin DH (1994) Intergration of carbon and nitrogen metabolism in plant and algal cells. Annu Rev Plant Physiol Plant Mol Biol 45:577–607
Klepper LA, Flesher D, Hageman RH (1971) Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves. Plant Physiol 48:580–590
Li HD, Gao HY (2007) Effects of different nitrogen application rate on allocation of photosynthetic electron flux in rumex K-1 leaves. J Plant Physiol Mol Biol 33(5):417–424
Li XP, Bjorkman O, Shi C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403:391–395
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-A practical guide. J Exp Bot 51(345):659–668
Mi GH, Chen JF, Chun L, Guo YF, Tian QY, Zhang FS (2007) Advances in study of factors affecting soil N mineralization in grassland ecosystems. Plant Nutr Fertil Sci 13(1):155–159
Mitsuya S, Takeoka Y, Miyake H (2000) Effects of sodium chloride on foliar ultrastructure of sweet potato (Ipomoea batatas Lam.) plantlets grown under light and dark conditions in vitro. J Plant Physiol 157(6):661–667
Munns R, James RA, Läuchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57(5):1025–1043
Nathawat NS, Kuhad MS, Goswami CL, Patel AL, Kumar R (2007) Interactive effect of N source on salinity on growth indices and ion content of Indian mustard. J Plant Nutr 30:569–598
Pompeiano A, Giannini V, Gaetani M, Vita F, Guglielminetti L, Bonari E, Volterrani M (2014) Response of warm-season grasses to N fertilization and salinity. Sci Hortic 177:92–98
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161(11):1189–1202
Robinson JM (1986) Carbon dioxide and nitrite photoassimilatory processes do not intercompete for reducing equivalents in spinach and soybean leaf chloroplasts. Plant Physiol 80:676–684
Shangguan ZP, Shao MA, Dyckmans J (2000) Effect of nitrogen nutrition and water deficit on net photosynthetic rate and chlorophyll fluorescence in winter wheat. J Plant Physiol 156(1):46–51
Skeffington MJS, Jeffrey DW (1988) Response of America maritime (Mill.)Willd and Plantago martima L. from an Irish salt marsh to nitrogen and salinity. New Phytol 110(3):399–408
Song J, Feng G, Tian CY, Zhang FS (2006) Osmotic adjustment traits of Suaeda physophora, Haloxylon ammodendron, and Haloxylon persicum, in field or controlled conditions. Plant Sci 170(1):113–119
Song Y, Zhang YX, Guo N, Sun GY (2013) Effects of lights intensity on chlorophyll fluorescence characteristics and energy allocation pathways in leaves of Populus simonii × P. nigra seedlings after chilling stress. J Anhui Agric Sci 41(10):4421–4423
Strasser BJ (1997) Donor side capacity of photosystem II probed by chlorophyll a fluorescence transients. Photosynth Res 52(2):147–155
Strasser RJ, Srivastava A, Govindjee (1995) Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol 61(1):32–42
Takahashi M, Shigeto J, Sakamoto A, Morikawa H (2017) Selective nitration of PsbO1, PsbO2, and PsbP1 decreases PSII oxygen evolution and photochemical efficiency in intact leaves of Arabidopsis. Plant Signal Behav 12(10):e1376157
Tian JC, Wang XC, Liu GT (2010) The coupling and regulation between photosynthesis and nitrogen, carbon metablolism in plant. Chin Bull Life Sci 13(4):145–147
Wang LY, Zhao KF (2004) Effect of NaCl stress on ion compartmentation, photosynthesis and growth of Salicornia bigelovii Torr. J Plant Physiol Mol Biol 30(1):94–98
Wang BS, Luttg U, Ratajczak R (2001) Effects of salt treatment and osmotic stress on V-ATPase and V-Pase in leaves of the halophyte Suaedasalsa. J Exp Bot 52(365):2355–2365
Wang CH, Xing XR, Han XG (2004) Advances in study of factors af fecting soil N mineralization in grassland ecosystems. Chin J Appl Ecol 15(11):2184–2188
Wang XY, Wei SS, Dong ST, Liu P, Zhang JW, Zhao B (2015) Regulation of nitrogen on protein expression of summer maize (Zea mays L.) leaves at filling stage. Sci Agric Sin 48(9):1727–1736
Wu H, Liu X, You L, Zhang LB, Zhou D, Feng JH, Zhao JM, Yu JB (2012) Effects of salinity on metabolic profiles, gene expressions, and antioxidant enzymes in Halophyte Suaeda salsa. J Plant Growth Regul 31(3):332–341
Xu N, Zhang HH, Gu SY, Li X, Zhu YW, Yang Y, Liu L, Zhang XL (2017) Effects of increased NO3 −–N application on PSII functionin leaves of Morus alba seedlings under Na2CO3 stress. Prata Cultural Sci 34(1):67–74
Yamori W, Noguchi KO, Hikosaka K, Terashima I (2010) Phenotypic plasticity in photosynthetic temperature acclimation among crop species with different cold tolerances. Plant Physiol 152:388–399
Yan SH, Ji J, Wang G (2006) Effects of salt stress on plants and the mechanism of salt tolerance. World Sci Technol Res Dev 28(4):70–76
Yang Y, Ma M, Zheng QS, Liu ZJ, Guo SW (2012) Response of canola seedlings to salt stress under different nitrogen forms. Plant Nutr Fertil Sci 18(5):1220–1227
Yuan JF, Tian CY, Feng G, Ma HY (2009) Effects of nitrate on the root growth and salt tolerance of Suaeda physophora seedlings under NaCl stress. Plant Nutr Fertil Sci 15(4):953–959
Zhang HH, Zhong HX, Wang JF, Sui X, Xu N, Gu SY (2016) Adaptive changes in chlorophyll content and photosynthetic features to low light in Physocarpus amurensis Maxim and Physocarpus opulifolius “Diabolo”. Peer J 4(3):e2125
Zhang HH, Xu N, Li X, GU SY (2017) Overexpression of 2-Cys Prx increased salt tolerance of photosystem II (PSII) in tobacco. Int J Agric Biol 19(4):735–745
Zhao N, Wang SJ, Ma XJ, Zhu HP, Sa G, Sun J, Li NF, Zhao CJ, Zhao R, Chen SL (2016) Extracellular ATP mediates cellular K+/Na+, homeostasis in two contrasting poplar species under NaCl stress. Trees 30(3):825–837
Zhou YH, Lam HM, Zhang JH (2007) Inhibition of photosynthesis and energy dissipation induced by water and high light stresses in rice. J Exp Bot 5(58):1207–1217
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6(2):66–71
Acknowledgements
The authors thank the Heilongjiang Academy of Agricultural Sciences for providing the seeds for this research.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Project Funding: The work was supported by the Fundamental Research Funds for the Central Universities (2572018BE05) and the National Natural Science Foundation of China (31500323; 31370426).
The online version is available at http://www.springerlink.com
Corresponding editor: Tao Xu.
Rights and permissions
About this article
Cite this article
Wang, H., Zhang, H., Liu, Y. et al. Increase of nitrogen to promote growth of poplar seedlings and enhance photosynthesis under NaCl stress. J. For. Res. 30, 1209–1219 (2019). https://doi.org/10.1007/s11676-018-0775-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-018-0775-6