Abstract
Tea tree oil is extracted from the leaves and twigs of Melaleuca alternifolia (Maiden & Betche) Cheel, and it is widely used in medicines, food preservatives, cosmetics and health care products. Traditional propagation of M. alternifolia from seeds does not necessarily transfer the desired characteristics from their mother trees, the seedlings are not uniform, and the multiplication rate from cuttings is relatively low. For these reasons, it is necessary to develop tissue culture techniques for this species. This study showed that an efficient explant initiation medium for M. alternifolia was MS 1/2 + BA 0.6 mg L−1 + NAA 0.1 mg L−1 + sucrose 30 g L−1, which yielded a 75.9 % initiation rate. An efficient multiplication medium was MS + BA 0.3 mg L−1 + NAA 0.15 mg L−1 + sucrose 30 g L−1, which yielded a 4.3 multiplication rate and 3.2 cm shoot length. The rooting medium was MS 1/2 + IBA 0.1–0.25 mg L−1 + sucrose 15 g L−1, which yielded a 100 % rooting rate, 2.94–3.32 roots per individual and 1.36–1.44 cm root length. Local red-core soil was suitable as a transplant medium, and yielded 98 % survival. This study improved the tissue culture technique for mass-propagation of M. alternifolia, enabling the production of high quality plants for market.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Melaleuca alternifolia Cheel, belonging to the family Myrtaceae, is a tree of 6 m height, and its origin is mainly in Australia. The leaves and twigs of M. alternifolia can be distilled for extraction of an essential oil called “tea tree oil”, which possesses anti-fungal and anti-bacterial properties (Buck et al. 1994; Banes-Marshall et al. 2001; Hammer et al. 2003; Bagg et al. 2006; Caldefie-Chézet et al. 2006; Carson et al. 2006; D’Arrigo et al. 2010; Tsao et al. 2010), anti-viral properties (Garozzo et al. 2009) and activity against cancer cells (Greay et al. 2010). For these reasons, tea tree oil is widely used in medicines, food preservatives, cosmetics and health care products. The traditional propagation for M. alternifolia by seeds does not allow complete inheritance of the desired characteristics of the mother trees and so the phenotypes of the progeny are not uniform (Chang and Huang 2003; Zhang and Xing 2003). The multiplication rate by cuttings is relatively low (Li et al. 2003; Guo 2007) and it is therefore necessary to develop optimized tissue culture techniques for M. alternifolia. Some researchers have studied tissue culture techniques for M. alternifolia, finding large differences in multiplication rates and rooting frequencies (Zhang et al. 2000; Wu et al. 2001; Zhou et al. 2001; Chang and Huang 2003; Kiong et al. 2007; Oliveira et al. 2010). They could not overcome the problem of low morphogenic capacity associated with shoot ageing (Wendling et al. 2014), even after 12 or more multiplication passages. In this study, the explants from selected trees of M. alternifolia were harvested for mass propagation by tissue culture, with the goal of producing high quality plants for markets.
Materials and methods
Sprouts were collected from the trunk base of elite individuals of M. alternifolia in Fujian, China. Apical buds and semi-lignified stems were used as explants.
Surface sterilization
The stems were initially washed under running tap water for 5–10 min, immersed in 70–75 % ethanol for 30 s under aseptic condition in a laminar flow cabinet, rinsed in sterilized water once, transferred to surface sterilization solution (100 mL of 0.1 % HgCl2 supplemented with 2 drops of Tween-20) for 12–15 min, and then rinsed in sterilized water four or five times. The stems were dissected to retain one node on each explant, each 1.5–2.0 cm in length, and were then transferred onto the explant initiation medium.
Culture conditions
The explants were initially incubated in darkness. The illumination intensity was then 1000–1500 lx for shoot multiplication and root induction, and 3000–6000 lx for plantlet hardening, for which the photoperiod was 12 h light/12 h darkness. All cultures were maintained at a room temperature of (24 ± 2) °C.
Experimental design
Explant initiation
The explant initiation medium comprised: (1) MS + BA 0.6 mg L−1 + NAA 0.1 mg L−1; or (2) MS 1/2 + BA 0.6 mg L−1 + NAA 0.1 mg L−1. All media contained sugar 30-g L−1 and carrageenan 6.0-g L−1 (produced in Quanzhou, Fujian, China), pH 6.0. There were 30 jars for each medium and the three replications within an experiment. One bud or shoot occupied one jar. The initiation rates were recorded after 30 days.
Shoot multiplication
The shoot multiplication medium comprised: (3) MS + BA 0.6 mg L−1 + NAA 0.1 mg L−1; (4) MS 1/2 + BA 0.6 mg L−1 + NAA 0.1 mg L−1; (5) MS + BA 0.3 mg L−1 + NAA 0.15 mg L−1; or (6) MS 1/2 + BA 0.3 mg L−1 + NAA 0.15 mg L−1. All media contained sugar 30 g L−1 and carrageenan 6.0 g L−1, pH 6.0. There were 30 jars (3 shoots per jar) for each medium and the three replications within an experiment. Multiplication rates (Sánchez and Vieitez 1991; Hung and Trueman 2011) and shoot height were recorded after 30 days.
Root induction
The rooting medium comprised: (7) MS 1/2 + IBA 0.1 mg L−1; (8) MS 1/2 + IBA 0.25 mg L−1; (9) MS 1/2 + IBA 0.5 mg L−1; or (10) MS 1/2 + IBA 1.0 mg L−1. All media contained sugar 15 g L−1 and carrageenan 7.0 g L−1, pH 6.0. There were 20 jars (3 shoots per jar) for each medium and the three replications within an experiment.
Plantlet hardening
After 20 days in rooting medium in the culture room, the plantlets within the bottles were transferred to a glasshouse. Rooting percentage, root number per rooted individual, root length and plantlet height were recorded after 15 days of hardening.
Plantlet transfer
The natural, local, red-core soil was used as the cultivation medium. The soil was put into plastic trays with drain holes, the trays were placed in the greenhouse, and the soil was sterilized with 0.03–0.05 % KMnO4. The hardened plantlets were rinsed under tap water to remove any remaining carrageenan. The plantlets were immersed in 1/1000 dilution of 70 % thiophanate methyl or 1/1000 dilution of 80 % Mancozeb solution for 15 min, then transplanted into the soil or medium and covered with a transparent plastic film to maintain high humidity. The plantlets were sprayed with water once daily. The plastic film was removed after 3 weeks and the spray frequency increased to 2–3 times per day.
Statistical analysis
Data were analyzed statistically with SPSS Statistics 17.0 software. Data were analyzed by analysis of variance (ANOVA) (for 3–6 means) or t test (for two means), with a post hoc Tukey’s test if the ANOVA was significant. Means are provided with standard errors, and means were considered significantly different at P < 0.05.
Results
Explant initiation medium
Shoots grew vigorously on medium No. 1, whereas shoots grew slowly without elongation on medium No. 2 (Table 1). Therefore, medium No. 1, MS + BA 0.6 mg L−1 + NAA 0.1 mg L−1, was selected for explant initiation for M. alternifolia.
Multiplication medium
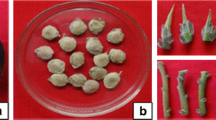
Shoot multiplication rate was highest, i.e. 8.9, in medium No. 3 (Table 2), but the shoots were short, 1.1 cm (Table 2). Multiplication rates on media Nos. 4 and 5 were moderate, i.e. 3.4 and 4.3, respectively. Shoots on medium No. 4 were short, 0.8 cm, with chlorotic leaves. The shoots on medium No. 5 were strong, with 3.2 cm length. Multiplication rate was lowest, 2.1, on medium No. 6, and the shoots had chlorotic leaves. Only the combination of multiplication rate and shoot length on medium No. 5 satisfied the demands for mass propagation for M. alternifolia. Therefore, medium No. 5, MS + BA 0.3 mg L−1 + NAA 0.15 mg L−1, was suitable for shoot multiplication of M. alternifolia (Fig. 1).
Rooting medium
No roots emerged on media Nos. 9 and 10, whereas 100 % rooting occurred on media Nos. 7 and 8 (Table 3). The root number per shoot on medium No. 7 was 3.3, which was significantly higher more than shoots on medium No. 8, 2.9 roots. However, the root length on medium No. 8 was 1.44 cm, significantly greater than that on medium No.7, 1.36 cm. The media Nos. 7 and 8 provided suitable root number and root length for mass plantlet production. They were, therefore, considered to be efficient rooting media for M. alternifolia (Figs. 2, 3).
Plantlet hardening and transfer
The survival rate of M. alternifolia was 98 % (Fig. 4).
Discussion
In this study, selected trees of M. alternifolia were used as explant sources. The optimal explant initiation medium was MS 1/2 + BA 0.6 mg L−1 + NAA 0.1 mg L−1 + sucrose 30 g L−1, which yielded an initiation rate of 75.9 %. Zhang et al. (2000) used aseptic seeds as explants. However, the phenotypic characteristics of seedlings are difficult to predict because the seeds do not necessarily inherit all of the desired characteristics of the mother tree.
The optimal multiplication medium for M. alternifolia was MS + 0.3 mg L−1 BA + 0.15 mg L−1 NAA + 30 g L−1 sucrose, which yielded a 4.3-fold multiplication rate and produced shoots of 3.2 cm height. Zhou et al. (2001) used the same basal MS medium supplemented with BA and NAA, but the concentrations of the plant growth regulators (PGRs) in that study were different. The multiplication rate (2.0) obtained by Zhou et al. (2001) was lower than that of our study, and it needed subculture in an additional medium to produce robust shoots. Oliveira et al. (2010) tested WPM and MS, but necrosis occurred on both of these media, especially WPM. They used liquid medium, but we did not attempt this method because shoots were curved in our previous experiment (data not shown), and this proved unsuitable for rooting.
The optimal rooting medium for M. alternifolia was 1/2 MS + 0.1–0.25 mg L−1 IBA + 15 g L−1 sucrose, which yielded 2.9–3.3 roots per rooted individual and roots that were 1.36–1.44 cm in length. In order to save the production cost, we can supplement 0.1 mg L−1 IBA in the rooting medium. Zhou et al. (2001) used the same basal medium, 1/2 MS supplemented with IBA, but the concentration of PGR was higher than that of the current study, and so would cost more in plant production. Oliveira et al. (2010) adopted MS without PGR as the rooting medium, obtaining 2.3 roots per individual, which is less than that of our study, 2.9–3.3 roots. A low concentration of IBA was beneficial to increase root numbers.
Kiong et al. (2007) studied callus induction of M. alternifolia. It is not desirable to regenerate plantlets from callus because it requires an additional medium to induce the callus and the technique can potentially allow the emergence of somaclonal variation (George 1993; Trueman and Richardson 2007). Wu et al. (2001) used 1-year-old seedlings as the explant source. It is difficult to determine whether the stock possessed desired characteristics (i.e. high content and high quality of essential oil) because the content of essential oil extracted from 1-year-old M. alternifolia is unstable and lower than that of mature individuals. A rooting rate of 93 % can be achieved by supplementing ABT (a rooting reagent produced by the China Academy of Forestry) at 2.0 mg L−1 and IBA at 0.4 mg L−1 in the medium (Wu et al. 2001), which is slightly lower than that of this study (100 %) in which IBA was added at 0.1–0.25 mg L−1.
The local red-core soil in this study provided suitable for plantlet hardening and yielded a 98 % survival rate. This soil was in expensive and easy to obtain, and it was also easy to disinfect. Cai et al. (2006) used M. alternifolia shoots as micro-cuttings and treated the shoots with 1000 mg kg−1 IBA, then inserted the cuttings into artificial medium containing perlite, red-core soil and peat (1:1:1), obtaining 92 % rooting. The disadvantages of micro-cutting propagation are that the technique is limited in spring, the artificial medium is expensive, and the survival rate is low compared with that in our study.
References
Bagg J, Jackson MS, Petrina Sweeney M, Ramage G, Davies AN (2006) Susceptibility to Melaleuca alternifolia (tea tree) oil of yeasts isolated from the mouths of patients with advanced cancer. Oral Oncol 42(5):487–492
Banes-Marshall L, Cawley P, Phillips CA (2001) In vitro activity of Melaleuca alternifolia (tea tree) oil against bacterial and Candida spp. isolates from clinical specimens. Br J Biomed Sci 58(3):139–145
Buck DS, Nidof DM, Addino JG (1994) Comparison of two topical preparations for the treatment of onychomycosis: Melaleuca alternifolia (tea tree) oil and clotrimazole. J Fam Pract 38(6):601–605
Cai YD, Zeng QR, Lin XP, He XJ, Zhang HT (2006) Study on cutting technology of Melaleuca alternifolia plantlet shoot via tissue culture. Guangdong For Sci Technol 22(3):32–35
Caldefie-Chézet F, Fusillier C, Jarde T, Laroye H, Damez M, Vasson MP, Guillot J (2006) Potential anti-inflammatory effects of Melaleuca alternifolia essential oil on human peripheral blood leukocytes. Phytother Res 20(5):364–370
Carson CF, Hammer KA, Riley TV (2006) Melaleuca alternifolia (Tea Tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev 19(1):50–62
Chang X, Huang Y (2003) Technique for raising seedlings of Melaleuca alternifolia. China For Sci Technol 17(6):34–36
D’Arrigo M, Ginestra G, Mandalari G, Furneri PM, Bisignano G (2010) Synergism and postantibiotic effect of tobramycin and Melaleuca alternifolia (tea tree) oil against Staphylococcus aureus and Escherichia coli. Phytomedicine 17(5):317–322
Garozzo A, Timpanaro R, Bisignano B, Furneri PM, Bisignano G, Castro A (2009) In vitro antiviral activity of Melaleuea alternifolia essential oil. Lett Appl Microbiol 49(6):806–808
George EF (1993) Plant propagation by tissue culture. Part 1. The technology. Exegetics Limited, England, p 574
Greay SJ, Ireland DJ, Kissick HT, Levy A, Beilharz MW, Riley TV, Carson CF (2010) Induction of necrosis and cell cycle arrest in murine cancer cell lines by Melaleuca alternifolia (tea tree) oil and terpinen-4-ol. Cancer Chemother Pharmacol 65(5):877–888
Guo Y (2007) Technique of cuttage seeding-raising of Melaleuca alternifolia. Prot For Sci Technol 3:26–27
Hammer KA, Dry L, Johnson M, Michalak EM, Carson CF, Riley TV (2003) Susceptibility of oral bacteria to Melaleuca alternifolia (tea tree) oil in vitro. Oral Microbiol Immunol 18(6):389–392
Hung CD, Trueman SJ (2011) In vitro propagation of the African mahogany Khaya senegalensis. New For 42(1):117–130
Kiong ALP, Dong H, Hussein S (2007) Callus induction from leaf explants of Melaleuca alternifolia. Int J Agric Res 2(3):227–237
Li X, Liang G, Hu Y, Huang S, Yang C (2003) Study on cutting propagation of Melaleuca alternifolia. Southwest Hortic 31(1):16–18
Oliveira Y, Pinto F, Silva SL, Guedes I, Biasi LA, Quoirin M (2010) An efficient protocol for micropropagation of Melaleuca alternifolia Cheel. Vitro Cell Dev Biol 46:192–197
Sánchez MC, Vieitez AM (1991) In vitro morphogenetic competence of basal sprouts and crown branches of mature chestnut. Tree Physiol 8(1):59–70
Trueman SJ, Richardson DM (2007) In vitro propagation of Corymbia torelliana × C. citriodora (Myrtaceae) via cytokinin-free node culture. Aust J Bot 55(4):471–481
Tsao N, Kuo CF, Lei HY, Lu SL, Huang KJ (2010) Inhibition of group A streptococcal infection by Melaleuca alternifolia (tea tree) oil concentrate in the murine model. J Appl Microbiol 108(3):936–944
Wendling I, Trueman SJ, Xavier A (2014) Maturation and related aspects in clonal forestry—part II: reinvigoration, rejuvenation and juvenility maintenance. New For 45(4):473–486
Wu Y, Wang Y, Cai Y, Huang J, Liang B (2001) In vitro propagation technique of Melaleuca alternifolia. Guangxi For Sci 30(2):68–69
Zhang M, Xing S (2003) The seedlings of Melaleuca alternifolia introduced and grown in the greenhouse. Pract For Technol 7:21–22
Zhang XC, Liang G, Yan Y, Yu Y, Yang G, Yang T (2000) Rapid propagation and polyploid induction in Melaleuca alternifolia. J Southwest Agri Univ 22(6):507–509
Zhou LH, Zhang HT, Gong Z, Zeng L, Zhou ZJ, Tan BX (2001) Propagation of Melaleuca alternifolia in industrial mode. Guangdong For Sci Technol l7(1):16–18
Author information
Authors and Affiliations
Corresponding author
Additional information
The online version is available at http://www.springerlink.com
Corresponding editor: Zhu Hong
Rights and permissions
About this article
Cite this article
Chen, B., Li, J., Zhang, J. et al. Improvement of the tissue culture technique for Melaleuca alternifolia . J. For. Res. 27, 1265–1269 (2016). https://doi.org/10.1007/s11676-016-0301-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-016-0301-7