Abstract
Hemidesmus indicus (L.) R. Br. ex Schult is commonly known as anantmul or Indian sarsaparilla. The roots of this plant, which display a wide range of medicinal, biological, and phytopharmaceutical properties, are used in the pharmaceutical and food industries. Conventionally, the plant is propagated by seed germination or vegetatively, but the efficacy of traditional methods has some limitations: plants derived from seed germination are prone to seed-borne diseases, or plantlet production using vegetative propagation is limited. In contrast, plant tissue culture allows for large-scale propagation and secondary metabolite production in vitro without sacrificing plants from their natural habitats. Many efforts have been made over 40 years of research to establish efficient micropropagation protocols to speed up cultivation of this plant, including callus-mediated in vitro propagation, somatic embryogenesis, and shoot multiplication using cotyledenory nodes, stem segments, shoot tips, and nodal explants. Among these explants, nodal explants are the most commonly used for H. indicus micropropagation. The application of adenine sulfate, citric acid, ascorbic acid, and arginine may be useful in preventing explant browning, premature leaf senescence, and shoot tip abscission during in vitro culture. This review provides insight into micropropagation, use of synthetic seeds for short-term germplasm preservation, and in vitro production of secondary metabolites such as 2-hydroxy-4-methoxybenzaldehyde, lupeol, vanillin, and rutin, from in vitro root and callus cultures. Furthermore, unexplored and possible innovative areas of research in Hemidesmus biotechnology are also discussed.
Key points
• Hemidesmus indicus has multiple therapeutic applications.
• H. indicus roots are used in confectionary and pharmacy.
• This review comprehensively assesses H. indicus tissue culture.
• Challenges and future research of H. indicus biotechnology are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hemidesmus indicus (L.) R. Br. ex Schult (syn. Periploca indica L.), a monophyletic plant (The Plant List 2020), is represented by 264 vernacular names in eight languages (FRLGHT 2020), but is most commonly known in English as Indian sarsaparilla. It is taxonomically distinct from “true” sarsaparilla Smilax febrifuga, in the Smilacaceae (Nandy et al. 2020). To date, H. indicus has been assigned to different families, including the Periplocaceae (Benerjee and Ganguly 2014), Asclepiadaceae (Efloras 2020), and Apocynaceae (The Plant List 2020), but has now been placed in the Apocynaceae following a phylogenetic reclassification (The Plant List 2020; Nandy et al. 2020). The growth form of the plant is a twining shrub with a woody rootstock, as a vine, and has opposite leaves and subsessile flowers in lateral cymes (Benerjee and Ganguly 2014; Efloras 2020). The plant has five partite calyces that have a glandular base, a greenish-purple corolla with five fleshy lobes that form below the sinus, free filaments, and granular pollen (Benerjee and Ganguly 2014; Efloras 2020).
The ethnobotanical, phytochemical, and pharmacological value of H. indicus has been extensively discussed by Nandy et al. (2020). This plant shows anticancer activity against colorectal cancer (Turrini et al. 2018), breast cancer (Suryavanshi et al. 2019), and leukemia (Turrini et al. 2019). An aromatic aldehyde (phenolic), 2-hydroxy-4-methoxybenzaldehyde (MBALD), accumulates in H. indicus roots, which are used as an ingredient in sherbet or flavored sweet drinks and bakery products (Patnaik and Debata 1996; Chakraborty et al. 2008; Fiori et al. 2014). MBALD, which is also the main constituent (97.9%) of the essential oil of H. indicus (Sreelekha et al. 2007), is produced by the shikimate pathway (Kundu et al. 2012). Due to the multipurpose nature of H. indicus, the National Medicinal Plant Board (NMPB) of India identified it as a “highly traded (500-1000 MT/year) medicinal plant” (NMPB 2020).
Indian sarsaparilla can be propagated by vegetative cuttings or seed germination, but the success of these methods is low, 60% and 46%, respectively, and seedlings produced using seed germination are prone to damping-off disease (Raghuramulu et al. 2005). Moreover, plants produced via vegetative propagation are prone to the transfer of diseases from stock plants to seedlings and are unsuitable for large-scale propagation. Hence, alternative methods to propagate plants would be useful for large-scale production not only of infection-free material and thus standard (cloned) biomass but also of source material for the production of secondary metabolites and essential oil. Since active ingredients are present in roots, field harvesting is essential. However, field harvesting is very difficult and laborious, so in vitro root culture offers an opportunity for the production of standardized quality root material for the pharmaceutical industry. In this review, we focus on the micropropagation, use of synthetic seeds, and in vitro production of secondary metabolites of Indian sarsaparilla.
Tissue culture and in vitro propagation
In vitro plant cell, tissue, and organ cultures are not only used for large-scale production of quality planting material, allowing for continuous supply to meet demand (Mukherjee et al. 2019; Wen et al. 2019; Teixeira da Silva et al. 2019; Mitra et al. 2020), but also useful for cryopreservation (Kulus and Zalewska 2014; Teixeira da Silva and Kulus 2014; Bi et al. 2017; Kulus 2019), secondary metabolite production (Isah et al. 2018; Teixeira da Silva et al. 2019), and genetic improvement (Chang et al. 2018; Sood et al. 2019). In vitro cultures derived from plant cells or tissues are a vital strategy for the large-scale production of pharmaceutically important plant secondary metabolites, which can reduce overexploitation of natural populations (Isah et al. 2018; Mukherjee et al. 2019; Mitra et al. 2020).
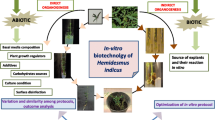
A complete tissue culture protocol for Indian sarsaparilla is provided in Fig. 1, while details of the choice of stock plant, explant collection, surface disinfection, and aseptic culture, as well as shoot induction and multiplication, rooting, and acclimatization, have been detailed in Tables 1 and 2.
Micropropagation of Hemidesmus indicus (Indian sarsaparilla) from nodal explants and subsequent acclimatization of plantlets. a Culture initiation from nodal explant on Murashige and Skoog (MS) medium with 8.88 μM BA, 8.81 μM AA, 135.5 μM AdS, 4.78 μM CA, and 4.36 μM Arg for 30 days. b, c Shoot multiplication during subculture on MS medium with 4.44 μM BA, 2.33 μM KIN, 0.57 μM IAA, 50 mg/l AA, 135.5 μM AdS, 4.78 μM CA, and 4.36 μM Arg for 30 days. d In vitro shoots rooted on ¼-strength MS medium with 14.66 μM IBA for 30 days. e Rooted plants in nursery poly-bags containing Soilrite®, manure, garden soil, and vermicompost (1:1:1:1, v/v) and maintained in a greenhouse for 6–7 weeks. f Hardened plantlets were transferred to pots containing soil for 3–4 weeks. g Acclimatized plantlets were transferred to the field (96% survival). Culture conditions were based on Shekhawat and Manokari (2016). Unpublished photographs. AA, ascorbic acid; AdS, adenine sulfate; Arg, arginine; BA, 6-benzyladenine; CA, citric acid; IAA, indole-3-acetic acid; KIN, kinetin
Explant selection for in vitro culture establishment
The physiological condition of the stock plant, the choice of explant, its size, position, or orientation may affect the outcome of in vitro propagation. Actively growing shoots from field-grown plants, which are likely to be the most responsive explant for in vitro conditions, were extensively used in several protocols for the tissue culture of Indian sarsaparilla (Table 2). However, only few reports are available on explants from seedlings raised in vitro (Raghuramulu et al. 2003; Saryam et al. 2012a, b; Purohit et al. 2014).
For Indian sarsaparilla, the easiest method is to use an explant with a predetermined meristem such as a shoot tip or node (Table 1). Sreekumar (1997) conducted a comparative study to select the optimal explant among roots, shoot tips, nodes, internodes, and leaves from 1-year-old field-grown plants and tested shoot regeneration on full-, half-, or quarter-strength Murashige and Skoog (1962) (MS) medium, B5 medium (Gamborg et al. 1968), Schenk and Hildebrandt (1972) (SH), or woody plant medium (WPM) (Lloyd and McCown 1980) supplemented with combinations of 2.22 μM 6-benzyladenine (BA) and 1.07 μM 1-naphthaleneacetic acid (NAA). Cultures were kept in the dark for 4 days, and then exposed to a 12-h photoperiod (50–60 μmol m−2 s−1). Sreekumar (1997) found that nodes were more effective for axillary shoot multiplication (92% of explants receptive, 9.37 shoots per node, approximately 2.8 cm long shoots) than other explant types when cultured on full-strength MS medium. In contrast, Sreekumar et al. (2000) reported best results for shoot multiplication (95% of explants receptive, 9.3 shoots per explant, 7.2 cm long shoot) on ½MS medium supplemented with 2.22 μM BA and 1.07 μM NAA. Misra et al. (2005), on the other hand, reported that nodes formed callus more effectively than leaves or roots from field-grown plants.
Even though nodes and shoot tips were the most frequently used explants for Indian sarsaparilla tissue culture (Table 1), stem segments (either nodes or internodes) (Heble and Chadha 1978; Sarasan and Nair 1991; Sarsan et al. 1994; Sreekumar 1997; Sreekumar et al. 2000), leaves (Sarasan and Nair 1991; Sarsan et al. 1994; Sreekumar 1997; Sreekumar et al. 2000; Misra et al. 2005; Ghatge and Dixit 2006; Ghatge 2007; Shanmugapriya and Sivakumar 2011; Pathak and Joshi 2017), roots from mature stock plants (Heble and Chadha 1978; Sreekumar 1997; Sreekumar et al. 2000; Misra et al. 2005), cotyledonary nodes from in vitro germinated seeds (Purohit et al. 2014), or root segments from seedlings raised in vitro (Raghuramulu et al. 2003) have also been employed. Sreekumar et al. (2000) noted that nodes collected from actively growing shoots on the second and third node (average of 9 shoots per node), counting from the shoot tip, were more responsive to axillary shoot multiplication than from nodes 4 to 8 on MS medium with 2.22 μM BA and 1.07 μM NAA. In contrast, Nagahatenna and Peiris (2007) found that the first and second nodes could not induce axillary shoots, the third node only produced callus at the base of the explant, while the fourth and fifth nodes induced the most axillary shoots (average of 2.5 shoots per node) on MS medium with 8.88 μM BA and 0.5 μM NAA.
Explant disinfection
Explant disinfection is one of the most essential steps of the tissue culture protocol because infection limits the success of all ensuing steps and practical applications (Teixeira da Silva et al. 2016). A summary of the disinfection protocols used for H. indicus in vitro regeneration is provided in Table 1. Ghatge (2007) tested mercuric chloride (HgCl2) for 1–7 min on leaf and nodal explants, finding that 0.1 mg l−1 HgCl2 for 5 min was effective, resulting in more than 80% aseptic cultures without any sign of browning of explants. A 10-fold higher concentration of HgCl2 and exposure for 2 min was sufficient to disinfect nodal segments (Sharma and Yelne 1995). When 0.1% HgCl2 for 1–5 min was used for shoot tips or 2 min for nodes, there was only 10–12% microbial contamination (Gopi 2014; Sindura 2014). However, none of these studies conducted statistical analyses, weakening the strength of their observed conclusions.
Light intensity and photoperiod
The source, spectrum, and intensity of light, as well as the photoperiod, are useful parameters to regulate plant growth in vitro (Batista et al. 2018). Despite this, no comparative studies on light conditions on tissue culture or secondary metabolite production are available for H. indicus, while many protocols provide poor or no details about the light source, photoperiod, or light intensity (Table 2). Future studies on H. indicus in vitro culture should optimize conditions such as the use of wide-spectrum light-emitting diodes (Miler et al. 2019) while also assessing the impact of culture vessel in photoautotrophic micropropagation (Xiao et al. 2011). Sreekumar (1997) was able to rapidly induce shoots from node, leaf, shoot tip, and root segments when inoculated in the dark for 4 days and then exposed to a 16-h photoperiod. Heble and Chadha (1978) were able to induce callus and shoot buds under continuous light, but plant production was poor.
Nutrient medium, carbon source, media additives, and plant growth regulators
The choice of nutrient medium, carbon source, media additives, and plant growth regulators (PGRs) all impact the success of in vitro growth and morphogenesis. Sreekumar (1997) and Ghatge (2007) tested various nutrient media with H. indicus, noting that the choice of nutrient medium affected the outcome of in vitro growth. Sreekumar (1997) found that MS medium was more responsive to shoot regeneration than other media, including B5 (Gamborg et al. 1968), Nitsch (Nitsch and Nitsch 1969), White (White 1934), SH (Schenk and Hildebrandt 1972), and WPM (Lloyd and McCown 1980). Similarly, Ghatge (2007) also found MS medium to be more responsive to shoot regeneration than B5 and Nitsch media (details in Table 2).
The carbon source, which provides additional carbohydrate and energy for in vitro explants that are not autotrophic, is a vital component of plant tissue culture media (Yaseen et al. 2013). The most frequently used carbon source for H. indicus in vitro culture (mainly shoot regeneration) is 3% sucrose (Table 1), but other concentrations of sucrose have also successfully been used, such as 2% (Patnaik and Debata 1996; Sreekumar 1997; Saha et al. 2003), 4% (Sreekumar 1997; Sreekumar et al. 1998), and 7.5% (Misra et al. 2003). Misra et al. (2005) used 4% sucrose for shoot induction from nodes and 3% sucrose for callus induction from leaves and roots. The development of photoautotrophic micropropagation will be useful for cost-effective and commercial-scale propagation of this plant (Xiao et al. 2011).
Similar to the vast majority of plants cultured in vitro, in H. indicus, the choice of PGR affects the outcome of regeneration. On MS medium supplemented with 13.32 μM BA, internode length and thickness of axillary shoots could be increased when the concentration of ammonium nitrate was reduced (Malathy and Pai 1998). Table 2 provides a detailed summary of how the choice of PGR affects organogenesis in H. indicus. Shekhawat and Manokari (2016) reported that in the most effective protocol for axillary shoot multiplication, about 272 shoots were obtained from a single node with a 98% survival of micropropagated plants (Table 2).
Misra et al. (2003) used 81.3 μM adenine sulfate (AdS) in shoot induction medium (SIM). Application of AdS prevented leaf abscission, callus formation, and accelerated shoot regeneration (Misra et al. 2003). Similarly, Nagahatenna and Peiris (2007) reported shoot tip abscision and premature leaf fall. Nagahatenna and Peiris (2007) used 81.3 μM AdS n in SIM, which was effective in the control of shoot tip abscission and premature leaf fall. In contrast, Patnaik and Debata (1996) also reported leaf abscission, but since there was no effect on plant growth, they did not use AdS or any other supplements. Saha et al. (2003) used 17.61 μM ascorbic acid (AA) and 14.61 μM glutamine in SIM while Shekhawat and Manokari (2016) applied 135.5 μM AdS, 8.81 μM AA, 4.78 μM citric acid, and 4.36 μM arginine in SIM, solving the problem of explant browning as well as shoot tip abscission and premature leaf fall. In all these cases, the objective was to reduce explant browning and oxidation.
In vitro shoot multiplication from a predetermined meristem (shoot tip or node)
In vitro shoot multiplication using a predetermined shoot meristem (apical or axillary bud) is the most popular and convenient method for micropropagation (Tables 1 and 2). The application of a predetermined meristem minimizes micropropagation steps and provides genetically uniform plants (Sandhu et al. 2018). There are only a few reports in which apical buds (shoot tips) were used for H. indicus micropropagation (Sarasan and Nair 1991; Sreekumar 1997; Sreekumar et al. 2000; Sudarmani and Hasina 2013; Gopi 2014; Sindura 2014). In most cases, axillary buds (nodal explants) were used for micropropagation (Patnaik and Debata 1996; Sreekumar 1997; Sreekumar et al. 2000; Misra et al. 2003; Saha et al. 2003; Nagahatenna and Peiris 2007, 2008; George 2009; Shanmugapriya and Sivakumar 2011; Saryam et al. 2012a, 2012b; Devi et al. 2014; Singh and Shalini 2015; Reddy et al. 2016; Shekhawat and Manokari 2016; Verma and Vashistha 2016; Pathak et al. 2017; Prashanti et al. 2017; Maity 2018b; Yadav et al. 2019).
Callus-mediated (indirect) regeneration and somatic embryogenesis
Callus-mediated regeneration has several advantages over direct regeneration from a predetermined meristem: callus can be utilized for investigating developmental biology (Ikeuchi et al. 2016) or somaclonal variation (Krishna et al. 2016), or utilized for cell suspension culture to produce secondary metabolites (Espinosa-Leal et al. 2018). There are few reports on callus-mediated regeneration for H. indicus (Table 2). Heble and Chadha (1978) made the first attempt to regenerate callus from shoot tips, leaves, and stems on Lin and Staba (1961) medium supplemented with 9 μM 2-4,dichlorophenoxy acetic acid (2,4-D) and 0.93 μM kinetin (KIN). When callus was transferred to Lin and Staba medium supplemented with 9.78 μM IBA, only stem-derived callus was able to regenerate adventitious shoots. Pathak and Joshi (2017) reported callus-mediated shoot regeneration from leaf explants when 20 μM BA and 1 μM IAA were added to MS medium. Yellowish nodular callus, which formed from nodes on MS medium containing 2.22 μM BA and 1.07 μM NAA (Maity 2018a), was able to produce shoots after transfer to MS medium with 0.88 μM BA.
Somatic embryos can be utilized for germplasm storage, micropropagation, or synthetic seed production. Only limited studies are available on somatic embryogenesis of H. indicus (Sarsan et al. 1994; Ghatge and Dixit 2006; Ghatge 2007; Nagahatenna and Peiris 2008; Cheruvathur et al. 2013), but several of these studies lacked sufficient histological or molecular evidence to support the unequivocal claim of somatic embryogenesis, while in most studies, acclimatization and survival of plantlets produced via somatic embryos was not quantified, except in the Cheruvathur et al. (2013) study. In the Cheruvathur et al. (2013) study, when callus was transferred to ½MS with 9.78 μM IBA, 92% of cultures produced about 32 somatic embryos per gram of callus, and upon transfer of these embryos to MS medium with 17.76 μM BA and 4.34 μM GA3, more than 90% of somatic embryos converted into plantlets.
Synthetic seeds
Production of synthetic seeds using alginate encapsulation is an efficient method of propagation and short- to mid-term storage (Sharma et al. 2013; Faisal and Alatar 2019; Qahtan et al. 2019). For H. indicus, there are only two reports on synthetic seed production (Cheruvathur et al. 2013; Yadav et al. 2019). Cheruvathur et al. (2013) used 4% sodium alginate and 100 μM CaCl2 to encapsulate somatic embryos, which were stored at 4 °C for 120 days, and the stored synthetic seeds show 100% plantlet conversion on MS medium supplemented with 9.3 μM KIN and 2.46 μM IBA. Yadav et al. (2019) used nodal cuttings with a single axillary bud encapsulated in 3% sodium alginate and 100 mM CaCl2 and stored at 4 °C for 60 days. Maximum regeneration frequency (84%) was observed on MS medium supplemented with 5.0 μM BA and 0.5 μM IBA up to the first week, but regeneration frequency decreased thereafter (Yadav et al. 2019).
Rooting and acclimatization
Rooting of in vitro raised shoots of H. indicus commonly takes place on full, ½, or ¼ MS nutrient medium, generally in the presence of IBA (Table 2). The induction of a profuse root system in vitro fortifies the chances of successful acclimatization and survival of plantlets when transferred ex vitro (Shekhawat and Manokari 2016). Despite the importance of the acclimatization step (Pospíšilová et al. 1999), parameters such as relative humidity, substrate choice, environmental conditions, treatment with antimicrobial agents, irrigation, light intensity, or relative humidity, which are some of the important factors that can determine the fate of acclimatized micropropagated plants, have not been reported for H. indicus. Misra et al. (2003) used ½MS with 9.78 μM IBA, 5.37 μM NAA, and 1.2 μM activated charcoal for in vitro rooting, yielding five roots. Saha et al. (2003) tested 14.61 μM glutamine along with 7.37 μ IBA in MS medium produced average 3.5 roots per shoot. In contrast, Shekhawat and Manokari (2016) reported 62 roots (in vitro rooting) from one shoot on ¼MS medium supplemented with 14.66 μM IBA, and about 45 roots per shoot (ex vitro rooting) when in vitro raised shoots were treated with 1954 μM IBA for 5 min. Although many reports describe the acclimatization step in a greenhouse, limited information is available about the survival and genetic fidelity of regenerants (Table 2). These crucial aspects need to be focused on in future research since they can affect the chemical composition of plants and thus influence the constituents of medicinally, pharmaceutically important compounds, or secondary metabolites (Shekhawat and Manokari 2016). Only a single study by Khan (2014) compared the anatomy of mother plants versus micropropagated plants, the latter forming more trichomes than the former. However, results were not quantified for cellular observations between stock plants and micropropagated plants. Hence, future histological studies on micropropagated plants can be useful, especially cell wall lignification and cuticle formation, for understanding the cellular basis of acclimatization. Saha et al. (2003) found a stable number of chromosomes (2n = 22) in micropropagated plants after acclimatization.
Phytochemical stability and in vitro production of secondary metabolites
Pathak et al. (2017) compared the chemical profile of in vitro–derived plants versus stock plants by high-performance thin-layer chromatography and quantified lupeol content. They found similar banding in plants derived from cytokinin-containing medium but variation in chemical constituents in shoots derived from auxin-supplemented medium. Highest lupeol content (0.187 mg g−1 dry weight (DW) was observed in in vitro shoots grown on MS medium supplemented with 10 μM BA and 5 μM KIN, equivalent to levels in stock plants (0.185 mg g−1 DW). Devi et al. (2014) conducted a phytochemical analysis of alkaloids, flavonoids, saponins, phenols, and tannins from stock plants followed by micropropagation, but a phytochemical analysis of micropropagated plants was not performed. Using gas chromatography, George (2009) found no phytochemical differences between micropropagated and stock H. indicus plants.
Heble and Chadha (1978) conducted pioneer studies in H. indicus on the production of secondary metabolites (cholesterol, campesterol, and sitosterol) from shoot tips, leaves, and stem-derived callus. Lupeol, vanillin, and rutin were reported from micropropagated plants (Misra et al. 2003), as well as from in vitro shoots and callus cultures (Misra et al. 2005). Secondary metabolite production, especially of MBALD, was reported by several researchers (Sreekumar 1997; Sreekumar et al. 1998, 2000; Gopi 2014; Sindura 2014). A higher concentration of MBALD (0.12% DW basis; ~ 2–3-fold more) was found in micropropagated plants than in stock plants (Sreekumar et al. 2000). Table 2 provides details of the culture conditions and medium composition of these studies. Ghatge (2007) reported a quantitative analysis of total phenolics, flavonoids, and alkaloids of callus from leaf and stock plant parts. The total phenolic, flavonoid, and alkaloid contents of leaves of stock plants (phenolics, 42.56 mg g−1 fresh weight (FW); flavonoids, 13.51 mg g−1 FW; alkaloids, 20.65 mg g−1 FW) were higher than callus culture (phenolics, 2.85 mg g−1 FW; flavonoids, 3.09 mg g−1 FW; alkaloids, 3.09 mg g−1 FW). Thus, optimization of the protocol for high-quality secondary metabolites from tissue culture is essential.
Conclusions and future perspectives
Most of the reports available for H. indicus are on in vitro shoot multiplication, except for a few studies on callus-mediated organogenesis, somatic embryogenesis, synthetic seed production, and MABLD production. Anatomical studies to confirm somatic embryogenesis would be useful for understanding the developmental biology of this medicinal plant. Physiological studies during in vitro culture and acclimatization are totally unexplored areas of research. The application of molecular markers, genetic engineering, cryoconservation for long-term preservation, temporary immersion systems, photoautotrophic systems, low cost systems of micropropagation, elicitation, and genetic engineering of in vitro cultures for the production of secondary metabolites are some areas of research that deserve a special focus to advance the biotechnology of this medicinal plant species.
Change history
22 August 2020
Following publication of the original article (Kher et al. 2020), the authors identified following mistake in the author affiliation.
References
Batista DS, Felipe SHS, Silva TD, de Castro KM, Mamedes-Rodrigues TC, Miranda NA, Ríos-Ríos AM, Faria DV, Fortini EA, Chagas K, Torres-Silva G, Xavier A, Arencibia AD, Otoni WC (2018) Light quality in plant tissue culture: does it matter? In Vitro. Cell Dev Biol Plant 54:195–215. https://doi.org/10.1007/s11627-018-9902-5
Benerjee A, Ganguly S (2014) Medicinal importance of Hemidesmus indicus: a review on its utilities from ancient Ayurveda to 20th century. Adv Biores 5:208–213. https://doi.org/10.15515/abr.0976
Beyl CA (2011) Getting started with tissue culture: media preparation, sterile technique, and laboratory equipment. In: Trigiano RN, Gray DJ (eds) Plant tissue culture, development, and biotechnology. CRC Press, Inc., Boca Raton, Florida, USA, pp 11–26. https://doi.org/10.1201/F9780203506561-7
Bi WL, Pan C, Hao XY, Cui ZH, Kher MM, Marković Z, Wang QC, Teixeira da Silva JA (2017) Cryopreservation of grapevine (Vitis spp.)—a review. In Vitro Cell Dev Biol Plant 53:449–460. https://doi.org/10.1007/s11627-017-9822-9
Chakraborty D, Sircar D, Mitra A (2008) Phenylalanine ammonia-lyase-mediated biosynthesis of 2-hydroxy-4-methoxybenzaldehyde in roots of Hemidesmus indicus. J Plant Physiol 165:1033–1040. https://doi.org/10.1016/j.jplph.2007.09.002
Chang S, Mahon EL, MacKay HA, Rottmann WH, Strauss SH, Pijut PM, Powell WA, Coffey V, Lu H, Mansfield SD, Jones TJ (2018) Genetic engineering of trees: progress and new horizons. In Vitro Cell Dev Biol Plant 54:341–376. https://doi.org/10.1007/s11627-018-9914-1
Cheruvathur MK, Najeeb N, Thomas TD (2013) In vitro propagation and conservation of Indian sarsaparilla, Hemidesmus indicus L. R. Br. through somatic embryogenesis and synthetic seed production. Acta Physiol Plant 35:771–779. https://doi.org/10.1007/s11738-012-1117-5
Devi B, Mohan C, Manjula P, Kiran KB, Naresh B, Prathibha DB (2014) Phytochemical and micropropagation studies in Hemidesmus indicus (L.) R. Br. J Indian Bot Soc 93:76–81
Efloras (2020) Hemidesmus indicus (L.) R. Br. http://www.efloras.org/florataxon.aspx?flora_id=5&taxon_id=115013. Accessed: 23 May, 2020
Espinosa-Leal CA, Puente-Garza CA, García-Lara S (2018) In vitro plant tissue culture: means for production of biological active compounds. Planta 248:1–18. https://doi.org/10.1007/s00425-018-2910-1
Faisal M, Alatar A (2019) Synthetic seeds: germplasm regeneration, preservation and prospects. Springer International Publishing, Cham. https://doi.org/10.1007/978-3-030-24631-0
Fiori J, Leoni A, Fimognari C, Turrini E, Hrelia P, Mandrone M, Iannello C, Antognoni F, Poli F, Gotti R (2014) Determination of phytomarkers in pharmaceutical preparations of Hemidesmus indicus roots by micellar electrokinetic chromatography and high-performance liquid chromatography–mass spectrometry. Anal Lett 47:2629–2642. https://doi.org/10.1080/00032719.2014.917423
FRLGHT (2020) Hemidesmus indicus (L.) Schult. In: FRLGHT, Indian Med. Plant Database. http://www.medicinalplants.in/searchpage/showdetails/xplant_id/e2d232e3dcd00751bdf511c1ac0d0195. Accessed: 23 May, 2020
Gamborg OL, Miller RAA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158. https://doi.org/10.1016/0014-4827(68)90403-5
George S (2009) PhD thesis “In vitro propagation, conservation and phytochemical studies of Decalepis habflltomi Wight & Arn., Hemidesmus indicus (L.) R. Br. and Utleria salicifolia Bedd. ex Hook. f.” Department of Biotechnology and Microbiology, Kannur University, Kerala, India, pp 1–189
Ghatge SR (2007) In vitro culture studies in medicinal plants viz. Hemidesmus indicus (L.) Schult and Rubia cordifolia L. PhD thesis, Department of Botany, Shivaji University, Kolhapur, Maharastra, India, pp 1–162
Ghatge S, Dixit GB (2006) Somatic embryogenesis and plant regeneration from leaf cultures of Hemidesmus indicus R. Br. a medicinal plant. In: International Symposium on Frontiers in Genetics and Biotechnology-Retrospect and Prospects, p 179
Ghatge SR, Kedage VV, Dixit GB (2008) Somatic embryogenesis and plant regeneration of a rare and multipurpose medicinal plant Hemidesmus indicus (L.) Schult. Plant Cell Biotechnol Mol Biol 9:71–74
Gopi S (2014) M.Sc. Thesis on “Induction and stablishmnt of transformed hairy root culturs of Sarsaparilla (Hemidesmus indicus L.) R. Br.” Departmnt of Plant Biotchnology, Faculty of Agriculture, Kerala Agriculture University, pp 1–78
Heble MR, Chadha MS (1978) Steroids in cultured tissues and mature plant of Hemidesmus indicus RBr. (Asclepiadiaceae). Zeit Pflanzenphysiol 89:401–406. https://doi.org/10.1016/S0044-328X(78)80036-1
Ikeuchi M, Ogawa Y, Iwase A, Sugimoto K (2016) Plant regeneration: cellular origins and molecular mechanisms. Development 143:1442–1451. https://doi.org/10.1242/dev.134668
Isah T, Umar S, Mujib A, Sharma MP, Rajasekharan PE, Zafar N, Frukh A (2018) Secondary metabolism of pharmaceuticals in the plant in vitro cultures: strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult 132:239–265. https://doi.org/10.1007/s11240-017-1332-2
Khan PA (2014) Tissue culture studies in Hemidesmus indicus (L.) R. Br. Golden Res Thoughts 4:1–6
Krishna H, Alizadeh M, Singh D, Singh U, Chauhan N, Eftekhari M, Sadh RK (2016) Somaclonal variations and their applications in horticultural crops improvement. 3. Biotech 6:1–18. https://doi.org/10.1007/s13205-016-0389-7
Kulus D (2019) Managing plant genetic resources using low and ultra-low temperature storage: a case study of tomato. Biodivers Conserv 28:1003–1027. https://doi.org/10.1007/s10531-019-01710-1
Kulus D, Zalewska M (2014) Cryopreservation as a tool used in long-term storage of ornamental species – a review. Sci Hortic 168:88–107. https://doi.org/10.1016/j.scienta.2014.01.014
Kundu A, Jawali N, Mitra A (2012) Shikimate pathway modulates the elicitor-stimulated accumulation of fragrant 2-hydroxy-4-methoxybenzaldehyde in Hemidesmus indicus roots. Plant Physiol Biochem 56:104–108. https://doi.org/10.1016/j.plaphy.2012.04.005
Lin ML, Staba EJ (1961) Peppermint and spearmint tissue cultures. I. Callus formation and submerged culture. Lloydia 24:139–145
Lloyd G, McCown B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Int Plant Propagators’ Soc Proc 30:421–427
Maity SK (2018a) A micropropagation technique of Hemidesmus indicus (Asclepiadaceae), a valuable medicinal plant. Flora Fauna 24:16–20
Maity SK (2018b) Rapid and large scale micropropagation technique of Hemidesmus indicus R. Br. (Asclepiadaceae) which is a valuable medicinal plant all over India. In: De D, Roy S, Bera GC (eds) Biotechnology and Nature. Santi Mudran, 32/3 Patuatola Lane, Kolkata-700009, pp 177–180
Malathy S, Pai JS (1998) In vitro propagation of Hemidesmus indicus. Fitoterapia 69:533–536
Miler N, Kulus D, Woźny A, Rymarz D, Hajzer M, Wierzbowski K, Nelke R, Szeffs L (2019) Application of wide-spectrum light-emitting diodes in micropropagation of popular ornamental plant species: a study on plant quality and cost reduction. In Vitro Cell Dev Biol Plant 55:99–108. https://doi.org/10.1007/s11627-018-9939-5
Misra N, Mehrotra S (2006) Effect of mutagens on production of secondary metabolites in callus culture of Indian Sarsaparilla (Hemidesmus indicus). Hortic Environ Biotechnol 47:23–27. https://doi.org/10.3923/jps.2008.146.156
Misra N, Misra P, Datta SK, Mehrotra S (2003) Improvement in clonal propagation of Hemidesmus indicus R. Br. through adenine sulphate. J Plant Biotechnol 5:239–244
Misra N, Misra P, Datta SK, Mehrotra S (2005) In vitro biosynthesis of antioxidants from Hemidesmus indicus R. Br. cultures. In Vitro Cell Dev Biol Plant 41:285–290. https://doi.org/10.1079/ivp2004627
Mitra M, Gantait S, Mandal N (2020) Coleus forskohlii: advancements and prospects of in vitro biotechnology. Appl Microbiol Biotechnol. (in press). https://doi.org/10.1007/s00253-020-10377-6
Mukherjee E, Gantait S, Kundu S, Sarkar S, Bhattacharyya S (2019) Biotechnological interventions on the genus Rauvolfia: recent trends and imminent prospects. Appl Microbiol Biotechnol 103:7325–7354. https://doi.org/10.1007/s00253-019-10035-6
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nagahatenna DSK, Peiris SE (2007) In vitro propagation of Hemidesmus indicus (L.) R . Br . (Iramusu) through nodal culture. Trop Agric Res 19:181–192
Nagahatenna DSK, Peiris SE (2008) Modification of plant architecture of Hemidesmus indicus (L.) R . Br . (Iramusu) by In vitro colchicine treatment. Trop Agric Res 20:234–242
Nandy S, Mukherjee A, Pandey DK, Ray P, Dey A (2020) Indian sarsaparilla (Hemidesmus indicus): recent progress in research on ethnobotany, phytochemistry and pharmacology. J Ethnopharmacol (in press) 112609. https://doi.org/10.1016/j.jep.2020.112609
Nitsch JP, Nitsch C (1969) Haploid plants from pollen grains. Science 163(3862):85–87. https://doi.org/10.1126/science.163.3862.85
NMPB (2020) Hemidesmus indicus (L.) R.Br. ex Schult. In: National Medicinal Plants Board, Ministry of AYUSH, Government of India. https://nmpb.nic.in/medicinal_list. Accessed: 23 May, 2020
Pathak AR, Joshi AG (2017) Indirect organogenesis from leaf explants of Hemidesmus indicus (L.) R. Br.: an important medicinal plant. Plant Biosyst 151:1–5. https://doi.org/10.1080/11263504.2015.1108938
Pathak AR, Joshi AG, Shrivastava N, Sharma P (2017) Regeneration and chemical profiling in Hemidesmus indicus (L.) R. Br. South African J Bot 113:413–420. https://doi.org/10.1016/j.sajb.2017.09.022
Patidar N (2017) Callusing and organogenesis in Shodhganga or Anantmoola (Hemidesmus indicus Linn.). M.Sc. Thesis, Departement of Plant Breeding and genetics, Rajmata Vijayaraje Scindia Krishi Vishwa Vidyalaya, Gwalior, pp 1–51
Patnaik J, Debata BK (1996) Micropropagation of Hemidesmus indicus (L.) R. Br. through axillary bud culture. Plant Cell Rep. 15:427–430
Pospíšilová J, Tichá I, Kadlechek P, Haisel D, Plzáková Š (1999) Acclimatization of micropropagated plants to ex vitro conditions. Biol Plant 42:481–497. https://doi.org/10.1023/A:1002688208758
Prashanti M, Kalpana K, Shivaprasad P, Singh S, Rojarani A, Reddy KJ (2017) Rapid in vitro propagation of medicinally important Hemidesmus indicus (L.) R. BR. through nodal explants. J Indian Bot Soc 96:76–81
Purohit P, Bais RT, Singh P, Khan S (2014) In vitro regeneration of Hemidesmus indicus L. R. Br an important endangered medicinal plant. UK J Pharm Biosci 2:25–31
Qahtan AA, Abdel-Salam EM, Alatar AA, Wang Q-C, Faisal M (2019) An introduction to synthetic seeds: production, techniques, and applications. In: Faisal M, Alatar AA (eds) Synthetic seeds: germplasm regeneration, preservation and prospects. Springer International Publishing, Cham, pp 1–20. https://doi.org/10.1007/978-3-030-24631-0_1
Raghuramulu D, Murthy KSR, Pullaiah T (2003) Regeneration of plants from root segments derived from aseptic seedlings of Hemidesmus indicus R.Br. Phytomorphology 53:293–298
Raghuramulu D, Murthy KSR, Pullaiah T (2005) Vegetative propagation of Hemidesmus indicus R.Br. by stem cuttings. Indian For 131:1505–1508
Reddy GS, Reddy YM, Saritha KV (2016) Effect of plant growth regulators on in vitro propagation of Hemidesmus indicus (L.) R. Br.- an important aromatic medicinal herb. Sci Spectr 1:366–370
Saha S, Mukhopadhyay MJ, Mukhopadhyay S (2003) In vitro clonal propagation through bud culture of Hemidesmus indicus (L) R Br: an important medicinal herb. J Plant Biochem Biotechnol 12:61–64. https://doi.org/10.1007/BF03263162
Sandhu M, Wani SH, Jiménez VM (2018) In vitro propagation of bamboo species through axillary shoot proliferation: a review. Plant Cell Tissue Organ Cult 132:27–53. https://doi.org/10.1007/s11240-017-1325-1
Sarasan V, Nair GM (1991) Tissue culture of medicinal plants: morphogenesis, direct regeneration and somatic embryogenesis. In: Prakash J, Pierik RLM (eds) Horticulture — new technologies and applications. Current Plant Science and Biotechnology in Agriculture, vol 12. Springer, Dordrecht, pp 237–240
Sarsan V, Soniya EV, Nair GM (1994) Regeneration of Indian sarsaparilla, Hemidesmus indicus R.Br., through organogenesis and somatic embryogenesis. Indian J Exp Biol 32:284–287
Saryam R, Seniya C, Khan S (2012a) In-vitro micropropagation of Hemidesmus indicus an important medicinal plant. Int J Compr Pharm 3:1–3
Saryam R, Seniya C, Khan S (2012b) In-vitro micropropagation of Hemidesmus indicus an important medicinal plant. J Pharm Res 5:5467–5469
Schenk RU, Hildebrandt AC (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot 50:199–204. https://doi.org/10.1139/b72-026
Shanmugapriya AK, Sivakumar T (2011) Regeneration of in-vitro plantlets in Hemidesmus indicus (L.) R. Br. through nodal and leaf explants. Int Multidiciplinary Res J 1:41–45
Sharma PC, Yelne MB (1995) Observations on in vitro propagation of Sariva (Hemidesmus indicus R. Br). Bull Medico-Ethano-Botanical Res 16:129–132
Sharma S, Shahzad A, Teixeira da Silva JA (2013) Synseed technology—a complete synthesis. Biotechnol Adv 31:186–207. https://doi.org/10.1016/j.biotechadv.2012.09.007
Shekhawat MS, Manokari M (2016) In vitro regeneration frequency, micro-morphological studies and ex vitro rooting of Hemidesmus indicus (L.) R. Br.: a multi-potent endangered climber. Indian J Plant Physiol 21:151–160. https://doi.org/10.1007/s40502-016-0216-5
Siddique NA, Bari MA (2006) Plant regeneration from axillary shoot segments derived callus in Hemidesmus indicus (L.) R. Br. (Anantamul) an endangered medicinal plant in Bangladesh. J Plant Sci 5:61–67
Siddique NA, Bari MA (2010) Plant regeneration from axillary shoot segments derived callus in Hemidesmus indicus (L.) R. Br. (Anantamul) an endangered medicinal plant in Bangladesh. J Plant Sci 1:42–48
Siddique NA, Bari MA, Khatun N, Rahman MH, Rahman MH, Huda S (2003) Plant regeneration from nodal segments derived callus in Hemidesmus indicus (L.) R. Br (Anantamul) an endangered medicinal plant in Bangladesh. J Biol Sci 3:1158–1163. https://doi.org/10.3923/jbs.2003.1158.1163
Sindura KP (2014) Establishment of in vitro root cultures of sarsaparilla (Hemidesmus indicus L.) R. Br. M.Sc. Thesis, Departmnt of Plant Biotchnology, Faculty of Agriculture, Kerala Agriculture University, pp 1–73
Singh K, Shalini AK (2015) Micropropagation and multiple shoot formation from nodal explants of Hemidesmus indicus (L.) R. Br. - a rare medicinal plant. Intrnational J Adv Res 3:725–728
Sood P, Singh RK, Prasad M (2019) Millets genetic engineering: the progress made and prospects for the future. Plant Cell Tissue Organ Cult 137:421–439. https://doi.org/10.1007/s11240-019-01587-6
Sreekumar S (1997) In vitro culture and secondary metabolite production in Hemidesmus indicus R . Br . PhD Thesis, Plant Biotechnology Division, Tropical Botanical Garden and Research Institute, Paldode, Thiruvanthapuram, Kerala, India, pp 1–180
Sreekumar S, Seeni S, Pushpangadan P (1998) Production of 2-hydroxy 4-methoxy benzaldehyde using root cultures of Hemidesmus indicus. Biotechnol Lett 20:631–635. https://doi.org/10.1023/A:1005354003727
Sreekumar S, Seeni S, Pushpangadan P (2000) Micropropagation of Hemidesmus indicus for cultivation and production of 2-hydroxy 4-methoxy benzaldehyde. Plant Cell Tissue Organ Cult 62:211–218. https://doi.org/10.1023/A:1006486817203
Sreelekha A, Jirovetz L, Shafi PM (2007) Comparative study of the essential oils from Hemidesmus indicus and Decalepis hamiltonii. Asian J Chem 19:4942–4944
Sudarmani P, Hasina M (2013) In vitro regeneration and sodium chloride salt tolerant Hemidesmus indicus (L.) R.Br. plant production. ANJAC Journal of Science 12:55–62
Suryavanshi S, Choudhari A, Raina P, Kaul-Ghanekar R (2019) A polyherbal formulation, HC9 regulated cell growth and expression of cell cycle and chromatin modulatory proteins in breast cancer cell lines. J Ethnopharmacol 242:112022. https://doi.org/10.1016/j.jep.2019.112022
Teixeira da Silva JA (2012a) Is BA (6-benzyladenine) BAP (6-benzylaminopurine)? Asian Aust J Plant Sci Biotechnol 6 (special issue 1):121–124
Teixeira da Silva JA (2012b) Callus, calluses or calli: multiple plurals? Asian Aust J Plant Sci Biotechnol 6(special issue 1):125–126
Teixeira da Silva JA, Kulus D (2014) Chrysanthemum biotechnology: discoveries from the recent literature. Folia Hortic 26:67–77. https://doi.org/10.2478/fhort-2014-0007
Teixeira da Silva JA, Kulus D, Zhang X, Zeng S-J, Ma G-H, Piqueras A (2016) Disinfection of explants for saffron (Crocus sativus L.) tissue culture. Env Exp Biol 14:183–198. https://doi.org/10.22364/eeb.14.25
Teixeira da Silva JA, Zeng S, Godoy-Hernández G, Rivera-Madrid R, Dobránszki J (2019) Bixa orellana L. (achiote) tissue culture: a review. In Vitro Cell Dev Biol Plant 55:231–241. https://doi.org/10.1007/s11627-019-09969-3
The Plant List (2020) Hemidesmus indicus (L.) R. Br. ex Schult. http://www.theplantlist.org/tpl1.1/record/tro-2604022. Accessed: 23 May, 2020
Turrini E, Catanzaro E, Muraro MG, Governa V, Trella E, Mele V, Calcabrini C, Morroni F, Sita G, Hrelia P, Tacchini M, Fimognari C (2018) Hemidesmus indicus induces immunogenic death in human colorectal cancer cells. Oncotarget 9:24443–24456. doi: 10.18632/oncotarget.25325
Turrini E, Catanzaro E, Ferruzzi L, Guerrini A, Tacchini M, Sacchetti G, Paganetto G, Maffei F, Pellicioni V, Poli F, Hrelia P, Mandrone M, Sestili P, Brigotti M, Fimognari C (2019) Hemidesmus indicus induces apoptosis via proteasome inhibition and generation of reactive oxygen species. Sci Rep 9:7199. https://doi.org/10.1038/s41598-019-43609-5
Verma S, Vashistha BD (2016) In vitro multiplication of Indian sarsaparilla, Hemidesmus indicus (L.) R. Br. – an important medicinal herb through shoot tip explants. Int J Innov Res Sci Eng Technol 5:1542–1548. https://doi.org/10.15680/IJIRSET.2016.0502072
Wen SS, Chen L, Tian RN (2019) Micropropagation of tree peony (Paeonia sect. Moutan): a review. Plant Cell Tiss Organ Cult 141:1–14. https://doi.org/10.1007/s11240-019-01747-8
White PR (1934) Potentially unlimited growth of excised tomato root tips in a liquid medium. Plant Physiol 9:585–600. https://doi.org/10.1104/pp.9.3.585
Xiao Y, Niu G, Kozai T (2011) Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ Cult 105:149–158. https://doi.org/10.1007/s11240-010-9863-9
Yadav V, Shahzad A, Ahmad Z, Sharma S, Parveen S (2019) Synthesis of nonembryonic synseeds in Hemidesmus indicus R. Br.: short term conservation, evaluation of phytochemicals and genetic fidelity of the regenerants. Plant Cell Tiss Organ Cult 138:363–376. https://doi.org/10.1007/s11240-019-01634-2
Yaseen M, Ahmad T, Sablok G, Standardi A, Hafiz IA (2013) Review: Role of carbon sources for in vitro plant growth and development. Mol Biol Rep 40:2837–2849. https://doi.org/10.1007/s11033-012-2299-z
Acknowledgments
The authors thank Professor T. Pullaiah (Department of Botany, Sir Krishnadevaraya University, Anantpur, AP, India), Dr. K. Sri Ramamurthy (Principal Scientist, Shivashakti Biotechnologies Ltd., Hyderabad, Telangana State, India), and Dr. Pratibha Mishra (Senior Principal Scientist, National Botanical Research Institute) for reprints.
Funding
Dr. Mahipal S. Shekhawat thanks the National Medicinal Plant Board, Ministry of AYUSH, Government of India (grant number NMPB/IFD/GIA/NR/PL/2018-19/187) for financial support.
Author information
Authors and Affiliations
Contributions
All authors have equal contribution in this review, and are responsible for the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kher, M.M., Shekhawat, M.S., Nataraj, M. et al. Indian sarsaparilla, Hemidesmus indicus (L.) R. Br. ex Schult: tissue culture studies. Appl Microbiol Biotechnol 104, 6463–6479 (2020). https://doi.org/10.1007/s00253-020-10714-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10714-9