Abstract
The present paper describes the role of process conditions in developing phase-pure α-Al2O3 coatings using solution precursor plasma spraying. Different precursor combinations were employed and two key parameters, namely plasma power and substrate pre-heat temperature, were varied. Detailed studies of in-flight formed particles and splat characteristics were found to correlate well with the Al2O3 coating characteristics. The Al2O3-forming precursor formulation was also found to be crucial in determining the phase constitution of the deposited coatings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thermally sprayed alumina coatings find wide-ranging industrial use in wear-resistant, dielectric, corrosion-resistant, and diffusion barrier applications (Ref 1, 2). However, most of these applications ideally demand a thermally stable α-Al2O3 phase, which is often not achieved through conventional thermal spray variants including plasma spraying, even if the starting powder feedstock comprises phase-pure α-Al2O3 alone (Ref 2-4). It is relevant to mention that conventional powder synthesis techniques, too, may not readily yield pure α-Al2O3 powders in a single step and necessitate process modifications, such as using high-calorific value fuels during combustion spray pyrolysis or post-calcination after synthesis (Ref 5). A recent report by the authors on the deposition of phase-pure, nanostructured, and thick α-Al2O3 coating in the as-deposited condition using SPPS has provided useful insights and demonstrated the possibility of achieving the above through thermal spray processing in a single step (Ref 6). This has encouraged the present detailed process parametric study to be carried out to understand the role of individual parameters in yielding the desired coating characteristics.
There has been considerable prior work on the unique properties of SPPS YSZ coatings resulting from novel microstructures achievable under certain controlled processing conditions (Ref 7-9). Recent studies from the authors’ laboratory have provided improved insight into how the microstructural characteristics of SPPS YSZ coatings can be manipulated by appropriately controlling the deposition parameters (Ref 10). It is pertinent to note that the SPPS coatings are influenced by a wide range of variables, comprising plasma spray (current, gas flow rates, nozzle type) and liquid injection parameters as well as other external parameters (Ref 7-11). Based on prior studies involving YSZ coatings, plasma power, substrate pre-heat temperature, and standoff distance have been found to be play a significant role (Ref 10). In addition, the precursor chemistry, concentration, and flow rate have also been shown to considerably influence coating properties as well as the resulting microstructural features (Ref 12, 13).
Based on the above studies on SPPS YSZ coatings, it is reasonable to presume that an appropriate combination of spray conditions would be the key to achieving the desired phase purity in SPPS alumina coatings. In this context, the starting precursor chemistry has also been reported to play a significant role in deciding the phase constitution, size, shape etc., of powders produced by conventional spray pyrolysis techniques (Ref 5, 14). Prior spray pyrolysis studies have shown that realization of phase-pure α-Al2O3 powder is possible, but only by adopting a post-heat treatment step (Ref 5, 14). The post-treatment step is presumably necessitated by the deficiency in thermal energy available to enable complete phase transition in a single step. Since the precursors can be exposed to significantly higher temperatures (~5000 °C) during SPPS compared to conventional spray pyrolysis, the possibility of formation of thermally stable α-Al2O3 phase in a single step is envisioned and has indeed been demonstrated by the authors (Ref 6).
Notwithstanding the above, a detailed study to understand process parameter impact on realizing phase-pure α-Al2O3 coatings by SPPS is lacking. The present study seeks to fill this gap by investigating aluminum acetate and aluminum nitrate as precursors. Such an understanding is crucial for depositing high-performance phase-pure α-Al2O3 coatings by the SPPS route and also, seeks to identify the right combination of process parameters and feedstock.
Experimental
Preliminary studies employing aluminum nitrate and aluminum acetate as precursors revealed that use of aluminum nitrate yielded coatings with substantial amount of γ-Al2O3 along with α-Al2O3, while use of aluminum acetate salt led to relatively lower γ-Al2O3 and higher α-Al2O3 phase content in the coatings. This was consistent with the fact that the carbonaceous organic precursor was expected to contribute additional thermal energy because of the associated exothermic reactions. This prompted the use of organic solvents like isopropanol and acetic acid, which are known to release substantial amount of heat energy during combustion (Ref 15), as additives in an effort to further promote α-Al2O3 formation. Initial trials by modification of the aluminum nitrate precursor with addition of as much as 20 wt.% isopropanol continued to yield coatings with substantially high γ-Al2O3 content, whereas addition of 5 wt.% acetic acid to aluminum acetate exhibited improved phase purity with only traces of γ-Al2O3 being noted. Based on the above observations, aluminum acetate mixed with 10 wt.% acetic acid and aluminum nitrate without any modification were specifically chosen as two extreme cases for a more detailed investigation to understand conditions favoring the formation of phase-pure α-Al2O3.
The precursors used were aluminum nitrate nonahydrate [Al(NO3)3·9H2O] and aluminum acetate [Al(CH3COO)3] for SPPS deposition of Al2O3 coatings. The respective aluminum salts were dissolved in demineralized water to prepare a 1 M solution and thoroughly mixed using a magnetic stirrer. Compared to the inorganic aluminum nitrate which readily dissolved in water, the organic aluminum acetate salt was stirred for about 4 h to form a clear transparent solution. Acetic acid was also added during the continuous stirring of aluminum acetate salt. SPPS coatings were deposited employing a 9 MB plasma spray system using a standard GP nozzle (Sulzer Metco AG, Switzerland) equipped with a precursor delivery unit (Model: SPS-II; Inframat Corporation, USA). Al2O3 coatings were deposited on sand-blasted SS304 specimens, while the splats were collected on mirror-polished SS304 specimens. Plasma power was varied between 35 and 49 kW, by changing plasma current while keeping the voltage constant. All other relevant parameters were kept constant, with the spray distance being chosen as 50 mm, based on preliminary trials conducted.
The process parameters employed to generate powders, splats, and coatings during the present study are summarized in Table 1. The above spray parameters were chosen after conducting some preliminary experiments using the conditions previously indicated by Gell et al. (Ref 16). The precursor injection parameters and spray conditions were kept constant for all the precursors unless otherwise specified and no attempt was made to vary injection parameters with spray condition either. However, it is to be acknowledged that that there could be an opportunity to further improve SPPS coating quality by optimizing the precursor injection variables for each spray condition and/or for a given precursor. The methodologies adopted for collection of particles formed mid-stream and splats have already been provided in an earlier study (Ref 12). The in-flight generated particles were collected in water rather than being allowed to impact a substrate and dried to enable their characterization. The splats and coatings were generated on substrates pre-heated to 400 and 500 °C, depending upon the type of precursor employed. The number of passes of the gun over the substrate was kept constant in case of all the coating specimens generated. Hence, the thickness of the deposited coating is directly indicative of the deposition rate. Substrate pre-heating to the desired temperature was carried out using the plasma spray torch with the precursor feeding turned off.
Thermogravimetric analysis of the precursor and the particles collected mid-stream was performed using a thermal analyzer (Model: STA 449F3, NETZSCH, Germany) under oxygen atmosphere up to 1200 °C at a heating rate of 10 °C per min. An x-ray diffraction system (Model: D8, Brukers AXS, Germany) was employed to identify the phase constitution of the collected particles as well as the coatings. The morphological features of the in-flight formed particles and splats, as well the cross-sectional microstructure of the coatings, were studied using a field emission scanning electron microscope (Model: S43000SE/N, Hitachi, Japan). Focused ion beam (FIB) milling studies were attempted to study the cross-sectional morphology of the in-flight formed Al2O3 particles. Transmission electron microscope (Model: 2000FX, FEI, Netherlands) was used to study the morphology and grain size of the Al2O3 particles.
Results and Discussion
Precursor Characteristics
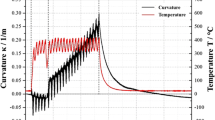
The starting precursors were subjected to TG/DTA analysis to identify the possible pathways for transition to a stable alumina phase. The results are depicted in Fig. 1. In case of both precursors, the eventual Al2O3 formation starts with removal of water and nitrate/acetate ligands that successively result in amorphous Al2O3, followed by transition γ, θ, δ-Al2O3 phases which, upon further heating, lead to formation of α-Al2O3 around 1200 °C (Ref 17, 18). In case of the aluminum nitrate precursor, the endothermic peaks around 120 °C correspond to dehydration of physisorbed water while another in the 240 to 300 °C range indicates removal of nitrate ligands and chemisorbed water. Around 400 °C, a small endothermic hump, possibly corresponding to dehydration of amorphous Al2O3, is seen. As the temperature reaches about 900 °C, the event corresponding to γ-Al2O3 formation is noted. As reported by Du et al. (Ref 18) for aluminum nitrate precursor, γ-Al2O3 transforms to α-Al2O3 phase upon heating to 1150 °C. However, continuous weight loss can be noted in case of aluminum nitrate precursor in Fig. 2 over the entire temperature range up to 1200 °C, which suggests that stable α-Al2O3 phase does not completely form within this temperature range.
The TG/DTA analysis of acetate-based precursor, too, exhibited water removal up to 250 °C, followed by exothermic decomposition of acetates and acetic acid from the salts as well as the solvent till about 400 °C. Minor exothermic peaks corresponding to possible transformation to amorphous phases could be observed in the 500 to 800 °C temperature range. At around 850 °C, events related to γ-Al2O3 formation are seen, as reported elsewhere in case of aluminum acetate (Ref 17). A minor peak at around 1055 °C can be related to the possible transformation to the desired α-Al2O3 phase, with the organic ligands and the additional presence of acetic acid plausibly accelerating the above transformation compared to aluminum nitrate precursor (Ref 17, 18) due to the substantial heat of combustion. It is also pertinent to note the relative mass loss getting stabilized in case of the modified aluminum acetate precursor in Fig. 1b, thereby implying formation of a more stable residue.
Analysis of Particles Generated in Flight
Particle Morphology
The investigation of in-flight formed particles collected before impact has been found to be educative to develop an understanding of the coating formation mechanism in SPPS (Ref 6, 12). The typical morphologies of particles collected mid-stream at different plasma power levels employing aluminum nitrate and modified aluminum acetate precursors are shown in Fig. 2 and 3, respectively. In case of aluminum nitrate precursor, it is amply evident from Fig. 2 that a certain minimum plasma power is essential before any noticeable pyrolysis of the precursor to form Al2O3 particles can occur in flight. At low plasma power levels of 35 kW, the specimens collected mid-stream appeared to be comprised almost entirely of the unpyrolyzed precursor and some hint of particle formation could be detected from the micrographs only at 42 kW of plasma power. With further increase in plasma power, the extent of pyrolysis was found to progressively increase and somewhat well-defined particles, although co-existing with unpyrolyzed matter, could be observed at 49 kW plasma power. In contrast to aluminum nitrate precursor, use of the modified aluminum acetate precursor appeared to certainly facilitate particle generation in flight at lower plasma power itself. Apparently, fragile and hollow particles could be noted at plasma powers as low as 35 kW, which further transformed into well-defined spherical particles at higher plasma power, as illustrated in Fig. 3. These observations are consistent with those made in case of in-flight collected YSZ particles in a separate study (Ref 12).
TEM analysis was also carried out on particles collected at two extreme power levels, namely 35 and 46 kW to gain further insight into particle formation. Results from the TEM studies are depicted in Fig. 4 and 5 for the aluminum nitrate and modified aluminum acetate precursors, respectively. At lower power levels using aluminum nitrate precursor, the in situ formed Al2O3 particles consisted of significant amount of amorphous features corresponding to aluminum hydroxides (Ref 18) along with some crystalline features as observed through the diffraction spots in Fig. 4b. With increase in plasma power, the extent of crystallinity improves as observed from the TEM micrograph in Fig. 4c and ring SAED pattern shown in Fig. 4d. In case of Al2O3 particles generated using modified aluminum acetate precursor, near-spherical particles surrounded by acicular/gel-like features were observed at low plasma power and were also found to be exhibit better crystallinity as observed from the diffraction pattern shown in Fig. 5a and b. At higher plasma power levels, the shell thickness was well-defined, as observed from the contrast difference in Fig. 5c, and the acicular amorphous features also diminished. Although, the relative number of diffraction spots was higher at 46 kW plasma power (Fig. 5d), some amorphous features could also be seen.
The inherent hollowness of the Al2O3 particles collected mid-stream using both precursors was apparent from the TEM analysis. Upon careful focused ion beam (FIB) milling at different time intervals, the shell thickness was observed to be approximately 100 nm as shown in Fig. 6. The shell thickness, which is likely to vary with process conditions, can not only govern the possible rupture of the particle upon continued high temperature exposure (manifesting in the form of continued mass loss during TG/DTA), but also influence the resulting coating microstructure by virtue of determining its behavior on impact with the substrate. At high power levels, the possibility of formation of hollow particles due to rapid surface solidification is particularly distinct as described by Jayanthi et al. (Ref 19). Similar hollow particles with relatively thick shells have been observed with mid-stream collected YSZ particles during SPPS (Ref 10). The different particle morphologies that can result during SPPS are summarized in Fig. 7, using micrographs from the exhaustive experiments conducted. It is clear from these micrographs that the morphologies can vary over a wide range, depending upon the choice of process parameters. For example, the irregularly shaped particle shown at the top of Fig. 7 corresponds to unpyrolyzed precursor, which typically results at conditions employing lower plasma power. Due to the relatively limited plasma energy available at low power levels (~35 kW), especially while using the aluminum nitrate precursor, it was found that the samples collected in flight comprised mainly unpyrolyzed mass.
Similarly, the hollow particles shown subsequently in Fig. 7 correspond to particles resulting from precursor droplets that experience high surface evaporation rates such as at high plasma power. Further, it is pertinent to note that the droplet trajectory within the plasma plume also greatly affects the pyrolysis action (Ref 8, 11) and can influence particle morphology significantly. Although it is desirable for the droplets to travel along the central axis through the core of the plasma, the precursor injection conditions, droplet size distribution etc. govern individual droplet trajectories and inevitably lead to considerable differences in plasma-droplet heat and momentum transfer thereby also resulting in distinct particle morphologies (Ref 8). It may also be mentioned that, as reported in case of spray pyrolysis, it is extremely challenging to achieve a coherent structure involving a continuous network of precipitated solids extending from periphery to center of the particle (Ref 20). Among the various oxide particles studied through spray pyrolysis, Al2O3 particles are reported to possess the highest void fraction (Ref 20).
Phase and TG/DTA Analysis
Phase analysis of the in flight formed particles collected at different plasma power levels using aluminum nitrate and modified aluminum acetate precursors are shown in Fig. 8a and b. As noted from the XRD patterns in Fig. 8a, significant amount of amorphous content could be observed at low plasma power levels along with some boehmite phase formation when the aluminum nitrate precursor is used. SEM observations also confirm irregularly shaped morphologies (top of Fig. 7) correspond to the amorphous content similar to those previously reported for aluminum hydroxides like boehmite and Al(OH)3 (Ref 21). With increase in plasma power, the γ-Al2O3 content was found to progressively increase with improvement in crystallinity also being noted. In case of modified aluminum acetate precursors, the crystalline γ-Al2O3 phase was found to be present even at low power and the crystallinity, too, was consistently better compared to particles collected at identical plasma power level using the aluminum nitrate precursor. The above findings are wholly consistent with the indications provided by the SEM micrographs (shown in Fig. 2, 3) and TEM observations (shown in Fig. 4, 5). Compared to conventional spray pyrolysis routes, which are known to exhibit more amorphous phases and only minor amounts of γ-Al2O3 in the as-prepared state (Ref 5, 14, 22), the presence of higher transition phases such as θ-Al2O3 and even traces of α-Al2O3, were observed. This was presumably due to the augmented heat input available from the organic ligands and solvent used. It is pertinent to note that the peak broadening observed in Fig. 8 is also a clear indication that the in flight collected particles comprise fine-grained features.
Based on the above observations, TG/DTA analysis was also carried out on “powder” specimens collected at varying plasma power levels to assess their thermal behavior and derive information regarding the presence of remnant unpyrolyzed matter. There was substantial mass loss in case of aluminum nitrate precursor, with the least mass loss of approximately 30% being noted at the highest power level of 49 kW. The excessive mass loss reveals the substantial presence of unpyrolyzed matter in the specimens subjected to TG/DTA analysis. The overall trend was similar in case of modified aluminum acetate precursor, although the extent of mass loss was lower. This is consistent with the various morphologies observed in Fig. 2 to 6 which, too, are suggestive of substantial unpyrolyzed mass being present.
In order to understand the changes in phase constitution that occur during the course of the TG/DTA analysis, XRD phase analysis was done at the conclusion of the TG/DTA test, similar to earlier studies (Ref 12). Irrespective of the precursor used, the in flight formed Al2O3 particles collected at low plasma power levels showed significant amount of transition phases, whereas particles collected at higher power levels revealed almost complete transformation to the α-Al2O3 phase, with only minor presence of δ*-Al2O3being noted. As reported elsewhere, the presence of δ*-Al2O3 can be attributed to processes involving kinetics rather than thermodynamics (Ref 22). Such transition phases surviving beyond 1200 °C are totally unexpected and their presence at the conclusion of TGA could be on account of the following:
-
1.
Exposure to high temperature was for too short a time to enable complete transformation to a stable phase
-
2.
Possible rupture of hollow particles during the tests to expose “new” precursor entrapped within
The above observations clearly suggest the promise of the SPPS route to provide a promising pathway for depositing predominantly α-Al2O3 coatings.
Splat Characteristics
Effect of Plasma Power
Typical splats of Al2O3 particles collected along the central axis of the plasma plume at different plasma power levels are shown in Fig. 9 and 10. As observed previously, the inadequate heat energy at low plasma power levels of 35 kW usually precludes complete in flight formation of crystalline particulates. The impact of remnant gel-like unpyrolyzed mass on to the substrate in such cases is not expected to lead to formation of well-defined splats, as normally observed in powder-based plasma spraying. This is evident from the splat morphologies at low plasma power as observed in Fig. 9a and 10a. Upon increase in plasma power level, molten splats can be observed along with significant amount of fine spherical particles (marked as ‘S’), which plausibly result from secondary pyrolysis of unpyrolyzed mass upon impacting the pre-heated substrate. Similar secondary pyrolysis on pre-heated substrates has also been observed and discussed by the authors previously during SPPS deposition of YSZ (Ref 23). Although fine-sized splats of less than 2-μm diameter have been previously reported during SPPS spraying (Ref 8-10, 12, 13), this study clearly reveals different splat morphologies resulting at varied plasma power levels.
It is pertinent to note that, for better inter-splat cohesion, disk-shaped flattened splats without any fragmentation are preferred (Ref 24). Compared to splats observed with aluminum nitrate precursor, the use of modified aluminum acetate precursor yielded splats with greater degree of melting even at low plasma power levels as shown in Fig. 10. It is relevant to mention that, apart from increasing specific enthalpy, the use of organic solvents has been reported to be advantageous in increasing kinetic energy of the particles in case of suspension plasma spraying (Ref 25). The reduced surface tension with use of organic solvents presumably assists defragmentation of droplets through swift vaporization of solvents and allows the plasma energy to be utilized for transformation and/or melting of the particles prior to impact with the substrate.
Effect of Substrate Pre-heating Temperature
Although the effect of substrate temperature on splat formation has been widely reported in conventional plasma spraying, very few such studies are available involving SPPS (Ref 8-10, 12, 24, 26). Previous studies with SPPS YSZ splats have suggested that a certain threshold substrate temperature above the precursor decomposition temperature may be desirable, if secondary pyrolysis of any unpyrolyzed precursor impacting the substrate is to be enabled (Ref 9, 23). Since the precursor decomposition temperature was noted to be 400 and 500 °C (Fig. 1) for aluminum nitrate and modified aluminum acetate precursors, respectively, typical splats were collected at these substrate temperatures for each precursor while maintaining 46 kW plasma power level. The morphologies of the ensuing splats are shown in Fig. 11.
The present study of splat morphologies clearly reveals that an increase in substrate pre-heat temperature facilitates formation of pan-cake type, disk-shaped splats. In contrast, the splats generated without pre-heat do not reveal any significant flattening. In cases of organic precursors, there is an improvement in the flattening of in flight formed Al2O3 particles even at room temperature, as shown in Fig. 11c, compared to aluminum nitrate precursor (Fig. 11a). As in case of in flight particle generation, a careful study of splat morphologies that can result during SPPS under varying processing conditions can also be very informative, Fig. 12 summarizes the splat morphologies obtained from the exhaustive experiments conducted.
Correlating Coating Characteristics with in-Flight Formed Particles and Splats
Coating Microstructure
It is clear from the above studies that there is a significant influence of precursor chemistry and plasma power on the in flight generation of particles. These parameters, along with substrate temperature, also have a considerable effect on splat formation and can be, consequently, expected to govern the coating characteristics. SPPS Al2O3 coatings were generated on stainless steel substrates and based on the splats studies and the precursor decomposition characteristics, the substrate pre-heat temperature was maintained at 400 °C for aluminum nitrate and 500 °C for modified aluminum acetate precursors. The cross-sectional microstructures of SPPS coatings deposited under different plasma power levels are shown in Fig. 13 and 14, for aluminum nitrate and aluminum acetate precursors, respectively. The relative coating thickness was almost double in case of modified aluminum acetate precursor, yielding a coating thickness of around 180 to 250 μm as against 90-120 μm in case of aluminum nitrate, for a constant precursor flow rate and an identical number of coating passes. The deposition rate was found to be 7 to 10 and 3 to 5 μm/pass for aluminum acetate and nitrate precursors, respectively. Due to higher cation concentration in aluminum acetate (22.5%) than aluminum nitrate (7.2%), the effective conversion to the oxide was higher in case of the former. High magnification observation revealed that Al2O3 coatings (shown in inset) generated from both precursors exhibited significant amount of porosity along with the distributed presence of unmelted, or partially melted, fine particles throughout the microstructure. There is an understandable influence of the above on the coating properties, resulting in substantial porosity and low microhardness values in the coated Al2O3 layer.
Phase Analysis
XRD analysis of the above coatings was also carried out and the results are shown in Fig. 15. Although the Al2O3 coatings exhibited minor differences in microstructure, there were distinct variations in phase constitution of the coatings formed using the two precursors. In case of aluminum nitrate precursor, substantial amounts of transition phases were noted along with traces of α-Al2O3 phase at plasma power below 46 kW (Fig. 15a). The presence of considerable amount of amorphous phases was also evident at lower power levels. The above trend significantly altered at a plasma power of 49 kW, wherein α-Al2O3 was found to be the major peak along with minor traces of transition aluminas as shown in Fig. 15a. The crystallite size measured using Scherrer’s formula indicated the grain size to be around 11-14 nm at lower plasma power levels up to 46 kW, which corresponded to the fine-grained structure stabilized in the γ-Al2O3 phase. At higher plasma power level of 49 kW, the crystallite size corresponding to α-Al2O3 phase was found to be around 50 nm.
In case of modified aluminum acetate precursor, α-Al2O3 phase was found to be the majority phase even at the lowest plasma power level of 35 kW, as shown in Fig. 15b. However, traces of transition phases were found at this low plasma power. The crystallite size was found to be around 43 to 50 nm at all plasma power levels. However, conventional way of producing α-Al2O3 phase involves precursor evaporation, alumina nucleation, coagulation, and partial sintering which inevitably be evidenced from the relatively bigger grains (Ref 27). It has been reported that, irrespective of the flame temperature, the lower free energy of nucleation usually favors formation of γ-Al2O3 phase at high solidification rates, as noted in conventional thermal spraying (Ref 4, 28, 29). In order to promote α-Al2O3 phase formation in the coatings, it has been suggested that conditions conducive to reducing the solidification rates be realized by maintaining a hot substrate, as high as 1450 °C, and also significant re-arrangement in the oxygen ions (Ref 28). In accordance with the above, the use of a modified aluminum acetate precursor (with an additional organic solvent), relatively higher degree of pre-heating of the substrate, and reduced spray distance, represent conditions that assist in augmenting the intrinsic heat flux on the substrate surface during deposition and thereby ensure formation of phase-pure α-Al2O3 coatings (Ref 28). A detailed plausible formation mechanism of phase-pure α-Al2O3 coatings has already been discussed elsewhere (Ref 6).
Conclusions
A detailed study was undertaken to develop an improved understanding of the role of process parameters associated with the SPPS process to achieve phase-pure α-Al2O3 coatings. This was achieved through analysis of the in flight generated particles during spraying and the splats formed upon particle impact with the substrate, under varying process conditions. Some of the important findings are summarized below:
-
The in flight generated particles collected mid-stream exhibit diverse characteristics, which are markedly influenced by the process conditions employed
-
The nature of splat formation also differs significantly based on the choice of processing conditions
-
Apart from plasma power, the chemistry of the starting precursors plays a key role in determining in flight particle generation and subsequent splat formation
-
The coating characteristics also correlate well with those of the in flight generated particles and the splats formed
The enhanced understanding of the role of process parameters has enabled single-step deposition of a phase pure, nanostructured, and thick α-Al2O3 coating, which is difficult to achieve by other thermal spray variants.
References
L. Pawlowski, The Relationship Between Structure and Dielectric Properties in Plasma-Sprayed Alumina Coatings, Surf. Coat. Technol., 1988, 35, p 285-298
C.-J. Li, G.-J. Yang, and A. Ohmori, Relationship Between Particle Erosion and Lamellar Microstructure for Plasma-Sprayed Alumina Coatings, Wear, 2006, 260(2), p 1166-1172
C.C. Stahr, S. Saaro, L.-M. Berger, J. Dubsky, K. Neufuss, and M. Herrmann, Dependence of the Stabilization of α-Alumina on the Spray Process, J. Therm. Spray Technol., 2007, 16(5-6), p 822-830
R. McPherson, On the Formation of Thermally Sprayed Alumina Coatings, J. Mater. Sci., 1980, 15, p 3141-3149
R.M. Laine, J.C. Marchal, H.P. Sun, and X.Q. Pan, Nano-α-Al2O3 by Liquid-Feed Flame Spray Pyrolysis, Nat. Mater., 2006, 5, p 710-712
G. Sivakumar, R.O. Dusane, and S.V. Joshi, A Novel Approach to Process Phase Pure α-Al2O3 Coatings by Solution Precursor Plasma Spraying, J. Eur. Cer. Soc., 2013, 33, p 2823-2829
P. Fauchais, R. Etchart-Salas, V. Rat, J.F. Coudert, N. Caron, and K. Wittmann-Teneze, Parameters Controlling Liquid Plasma Spraying: Solutions, Sols, or Suspensions, J. Therm. Spray Technol., 2008, 17(1), p 31-59
M. Gell, E.H. Jordan, M. Teicholz, B.M. Cetegen, N.P. Padture, L. Xie, D. Chen, X. Ma, and J. Roth, Thermal Barrier Coatings Made by the Solution Precursor Plasma Spray Process, J. Therm. Spray Technol., 2008, 17(1), p 124-135
T. Bhatia, A. Ozturk, L. Xie, E.H. Jordan, B.M. Cetegen, M. Gell, X. Ma, and N.P. Padture, Mechanisms of Ceramic Coating Deposition in Solution-Precursor Plasma Spray, J. Mater. Res., 2002, 17(9), p 2363-2372
G. Sivakumar, R.O. Dusane, and S.V. Joshi, Understanding the Formation of Vertical Cracks in Solution Precursor Plasma Sprayed Yttria-Stabilized-Zirconia Coatings, J. Am. Cer. Soc., 2014, 97(11), p 3396-3406
P. Fauchais, G. Montavon, R.S. Lima, and B.R. Marple, Engineering a New Class of Thermal Spray Nano-Based Microstructures from Agglomerated Nanostructured Particles, Suspensions and Solutions: An Invited Review, J. Phys. D Appl. Phys., 2011, 44, p 1-53
G. Sivakumar, R.O. Dusane, and S.V. Joshi, In situ Particle Generation and Splat Formation During Solution Precursor Plasma Spraying of Yttria-Stabilized Zirconia Coatings, J. Am. Cer. Soc., 2011, 94(12), p 4191-4199
D. Chen, E.H. Jordan, and M. Gell, Effect of Solution Concentration on Splat Formation and Coating Microstructure Using the Solution Precursor Plasma Spray Process, Surf. Coat. Technol., 2008, 202, p 2132-2138
B. Jun, S. Lee, and G.L. Messing, Synthesis of Nano-Scaled α-Al2O3 Particles by Combustion Spray Pyrolysis, Key Eng. Mater., 2006, 317-318, p 207-210
R.H. Perry and D.W. Green, Perry’s Chemical Engineers’ Handbook, McGraw-Hill, New York, 1997
M. Gell, X. Ma, E. Jordan, N.P. Padture, L. Xie, D. Xiao, and A. DeCarmine, Coatings, Materials, Articles and Methods of Making Thereof, US Pat. No. 7,563,503B2, 2009.
T. Sato, S. Ikoma, and F. Ozawa, Thermal Decomposition of Organic Basic Aluminium Salts-Formate and Acetate, Thermochim. Acta, 1984, 75, p 129-137
X. Du, Y. Wang, X. Su, and J. Li, Influences of pH Value on the Microstructure and Phase Transformation of Aluminum Hydroxide, Powder Technol., 2009, 192, p 40-46
G.V. Jayanthi, S.C. Zhang, and G.L. Messing, Modeling of Solid Particle Formation During Solution Aerosol Thermolysis: The Evaporation Stage, J. Aerosol Sci. Technol., 1993, 19(4), p 478-490
S. Jain, D.J. Skamser, and T.T. Kodas, Morphology of Single-Component Particles Produced by Spray Pyrolysis, J. Aerosol Sci. Technol., 1997, 27(5), p 575-590
F. Gao-feng, W. Jing, X. Bing, G. Hong, X. Xiu-lin, and C. Hao, Influence of Hydrothermal Temperature on Structure and Microstructure of Boehmite, Trans. Nonferrous Met. Soc. China, 2010, 20, p s221-s225
T. Hinklin, B. Toury, C. Gervais, F. Babonneau, J.J. Gislason, R.W. Morton, and R.M. Laine, Liquid-Feed Flame Spray Pyrolysis of Metalloorganic and Inorganic Alumina Sources in the Production of Nanoalumina Powders, Chem. Mater., 2004, 16, p 21-30
S.V. Joshi, G. Sivakumar, T. Raghuveer, and R.O. Dusane, Hybrid Plasma Sprayed Thermal Barrier Coatings Using Powder and Solution Precursor Feedstock, J. Therm. Spray Technol., 2014, 23(4), p 616-624
S. Sampath and X. Jiang, Splat Formation and Microstructure Development During Plasma Spraying: Deposition Temperature Effects, Mater. Sci. Eng. A, 2001, 304-306, p 144-150
P. Fauchais, V. Rat, C. Delbos, J.F. Coudert, T. Chartier, and L. Bianchi, Understanding of Suspension DC Plasma Spraying of Finely Structured Coatings for SOFC, IEEE Trans. Plasma Sci., 2005, 33(2), p 920-930
A. Elsebaei, J. Heberlein, M. Elshaer, and A. Farouk, Comparison of In-Flight Particle Properties, Splat Formation, and Coating Microstructure for Regular and Nano-YSZ Powders, J. Therm. Spray Technol., 2010, 19(1-2), p 2-10
A.I.Y. Tok, F.Y.C. Boey, and X.L. Zhao, Novel Synthesis of Al2O3 Nano-Particles by Flame Spray Pyrolysis, J. Mater. Process. Technol., 2006, 178, p 270-273
R. McPherson, Formation of Metastable Phases in Flame and Plasma Prepared Alumina Coatings, J. Mater. Sci., 1973, 8, p 851-858
G. Bolelli, J. Rauch, V. Cannillo, A. Killinger, L. Lusvarghi, and R. Gadow, Microstructural and Tribological Investigation of High-Velocity Suspension Flame Sprayed (HVSFS) Al2O3 Coatings, J. Therm. Spray Technol., 2009, 18(1), p 35-49
Acknowledgments
The authors would like to thank Director, ARCI for his kind permission to publish this work. The support extended by Dr. Y. Srinivasa Rao, Dr. Joydip Joardar, Mr. A. Sathyanarayana, Mr. GVR. Reddy, Mr. L. Venkatesh, and Mr. K. Ramesh Reddy during experimental studies and characterization is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivakumar, G., Ramakrishna, M., Dusane, R.O. et al. Effect of SPPS Process Parameters on In-Flight Particle Generation and Splat Formation to Achieve Pure α-Al2O3 Coatings. J Therm Spray Tech 24, 1221–1234 (2015). https://doi.org/10.1007/s11666-015-0284-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-015-0284-5