Abstract
The present work deals with the study of tribo-pair interaction in lubricated sliding contacts. By considering the environmental issues, the sunflower oil was extracted from the sunflower seeds and used as a base lubricant. The two types of the nanoadditives, i.e., CuO and CeO2, varying concentrations from 0.10 to 0.50% w/v were used to formulate the nanolubricants. The compatibility/synergism of the nanoadditives was examined from antifriction and antiwear behavior study with four-ball tester. Also, sunflower oil was modified by the chemical method to improve its fatty acid structure. A comparative tribological and compatibility study was also done in modified oil at similar concentration levels with both types of nanoparticles. The tribological test result exhibits 0.10% w/v concentration of the nanoadditive as optimum due to lowest wear scar and coefficient of friction. Higher concentration of the nanoparticles impaired the base oil performance. Different analytical tools were used to characterize the oil modification and worn surfaces. Moreover, the role of subsurface of the contacting material with the tribological performance has been reported.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Excessive wear and friction are the primary phenomena responsible for the failure of most of the mechanical systems in relative motion, by material and energy loss (Ref 1). Therefore, proper lubricant must be applied at the interface in appropriate quantity at the right time. At present, a variety of mineral and synthetic oils is available commercially to perform the intended function. Moreover, the adverse effect of these petroleum products on the environment from the point of extraction to dump forced the researchers to move toward green or biolubricants (Ref 2). Concern for environmental health has shown continuous extensive development in the field of biolubricant from last 2 decades for energy conservation and sustainability (Ref 3). The aims for such development attempted to enhance the tribo-performance of the biolubricant and improve the physical properties as well. It has been reported that the biolubricant possesses good lubricity, adherability, non-toxic and environmentally benign (Ref 3, 4). In spite of these, the biolubricants are not as good as mineral and synthetic oils concerning tribological properties and some physical properties like oxidation stability and low-temperature performance (Ref 4). But the use of additive and/or self-modification of oil may improve such shortcomings. The biolubricants consist of both saturated and unsaturated fatty acids; however, a degree of saturation varies distinctly for different biolubricants which affect the tribological properties. Reeves et al. (Ref 5) reported the role of fatty acid composition on tribo-performances. They observed that dominating oleic acid content in the vegetable oil influences the friction and wear resistance property of the base oils, i.e., higher oleic acid content lowers the wear and friction. The presence of more unsaturation, i.e., double bond (C=C), shows instability of biolubricants, which affects tribo-physical properties. Therefore, chemical modification of base oil by epoxidation process is one of the economical methods to improve the physical properties and stability of the biolubricants (Ref 6). In this process the double bond act as a reactive site and it is converted into single bond where it is attached to an oxygen atom and thus forms a ring-like structure (called oxirane ring) (Ref 7). It is also reported that the epoxidation process was influenced by the molar ratio of reactants, amount, and type of catalyst, stirring speed and reaction temperature (Ref 8). Moreover, these controlled parameters especially the catalyst type have a significant role in the percentage conversion/epoxy yield (Ref 8, 9).
The lubricant performance synergistically depends on the type of the base oil, additive and formulation techniques (Ref 10). Pure biolubricants are not capable of performing up to the level of mineral or synthetic oils. However, with the small amount of additive in the base oil could provide equivalent or better antiwear and extreme pressure properties (Ref 11). Conventional/commercial liquid additives such as ZDDP enhance the tribological performance by changing the base oil properties, which improve oil–metal interaction. But these additives have some environmental concern; therefore, in the recent research ultrafine particles (nano- and micro-sized) have got much attention as a lubricant additive. The various researchers explored different categories of nanoparticles such as metal oxides, graphite, MoS2, graphene oxide, polymer (PTFE), nanodiamonds, magnetic nanoflakes, talc, metals and nonmetals being used as a lubricant additive for tribological applications (Ref 12,13,14,15,16,17). It was also reported that, as an additive, the shape, size and concentration of the nanoparticles have a critical role in lubrication (Ref 16,17,18). Some researchers also worked on the synthesis of zero SAPS (sulfated ash, phosphorus, sulfur) additive for the lubricants (Ref 19).
In view of the above and the past works, the few question that arises for nanoparticle-based lubricants are: (1) Whether all the nanoparticles have good compatibility with the base oils to improve tribological performance?; (2) what is the effect of nanoparticles parameter such as concentration on tribological performance?; (3) is the affinity of different nanoparticles same for mineral and vegetable oils? The present work deals with the same queries and tried to observe the solution from experimental results. In the current work, sunflower oil (obtained by after seed extraction) was selected as the base oil and modified chemically by epoxidation process. CeO2 and CuO nanoparticles were used as an additive in pure sunflower oil (PSO) and epoxidized sunflower oil (ESO). It has been reported in the literature that CeO2 nanoparticles may have a toxic impact on the environment if concentration is higher than 2000 mg/L, and this may inhibit the plant growth (Ref 20). However, a low dose of CeO2 (less than 2000 mg/L) stimulates the plant growth (Ref 20, 21). The rare earth elements like CeO2, La2O3 and Sm2O3 are being used in the agriculture fertilizers in China, in a low dose, and this has helped in raising the plant products such as wheat, scallion and onion (Ref 22). Further, it has been reported that the nano-CeO2 has been used as a potential candidate for the treatment of Parkinson-like diseases, cancer treatment, neurodegenerative conditions, etc. (Ref 23,24,25). Considering the aforementioned issues, in the present study the concentration of CeO2 was used up to 500 mg/L (i.e., 0.5% w/v), and it is implied that a low concentration of CeO2 nanoparticle in the lubricant may not have any toxic effect on the environment. The nanoparticle concentration was varied as 0.10, 0.25 and 0.50% w/v to formulate the nanolubricants. The nanolubricant was tested using four-ball test rig, and the worn surfaces were studied with different analytical tools. Also, the possible reason for the improvement or deterioration in the tribological properties is discussed in the later part.
Experimental Section
Materials
The sunflower seeds were purchased from the local market and kept it in an oven for 60 min to remove the moisture. Thereafter, these moisture-free seeds were extracted with mechanical expeller and filtered it to obtain the sunflower oil. The reactant formic acid (98%) and hydrogen peroxide (30%) were procured from Avra Synthesis Private Limited, India, and Merck Limited, India, respectively. CeO2 (≤90 nm) and CuO (≤150 nm) nanoparticles were purchased from Sigma-Aldrich, USA.
Methodology
Chemical Modification of Oil
The epoxidation process was adopted to modify the fatty acid structure of the sunflower oil, mainly by removing double bond between the carbon atoms. The molar ratio of PSO/formic acid/hydrogen peroxide was taken as 1:2:20. Here formic acid acts as an oxygen carrier and hydrogen peroxide as the oxygen donor. Sulfuric acid was used as a catalyst. The measured volume of PSO was poured into the three-neck round-bottom flask through one of the inclined necks, while thermometer was fixed with another neck to measure the reaction temperature. At first, poured PSO along with formic acid was heated up to 65 °C and stirred vigorously at 600 rpm. Then, about 2 mL of sulfuric acid was added slowly to boost the reaction rate. The calculated amount of hydrogen peroxide was added to the heated solution dropwise for 30 min. The exothermic reaction takes place just after the addition of hydrogen peroxide, which sudden shoots the reaction temperature. This increased temperature was controlled with ice/water bath. A water-jacketed reflux condenser was used to liquefy the evaporated reactant from the solution. The reaction took place at about 6 h. After completion of the reaction, the heated solution was immediately extracted with diethyl ether and followed by washing with deionized water about 8-10 times to remove any free fatty acid using separating funnel. The obtained final oil was named as epoxidized sunflower oil, i.e., ESO.
Preparation of Nanoparticle-Based Lubricant and Their Tribological Test
Nanolubricant Formulation
The nanoparticles with a concentration of 0.10, 0.25 and 0.50% w/v were blended in PSO and ESO for 1 h using magnetic stirrer (IKA HS4, Germany). Two-hour ultrasonication of blended sample was done to keep the nanoparticles stable and uniformly dispersed.
Specimen
The AISI 52100 steel balls (0.95% C, 0.15% Si, 0.24% Mn, 0.02% P, 0.018% S, 1.3% Cr, and remaining Fe) of 12.7 mm diameter having hardness of 59 to 61 HRC, and surface roughness of (Rq) 27.3 nm were used in each test.
Tribo-Testing
A four-ball tester was employed to examine the tribological performance of the PSO and ESO with/without nanoadditives. One of the balls was fixed in the rotating spindle via collet, and rest of three fixed in the stationary ball pot. Before the test, all balls, ball pot, collet and other assembly were cleaned thoroughly with acetone to remove contamination on the surface. The test was carried out according to the ASTM D4172 standard at the load of 392 N, spindle speed of 1200 rpm, temperature 75 °C for 60 min run time. The wear of the material was recognized by mean wear scar diameter (WSD) of three lower balls. The coefficient of friction was recorded automatically with a computer attached to the test rig. All the tests were performed at least thrice for each composition to get repeatability in the test results.
Characterization of Oil and Worn Surfaces
Nuclear magnetic resonance (NMR; Bruker 500 AVANCE III HD, Rheinstetten, Germany) spectroscopy was used to confirm the formation of the epoxy group in ESO. For NMR, the test solution was prepared in CDCl3 (99.8% deuterated chloroform) because it is non-reactive and will not exchange its deuterium with protons of the oil molecules. Fourier transform infrared (FTIR; Bruker Alpha Eco-ATR, Germany) spectrometer elucidates the presence and absence of absorption peaks in the range of 4000-500 cm−1 wavenumber to confirm the epoxidation. The oxidation stability of the ESO was examined with iodine value test according to AOCS Cd 1-25. The lower iodine value referred to enhanced oxidation stability and vice versa.
The topography of the worn surface was studied with scanning electron microscope (SEM; EVO 18 Research, Zeiss, Germany). Energy-dispersive spectroscopy (EDS) coupled with SEM was used to analyze the element available on the worn track after the test run. The severity of the wear was ascertained by the roughness of worn surface (70 × 70 µm area) using scanning probe microscope (SPM; NTEGRA Prima, NT-MDT Spectrum Instruments, Russia) in semi-contact mode.
Results and Discussion
Oil Modification
The fatty acid chain of vegetable oil consist both saturation and unsaturation. The unsaturation, in the form of a double bond, limits its full use due to inferior oxidation and thermal stability. Therefore, both of these demerits can be overcome by epoxidation. Figure 1 shows proton NMR and FTIR spectra of PSO and ESO. Table 1 indicates the presence and absence of different peaks over the range of frequency, which represents CHn (n = 0-3) carbons allocation. Proton spectra at δ 5.2-5.3 ppm were due to unsaturated carbon, which found in the vegetable oils (Fig. 1a). However, this peak was almost vanished and formed a new peak at δ 3.1-3.2 ppm which was not present earlier as shown in Fig. 1(b). At this range of frequency, oxygen radical attached with –CH– protons to form epoxy or oxirane ring by eliminating double bonds (Ref 26). Hence, it can be inferred that PSO has been successfully converted into ESO. Further confirmation of epoxidation of PSO was carried out with FTIR. In Fig. 1(c), the absorption peak due to the presence of unsaturation was obtained at the wavenumbers of 3008 cm−1 due to C-H stretch and 1643 cm−1 due to C=C stretch. The disappearances of these peaks were observed in ESO (Fig. 1d) and appearance of a new peak at 835.7 cm−1. This is the evidence of successful epoxidation. Salimon et al. (Ref 27) also explained the similar results of ricinoleic acid indicating the formation of quaternary carbons of oxirane ring. Table 2 summarizes the different phenomena persisting over the range of frequencies. In the range of 3350-3420 cm−1, a broad peak of O-H was also observed which shows the presence of hydroxyl functional group in ESO. In iodine value test, PSO has shown the higher value of 125, which indicates a higher amount of unsaturation present in the pure base oil; however, after epoxidation, it reduced to 7. Low iodine value of vegetable oil has a high degree of saturation and vice versa. It can also be correlated with the thermo-oxidative stability of the sunflower oil, i.e., modified oil has good stability as compared to the unmodified oil.
Nanoadditive Dispersion
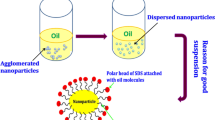
The suspension stability of the nanoparticles in lubricant reflects the tribological properties. It means uniform and longer suspension stability of the nanoparticles in the oil, which provides better tribo-performance. Figure 2 depicts the nanoadditive dispersion images. Here, 0 h (zero hours) represents the nanolubricant just after the stirring and ultrasonication. After 24 h, both the nanoparticles dispersed uniformly in oil. However, after 40 h slight settling of CuO particles was noticed, while CeO2 is in good suspension. After 50 and 78 h, CuO and CeO2 nanoparticles are entirely settle down at the bottom of lubricant jar due to acquired higher surface energy along with the influence of gravity.
Wear Behavior of Sliding Contacts
A series of wear test was performed to examine the tribological performance of different nanolubricants. The concentration was optimized by mean WSD. Figure 3 and Table 3 show the variation in WSD and mean wear volume (MWV) with different concentrations of the nanoadditives, respectively. Epoxidation of the PSO dramatically reduced the WSD due to removal/reduction in unsaturation and formation of an epoxy ring that has improved the oil–metal interaction. PSO and ESO, without additive, have shown the WSD of 873 and 690.6 µm, respectively. However, the addition of nanoparticles affects the wear performance significantly. It is clear that CuO nanoparticles neither show any positive improvement in WSD with PSO nor with ESO. It has increased the wear in both base oils at all the concentrations (Fig. 3a). Moreover, minimal reduction was observed for PSO with 0.5% CuO, but not so significant to consider it as optimum for antiwear. On the contrary, the CeO2 nanoadditive has shown benchmarking performance with small concentration. For both the base oils, WSD has increased gradually with increase in the concentration. The CeO2 concentration of 0.1% w/v is considered as optimum due to lowest WSD. It can be speculated that the sufficient number of particles may be available between the mating surfaces at the concentration of 0.1% w/v to reduce asperity–asperity contact, thus reducing the real area of contact (Ref 28). Beyond this optimum concentration, nanoparticles are detrimental to the antiwear property. The antiwear mechanisms are discussed in detail in the later part. The MWV for different compositions is depicted in Fig. 3(b). The MWV is calculated with Eq 1. Equation 2-5 are the supporting relationships to estimate MWV.
where mean WSD
r1, r2, ν1, ν2 and E1, E2: radius, Poisson’s ratio and Young’s modulus of top and bottom balls.
The calculated MWV as presented in Table 3 shows good agreement with a trend of WSD variation. The minimum MWV was obtained at the optimum concentration of nanoadditives.
Friction Behavior of Sliding Contacts
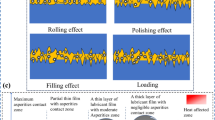
Figure 4 represents friction variation for different nanoparticle concentrations. Without nanoadditive, the COF for ESO was reduced by almost 50% in comparison with PSO (Fig. 4a). It may be because of increased oil–metal interaction due to the presence of an epoxy ring, which forms a thicker adsorbed layer on the mating surfaces. However, with additives, the nanolubricant behaves distinctly at different concentrations. At 0.1% w/v concentration, COF continuously decreases till the end of the test for all compositions. On the contrary, at 0.25 and 0.5% w/v concentration, friction shows either constant or increasing trend at the end of the test (Fig. 4b and c). It was observed that CeO2 with ESO reduced the friction for all concentrations up to the asymptotic value. In the case of CuO admixtures, 0.1% w/v was optimum with PSO (lowest COF), whereas there was no positive result with ESO at either concentration. Moreover, CeO2 with PSO and ESO has shown significant improvement at lower concentrations, which gradually increased with concentration. Therefore, in the case of CeO2 admixture, 0.1% w/v was optimum concentration. The friction improvement/deterioration is shown in Fig. 5. The top surface of the material, generally an amorphous and crystalline structure which is called as Beilby layer (Ref 29). Under high contact stress of 3.4 GPa (Ref 17), this layer begins to fracture and generate tiny secondary particles. The oil molecules penetrated deeply by crossing the fractured top layer to interact with compound oxide and a deformed layer of the ball material and form a strong adhesive adsorbed layer. This layer has quite good strength than a nearby ordered layer, to separate the asperities. With lower concentrations of 0.1% w/v, these tiny particles support the nanoadditives and fill the surface dimples and valleys to make the interface smoother. These nanosized particles begin to roll and slide over the surface by keeping the counter surface away; thus, reduction in the friction was observed. However, at higher concentrations, the nanoparticles come closer to each other and tend to agglomerate. These nanoparticles begin to engrave the compound oxide layer and deformed layer by shattering the asperities; thus, this has increased the friction.
Figure 6 and Table 4 illustrate the interfacial shear stress (product of friction coefficient and flow stress of material) for all nanolubricant compositions and corresponding values, respectively. PSO shows the higher interfacial stress of 127.7 MPa compared to ESO (i.e., 59.1 MPa). For nanoadditive-based oils, the variation of the interfacial stress is similar to the friction behavior. This is because, the shear stress at the interface was derived with the help of coefficient of friction (COF), and it is in direct proportion.
Worn Surface Study
SEM micrographs of worn surfaces lubricated with different nanolubricants at optimum concentration are depicted in Fig. 7. The worn surface lubricated with PSO and ESO shows deep furrows and scratches as shown in Fig. 7(a) and (b). ESO shows good metal–oil molecule interaction because of the presence of oxirane rings due to chemical modification. Also, a transfer film was observed in the case of ESO (Fig. 7b), probably because of contact fatigue and adhesive fatigue (Ref 30). It is the indication of a close contact, i.e., boundary lubrication situation (Ref 31). With the addition of small amount of nanoparticles in the oil, the surfaces separated to a great extent. Therefore, smoother worn surface has been observed in comparison with base oils (Fig. 7c-f). The CuO admixtures at optimum concentration show shallow furrows and microgrooves (Fig. 7c and d). However, in Fig. 7(e) and (f), the worn surface lubricated with CeO2 admixtures shows smoother and intermittent scratch mark because of abrasion by hard CeO2 nanoparticles. Wäsche et al. (Ref 32) reported that in situ secondary particles generated due to micro-shearing of the upper surface of nanoparticles during sliding. These particles are tiny as compared to the nanoparticles and help in the mending of the surfaces along with nanoparticles to avoid asperity–asperity contact, thus improving antiwear performance. The SEM results also confirm that the CeO2 nanoparticles have good compatibility with both PSO and ESO than CuO nanoparticles concerning antiwear.
Elemental analysis of the rubbed surface was carried out with EDS coupled with SEM in Fig. 8. The Cu and Ce traces are obtained on the worn track in few samples, which have been transferred by CuO and CeO2 nanoparticles at the interface during sliding. The adsorbed film keeps the mating surface away, and nanoparticles fill the surface pits and dimples to act as self-repair agent, thus improving the tribo-performance. The presence of oxygen on the worn surface may be due to the traces of additive or epoxy product.
Figure 9 represents SPM roughness images of the worn surfaces lubricated with nanolubricant at an optimum concentration. It is observed that PSO and ESO, without the additive, show a higher value of r.m.s. line roughness (Rq), i.e., 260.8 and 466 nm, respectively. However, for both the nanoadditives at 0.1% w/v, Rq was reduced significantly. The roughness with CeO2 nanoadditive with PSO and ESO shows good agreement with the SEM results. SPM result also confirms that at lower concentrations, CeO2 has good compatibility with PSO and ESO. At 0.1% w/v concentration, Rq value for PSO with CeO2 was 70.9 nm and ESO with CeO2 show 60.1 nm. It is almost fourfold and sevenfold less as compared to PSO and ESO, respectively.
Table 5 summarizes the compatibility of the nanoparticles with PSO and ESO based on the test results. Both improved and impaired tribological properties are observed with the nanoadditive-based lubricants at the sliding interface. In the present study, the tribo-test results exhibited the good compatibility of the CeO2 nanoadditive with both the PSO and ESO; however, CuO nanoadditives were incompatible with either pure or modified sunflower oil. It is speculated that size of the nanoparticle reflects on the lubricant performance. The average size of the CuO nanoparticles is more than 1.5 times than that of CeO2 nanoparticles. Therefore, CuO nanoparticle tends to agglomerate and form lump between the tribo-pairs due to gravity during the test run. However, the suspension stability of the CeO2 nanoparticles (due to comparable smaller size) is better, and it has improved the tribo-pair interaction.
Proposed Antiwear Mechanism and Model to Improve Tribo-Performance
It is known that no surface is perfectly smooth. It contains numerous asperities, dimples and valleys. In the case of close dynamic contacts (boundary lubrication), the protecting film formed by the lubricant becomes thinner which induces asperity–asperity collision. In such situations, nanoadditive may assist the lubricant in carrying the partial load and also separate the mating surfaces and avoid adhesion (Ref 33). To obtain improved antiwear property, three mechanisms may involve: (1) nanoadditives may melt, adhered on the shearing surfaces and react with the mating surfaces to form a protective film; (2) nanoadditives act as nanobearing between the mating surfaces; (3) tribo-sintering of the nanoadditives on the surface (Ref 34). In case of CuO and CeO2, first mechanism can be ruled out because of higher melting point (more than 1000 °C) of these oxide particles. The second mechanism may involve, i.e., nanobearing effect. The nanoparticles may roll–slide by separating the asperities and able to carry a proportion of the load. The EDS spectra of the worn surface also confirm the presence of the atomic content of the nanoadditives. Third, sinterization mechanism may also comprise in achieving improved tribo-performance (Ref 34). In this phenomenon, nanoparticles start to deposit in the dimples and valleys surrounded by the asperities under higher contact stress and elevated the localized temperature. It forms a compact protective layer which separates the mating surfaces, thus improving the antiwear properties.
Figure 10 depicts the tribological model of the ball-on-ball configuration under the sliding condition similar to our present study. Generally, two layers, i.e., adsorbed and ordered layer, have a significant role in reducing the wear and friction for the system working under elastohydrodynamic lubrication. However, adsorbed layer dominates over ordered layer in boundary lubrication regime. The molecules in the adsorbed layer are much stronger and solid in comparison with the base oil (that form ordered layer over the adsorbed layer) due to strong adhesion force between oil molecules and metal. The proportion of ordered layer thickness to total film thickness becomes larger if layer is thinner. For larger proportion, ordered layer plays a vital role. However, after rupture of the ordered layer, monomolecular adsorbed layer plays a lead role as in the case of boundary lubrication (Ref 30). In the case of nanolubricant, one more layer induced during the sliding, i.e., layer of nanoparticles. The molecules of the oil interact with the ball surface and an adsorbed layer formed over the ball surfaces by physisorption and chemisorption reaction. This adsorbed layer continuously generated and tries to keep mating surfaces away. The nanoparticles in oil repair the bulk material surface which earlier deteriorated under high contact stress. The layer of nanoparticles not only bears the load but also separates the mating surfaces. This condition was good up to an optimum concentration. Beyond this optimum concentration, the nanoparticles begin to act as an abrasive agent and causes a ploughing of different layers (Fig. 5). It causes severe wear and high friction.
Conclusions
It is not necessary that an improved tribological performance is always observed with the addition of the nanoparticles in the base oil. The nanoparticle’s parameter has a significant role in reflecting the lubricant performance. CeO2 nanoparticles have shown good compatibility with both PSO and ESO and reduced the WSD significantly in the antiwear test. The concentration of 0.1% w/v considered as an optimum in both the CeO2-based oils due to lowest WSD. On the contrary, CuO deteriorated the antiwear property with both PSO and ESO. Roughness behavior of the worn surfaces has shown good agreement with WSD variation. The higher roughness value was obtained for the PSO (Rq = 260.8 nm) and ESO (Rq = 466 nm), while a significant reduction in case of nanoadditive-based biolubricants. Friction property of nanolubricant improved at the lowest concentration (0.1% w/v for CeO2) in both PSO and ESO. Also, COF values increased gradually with increase in concentration. Moreover, CuO nanoadditive did not show any positive effect with ESO but showed good performance with PSO at 0.1 and 0.25% w/v concentration. Therefore, 0.1% w/v concentration of nanoparticles in both types of nanoadditives was considered as optimum.
References
S.M. Alves, B.S. Barros, M.F. Trajano, K.S.B. Ribeiro, and E. Moura, Tribological Behavior of Vegetable Oil-Based Lubricants with Nanoparticles of Oxides in Boundary Lubrication Conditions, Tribol. Int., 2013, 65, p 28–36
S.-W. Zhang, Green Tribology: Fundamentals and Future Fevelopment, Friction, 2013, 1(2), p 186–194
P. Nagendramma and S. Kaul, Development of ecofriendly/biodegradable lubricant: an overview, Renew. Sustain. Energy Rev., 2012, 16, p 764–774
N.J. Fox and G.W. Stachowiak, Vegetable oil-based lubricants—a review of oxidation, Tribol. Int., 2007, 40(7), p 1035–1046
C.J. Reeves and P.L. Menezes, Evaluation of boron nitride particles on the tribological performance of avocado and canola oil for energy conservation and sustainability, Int. J. Adv. Manuf. Technol., 2017, 89(9), p 3475–3486
S.Z. Erhan, B.K. Sharma, and J.M. Perez, Oxidation and low temperature stability of vegetable oil-based lubricants, Ind. Crops Prod., 2006, 24, p 292–299
S.-F. Snežana, J. Milovan, and S.P. Zoran, Kinetics of in situ epoxidation of soybean oil in bulk catalyzed by ion exchange resin, J. Am. Oil Chem. Soc., 2001, 78(7), p 725–731
B.M. Abdullah and J. Salimon, Epoxidation of vegetable oils and fatty acids: catalysts, method and advantages, J. Appl. Sci., 2010, 10(15), p 1545–1553
S.-F. Snežana, J. Milovan, and B. Olga, Epoxidation of castor oil with peracetic acid formed in situ in the presence of an ion exchange resin, Chem. Eng. Process., 2012, 62, p 106–113
W.L.Y. Hsien, Utilization of vegetable oil as bio-lubricant and additive, Towards Green Lubr. Mach., Springer Briefs in Green Chemistry for Sustainability, 2015, p 7–17. https://doi.org/10.1007/978-981-287-266-1_2.
P.-P. Laura, T.-T. Jaime, G. Andrés, M. Demófilo, A.G. Jesús, M. David, P. Eduardo, and C. Pablo, Antiwear and extreme pressure properties of nanofluids for industrial applications, Tribol. Trans., 2014, 57(6), p 1072–1076
B.K. Prasad, S. Rathod, M.S. Yadav, and O.P. Modi, Sliding wear behavior of a cast iron: influence of MoS2 and graphite addition to the oil lubricant, J. Mater. Eng. Perform., 2011, 20, p 445–455
B.K. Prasad, Lubricated sliding wear behavior of a cast iron: effect of graphite and/or talc fraction in oil, J. Mater. Eng. Perform., 2010, 19, p 413–420
M.K. Dubey, J. Bijwe, and S.S.V. Ramakumar, PTFE based nano-lubricants, Wear, 2013, 306, p 80–88
G.-J. Lee, J.-J. Park, M.-K. Lee, and C.K. Rhee, Stable dispersion of nanodiamonds in oil and their tribological properties as lubricant additives, Appl. Surf. Sci., 2017, 415, p 24–27
A.H. Battez, R. González, J.L. Viesca, J.E. Fernández, J.M.D. Fernández, A. Machado, R. Chou, and J. Riba, CuO, ZrO2 and ZnO nanoparticles as antiwear additive in oil lubricants, Wear, 2008, 265, p 422–428
R.N. Gupta and A.P. Harsha, Synthesis, characterization, and tribological studies of calcium–copper–titanate nanoparticles as a biolubricant additive, Trans ASME J Tribol, 2017, 139(2), p 021801-1–021801-11
W. Dai, B. Kheireddin, H. Gao, and H. Liang, Role of nanoparticles in oil lubrication, Tribol. Int., 2016, 102, p 88–98
R.B. Rastogi, J.L. Maurya, and V. Jaiswal, Zero SAPs and ash free antiwear additives: schiff bases of salicylaldehyde with 1,2-Phenylenediamine, 1,4-Phenylenediamine, and 4,4′-Diaminodiphenylenemethane and their synergistic interactions with borate ester, Tribol. Trans., 2013, 56, p 592–606
X. Yang, H. Pan, P. Wang, and F.-J. Zhao, Particle-specific toxicity and bioavailability of cerium oxide (CeO2) nanoparticles to Arabidopsis thaliana, J. Hazard. Mater., 2017, 322, p 292–300
Y. Ma, L. Kuang, X. He, W. Bai, Y. Ding, Z. Zhang, Y. Zhao, and Z. Chai, Effects of rare earth oxide nanoparticles on root elongation of plants, Chemosphere, 2010, 78(3), p 273–279
X. Pang, D. Li, and A. Peng, Application of rare earth elements in the agriculture of China and its environmental behavior in soil, J. Soils Sediments, 2001, 1(2), p 124–129
A. Pinna, L. Malfatti, G. Galleri, R. Manetti, S. Cossu, G. Rocchitta, R. Migheli, P.A. Serra, and P. Innocenzi, Ceria nanoparticles for the treatment of Parkinson-like diseases induced by chronic manganese intoxication, RSC Adv, 2015, 5, p 20432–20439
S.A. Kuddus, Nanoceria and its perspective in cancer treatment, J Cancer Sci Ther, 2017, 9(3), p 368–373
D. Barbara, S. Sandro, B. Elisabetta, D.L. Silvia, R.A. Phani, F. Stefano, A. Fernanda, P.C. Maria, and C. Annamaria, Cerium oxide nanoparticles trigger neuronal survival in a human alzheimer disease model by modulating BDNF pathway, Curr Nanosci, 2009, 5(2), p 167–176
A. Adhvaryu and S.Z. Erhan, Epoxidized soybean oil as a potential source of high temperature lubricants, Ind. Crops Prod., 2002, 15, p 247–254
J. Salimon, N. Salih, and E. Yousif, Synthetic biolubricant basestocks from epoxidized ricinoleic acid: improved low temperature properties, J. Chem. Chem. Eng., 2011, 60(3), p 127–134
H. Ghaednia and R.L. Jackson, The effect of nanoparticles on the real area of contact, friction, and wear, Trans. ASME J. Tribol., 2013, 135, p 041603-1–041603-10
P. Sahoo, Engineering Tribology, Chapter 2, 3rd ed., PHI, learning pvt ltd, Delhi, 2005, p 5–20
D.X. Peng, Y. Kang, R.M. Hwang, S.S. Shyr, and Y.P. Chang, Tribological properties of diamond and SiO2 nanoparticles added in paraffin, Tribol. Int., 2009, 42, p 911–917
H. Ghaednia, R.L. Jackson, and J.M. Khodadadi, Experimental analysis of stable CuO nanoparticle enhanced lubricants, J. Exp. Nanosci., 2015, 10, p 1–18
R. Wäsche, M. Hartelt, V.-D. Hodoroaba, Analysis of nanoscale wear particles from lubricated steel-steel contacts, Tribol. Lett., 2015, 58(3), p 1–10. Article number 49
B. Li, X. Wang, W. Liu, and Q. Xue, Tribochemistry and antiwear mechanism of organic-inorganic nanoparticles as lubricant additives, Tribol. Lett., 2006, 22, p 79–84
V. Jaiswal, R.B. Rastogi, R. Kumar, L. Singh, and K.D. Mandal, Tribological studies of stearic acid-modified CaCu2.9Zn0.1Ti4O12 nanoparticles as effective zero SAPS antiwear lubricant additives in paraffin oil, J. Mater. Chem. A, 2014, 2, p 375–386
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, R.N., Harsha, A.P. Friction and Wear of Nanoadditive-Based Biolubricants in Steel–Steel Sliding Contacts: A Comparative Study. J. of Materi Eng and Perform 27, 648–658 (2018). https://doi.org/10.1007/s11665-018-3175-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-018-3175-3