Abstract
A series of new Mg-8Li-xCa-yGd (x = 0, 1, 2; y = 0, 2; wt.%) alloys were prepared, and the microstructure and mechanical properties were investigated. The mechanical properties were characterized by tensile, compression and bending tests at room temperature. The results show that Mg-8Li-1Ca alloy is composed of alpha(Mg), beta(Li) and CaMg2 phases. In addition to the same phases in Mg-8Li-1Ca, there also exists CaLi2 phase in Mg-8Li-2Ca. In addition to the same phases in Mg-8Li-2Ca, GdMg5 phase is also formed in Mg-8Li-1Ca-2Gd alloy due to the addition of Gd. Both Ca and Gd have refining effect in the alloys, and the refining effect of Ca is better than that of Gd. The additions of Ca and Gd can improve the tensile strength and yield strength, but decrease the elongation and the bending strength. Comparing the mechanical properties of the investigated alloys, Mg-8Li-1Ca-2Gd possesses the best mechanical properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mg-Li alloys are among the lightest metallic engineering materials and possess high specific strength, specific stiffness, good electromagnetic screen and superior shock resistance ability (Ref 1, 2).
Adding a small amount of lithium to magnesium can decrease the c/a ratio of the hexagonal closed-packed lattice of Mg. When Li addition is between 5.3 and 10.3 wt.%, a body-centered-cubic phase (BCC, β-Li phase) is introduced into hexagonal closed-packed Mg-based alloy (HCP, α-Mg phase), which results in the formation of (α + β) duplex alloy. The formation of β-Li phase in Mg alloys can improve the ductility effectively but decrease the strength (Ref 3, 4). Great efforts have been made to enhance the comprehensive mechanical properties of Mg-Li alloys. Alloying is a common and effective method to improve mechanical properties (Ref 5, 6). A suitable amount of calcium addition has a good effect on grain refinement in Mg-Zr and Mg-Al alloys, causing the improvement of mechanical properties (Ref 7). In Mg-Gd-Zr alloy, with the addition of Ca content, the yield strength increases, and the elongation decreases (Ref 8). Rare earth (RE) elements can ameliorate the microstructure and enhance the mechanical properties effectively in Mg-Li alloys (Ref 9,10,11). Gd, as a heavy RE element, is beneficial for the improvement of both the mechanical properties and the corrosion resistance (Ref 12). A minor addition of Gd in Mg-Zn-Zr alloy can effectively refine grains and change phase compositions. The addition of Gd in as-rolled Mg-Zn-Zr alloy can effectively weaken the texture and modify the tensile fracture type from quasi-cleavage fracture to ductile fracture (Ref 13).
In this work, the as-cast Mg-8Li-xCa-yGd (x = 0, 1, 2; y = 0, 2; wt.%) alloys are prepared, and the influences of Ca and Gd on the microstructure and mechanical properties are investigated.
Experimental Procedures
The investigated as-cast alloys were prepared by melting commercially pure (CP) Mg, CP Li, CP Ca and Mg—20 wt.% Gd master alloy in the melting furnace under the protective atmosphere of Ar. After melting, the melt was poured into a permanent mold. The book-shape as-cast ingots with dimensions of 230 mm × 145 mm × 40 mm were obtained. Then, the specimens for microstructure analysis and mechanical properties testing were cut from the ingot. The actual chemical compositions of the as-cast ingots measured by inductively coupled plasma analyzer (ICP, JY Ultima2) are listed in Table 1.
The specimens for microstructure observation were etched with a solution of 5 vol.% nital. The microstructures were observed by optical microscope (OM) and scanning electron microscope (SEM). The fracture microstructure was also observed by SEM. The phases of the alloys were analyzed by x-ray diffraction (XRD).
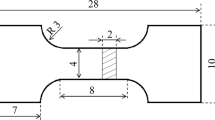
The tensile and compression tests were carried out on an electronic universal uniaxial testing machine. The initial strain rate during tensile tests was 1 × 10−3 s−1, and the dimensions of the tensile specimen are shown in Fig. 1, in which the orientation of width, 15 mm, was parallel to the orientation of width, 40 mm, in the as-cast ingot. The compression specimens were cylinders, with dimensions of Φ 10 mm × 15 mm, in which the orientation of height, 15 mm, was parallel to the orientation of height, 145 mm, in the as-cast ingot. The compression rate was 1 × 10−3 s−1. The dimensions of the three-point bending specimen were 30 mm × 18 mm × 3 mm, in which the orientation of length, 30 mm, is parallel to the orientation of width, 40 mm, in the as-cast ingot. The loading rate was 1.5 × 10−3 s−1. During mechanical properties testings, all the test values were the average of at least three measurements.
Results and Discussion
Microstructure
Figure 2 shows the XRD patterns of the investigated alloys. Mg-8Li alloy is composed of α-Mg and β-Li. CaMg2 phase appears in Mg-8Li-1Ca alloy. As the content of Ca increases to 2 wt.%, although the XRD peaks of CaLi2 phase are not obvious, combined with the results of Fig. 3 and 4 and Ref 14, it can be deduced that there exists CaLi2 phase in Mg-8Li-2Ca alloy. The solid solubility of calcium in magnesium is very small, less than 0.82%. When the content of Ca is larger than 0.82%, the excess Ca would react with Mg and Li to form new phases (Ref 14). After the addition of Gd, the amount of CaMg2 and CaLi2 phase decreases, and GdMg5 phase appears. It is known that the larger electronegativity difference means an easier formation of the compound, and the compound is more stable. The electronegativity values of the four elements in the alloys are listed in Table 2. With the addition of Ca, it reacts with Mg to form Mg2Ca preferentially because the electronegativity difference between Ca and Mg is larger than that between Ca and Li. When the content of Ca is large, Li2Ca and Mg2Ca both exist in the alloy. The solid solubility of Gd is much smaller in β-Li than that in α-Mg (Ref 15). Accordingly, although the electronegativity difference between Gd and Mg is less than that between Gd and Li, the supersaturated Gd preferentially reacts with Mg to form Mg-Gd compound in α-Mg matrix. The compound phases in this experiment are also consistent with the results of other literature (Ref 16,17,18).
Figure 3 shows the optical microstructures of the alloys. To observe the detailed microstructures, the high-magnification SEM images of Mg-8Li-2Ca and Mg-8Li-1Ca-2Gd alloys are shown in Fig. 4. In Mg-8Li alloy, the long-strip and blocky α-Mg phase is distributed in β-Li phase, as shown in Fig. 3(a) and (b). With the addition of 1 wt.% Ca, a network phase appears mainly at the phase boundaries between α-Mg and β-Li, as shown in Fig. 3(c) and (d). According to the XRD results of Fig. 2, the network phase is CaMg2. When the content of Ca increases to 2 wt.%, the amount of CaMg2 increases relatively. Additionally, many particles are uniformly distributed in β-Li phase, as shown in Fig. 3(e), (f), and 4(a). According to Ref 14, it can be concluded that this granular phase in Mg-8Li-2Ca alloy is CaLi2. In Mg-8Li-1Ca-2Gd, the amount of CaMg2 phase is close to that in Mg-8Li-1Ca, and some white particles, whose size is larger than that of CaLi2, appear in α-Mg and β-Li phases, as shown in Fig. 4(b). Combined with the XRD result, it can be concluded that the white granular phase in Fig. 4(b) is GdMg5. Quantitatively analysis reveals that the percentages of CaMg2 in Mg-8Li-1Ca, Mg-8Li-2Ca and Mg-8Li-1Ca-2Gd alloys are about 2.8 ± 0.2, 4.5 ± 0.3, and 2.6 ± 0.2%, respectively. Comparing Fig. 3(d), (f), and (h), it can be observed that the size of CaMg2 phase in Mg-8Li-2Ca is the finest, and that in Mg-8Li-1Ca-2Gd is the coarsest.
Quantitatively analysis indicates that the average α-Mg sizes of Mg-8Li, Mg-8Li-1Ca, Mg-8Li-2Ca and Mg-8Li-1Ca-2Gd are 30.5 ± 2.8, 25.3 ± 2.0, 15.5 ± 1.8, and 36.2 ± 3.1 μm, respectively. Comparing the microstructures of the four alloys, Ca and Gd both have refining effects in Mg-8Li alloy, and Mg-8Li-2Ca alloy possesses the finest microstructure. With the addition of Ca, Ca would segregate at the grain boundaries of α-Mg phase during the solidification, increasing the composition fluctuation (Ref 19). Therefore, the degree of supercooling increases, and the nucleation rate increases, causing the refining effects. Furthermore, CaMg2 forms at the grain boundary and connects into a network, which also prevents the grain growth. Gd and Mg possess the same crystal structure (HCP) and similar lattice constants, and the melting point of GdMg5 is higher than that of Mg. Therefore, during the solidification, GdMg5 may serve as the heterogeneous nucleation, causing the grain refinement.
Tensile Properties
Figure 5 shows the stress–strain curves of the four alloys. The values of tensile strength (σ b ), yield strength (σ 0.2) and elongation (ε) are listed in Table 3. It can be found that the yield strength increases with the increase in Ca content. When the content of Ca is 1 wt.%, there is little effect on tensile strength of the alloy. The addition of 2 wt.% Ca is much more effective in the improvement of tensile strength. The addition of 2 wt.% Gd can further increase the strength, making Mg-8Li-1Ca-2Gd possesses the highest yield strength and tensile strength among the four alloys. The alloys with Ca or/and Gd possess much lower elongation values compared with Mg-8Li alloy. The elongation of Mg-8Li-1Ca is the minimum. Compared with Mg-8Li-1Ca and Mg-8Li-1Ca-2Gd, the elongation of Mg-8Li-2Ca is relatively high, which is about 2 times larger than that of Mg-8Li-1Ca.
In Mg-8Li-1Ca, the network CaMg2 that exists at the phase boundaries between α-Mg and β-Li is a brittle compound. Although the microstructure of the alloy is refined because of Ca addition, the existence of large-size and brittle CaMg2 makes the strengthening effect become unobvious and the ductility decrease seriously. When Ca content is 2 wt.%, the granular CaLi2 phase appears in the alloy, making the amount of network CaMg2 decrease and the refining effect become more obvious. Therefore, the strength and elongation of Mg-8Li-2Ca are both improved obviously compared with those of Mg-8Li-1Ca. Based on Mg-8Li-1Ca, adding 2 wt.% Gd leads to forming granular GdMg5 and decreasing the amount of brittle network CaMg2. What is more, the microstructure is further refined. Therefore, the strength is improved obviously. The strength of Mg-8Li-1Ca-2Gd is the highest among the four alloys. Because the amount of brittle network CaMg2 in Mg-8Li-1Ca-2Gd alloy is somewhat larger than that in Mg-8Li-2Ca alloy, the elongation of Mg-8Li-1Ca-2Gd alloy is less than that of Mg-8Li-2Ca alloy.
Figure 6 shows the tensile fracture morphology of the alloys. There are a large number of deep dimples in the fracture of Mg-8Li alloy, showing a ductile fracture mode. There exist many cleavage planes, cleavage steps and obvious tear ridges in the fracture of Mg-8Li-1Ca, showing a quasi-cleavage mode. In Mg-8Li-2Ca alloy, cleavage planes and cleavage steps disappear, and small and shallow dimples appear, showing a type of mixed fracture mode. The network CaMg2 phase distributing at phase boundary splits the matrix, causing a poor ductility. When Ca content increases to 2 wt.%, the dispersive CaLi2 phase makes the ductility of the alloy increase. Compared with the fracture morphology of Mg-8Li-1Ca, due to the addition of Gd, the amount of cleavage planes, cleavage steps and tear ridges in the fracture of Mg-8Li-1Ca-2Gd decreases. The tensile fracture is a mixture of quasi-cleavage and tough dimple. With the addition of Gd, the grains are refined effectively. Furthermore, the formation of GdMg5 phase makes the amount of brittle CaMg2 phase decrease. As a result, the ductility of Mg-8Li-1Ca-2Gd is better than that of Mg-8Li-1Ca, which is consistent with results of stress–strain curves.
Compression Properties
During the compression process, all the specimens are not broken, only with a lot of small cracks on the surface. Figure 7 and 8 show the stress–strain curves and compressive yield strength of the four alloys. From Fig. 7, it can be found that the additions of Ca and Gd both increase the compressive yield strength. Mg-8Li-2Ca possesses the highest yield strength. Comparing the compressive strength values with the tensile strength values, it can be found that the addition of Ca performs more favorable effects on compressive strength than tensile strength. As for magnesium alloy, the deformation mechanism of compression is different from that of tensile. The piling up of dislocations during compression deformation is faster than that during tensile deformation (Ref 20). Compared with GdMg5 phase in Mg-8Li-1Ca-2Gd, the size of particular CaLi2 in Mg-8Li-2Ca is smaller and more uniform, and the distribution of it is more dispersive. The blocking effects of CaLi2 particles on the dislocation movement are stronger (Ref 21). As a result, the compressive yield strength of Mg-8Li-2Ca is better than that of Mg-8Li-1Ca-2Gd.
Bending Properties
Figure 9 shows the bending deformation curves of the four alloys. The bending strength of Mg-8Li is the highest. The formation of brittle CaMg2 phase leads to the deterioration of bending strength. The dispersive CaLi2 phase strengthens the matrix, and the strength and ductility are improved. Comparing the bending curves of the four alloys, it can be obviously found that the unfavorable effect of Gd on the bending strength is less than that of Ca. It can be attributed to the three factors caused by the addition of Gd, i.e., the decrease of brittle CaMg2 phase, the formation of granular GdMg5 phase and the refinement effect.
Conclusions
-
1.
Mg-8Li-1Ca alloy is mainly composed of α-Mg, β-Li and CaMg2. The network CaMg2 phase distributes at the boundaries between α-Mg and β-Li. When Ca content increases to 2 wt.%, granular CaLi2 phase forms in β-Li phases and the amount of network CaMg2 phase decreases. Adding 2 wt.% Gd to Mg-8Li-1Ca alloy leads to the formation of granular GdMg5 phase. The additions of Ca and Gd both have refining effect in the alloys, and the refining effect of Ca is better than that of Gd.
-
2.
The additions of both Ca and Gd can increase the tensile strength, but decrease the elongation. The strengthening effect of Gd is better than that of Ca. The existence of network CaMg2 is unfavorable for elongation. Compared with Mg-8Li-1Ca and Mg-8Li-1Ca-2Gd, Mg-8Li-2Ca possesses a relatively higher elongation.
-
3.
The additions of Ca and Gd both increase the compressive yield strength. The addition of Ca performs more favorable effects on compressive strength than tensile strength. The strengthening effect of Ca is better than that of Gd. Mg-8Li-2Ca possesses the highest compressive yield strength.
-
4.
The additions of Ca and Gd both make the bending strength deteriorate. The unfavorable effect of Gd on the bending strength is less than that of Ca.
-
5.
Comparing the tensile strength, elongation, compression strength and bending strength, Mg-8Li-1Ca-2Gd possesses the best mechanical properties among the four alloys.
References
R.Z. Wu, Y.D. Yan, G.X. Wang, L.E. Murr, W. Han, Z.W. Zhang, and M.L. Zhang, Recent Progress in Magnesium-Lithium Alloys, Int. Mater. Rev., 2015, 60, p 65–100
G.B. Wei, Y. Mahmoodkhani, X.D. Peng, A. Hadadzadeh, T.C. Xu, J.W. Liu, W.D. Xie, and M.A. Wells, Microstructure Evolution and Simulation Study of a Duplex Mg-Li Alloy During Double Change Channel Angular Pressing, Mater. Des., 2016, 90, p 266–275
Y. Zeng, B. Jiang, D.H. Huang, J.H. Dai, and F.S. Pan, Effect of Ca Addition on Grain Refinement of Mg-9Li-1Al Alloy, J. Magnes. Alloys, 2013, 1, p 297–302
R. Mahmudi, M. Shalbafi, M. Karami, and A.R. Geranmayeh, Effect of Li Content on the Indentation Creep Characteristics of Cast Mg–Li–Zn Alloys, Mater. Des., 2015, 75, p 184–190
T.L. Zhu, C.L. Cui, T.L. Zhang, R.Z. Wu, S. Betsofen, Z. Leng, J.H. Zhang, and M.L. Zhang, Influence of the Combined Addition of Y and Nd on the Microstructure and Mechanical Properties of Mg-Li Alloy, Mater. Des., 2014, 57, p 245–249
R.Z. Wu, Z.K. Qu, and M.L. Zhang, Effects of the Addition of Y in Mg-8Li-(1,3)Al Alloy, Mater. Sci. Eng. A, 2009, 516, p 96–99
T. Wang, R.Z. Wu, M.L. Zhang, L. Li, J.H. Zhang, and J.Q. Li, Effect of Calcium on the Microstructures and Tensile Properties of Mg-5Li-3Al Alloys, Mater. Sci. Eng. A, 2011, 528, p 5678–5684
L.L. Shi, Y.D. Huang, L. Yang, F. Feyerabend, C. Mendis, R. Willumeit, K.U. Kainer, and N. Hort, Mechanical Properties and Corrosion Behavior of Mg-Gd-Ca-Zr Alloys for Medical Applications, J. Mech. Behav. Biomed., 2015, 47, p 38–48
M.E. Moussa, M.A. Waly, and A.M. El-Sheikh, Effect of Ca Addition on Modification of Primary Mg2Si, Hardness and Wear Behavior in Mg-Si Hypereutectic Alloys, J. Magnes. Alloys, 2013, 2, p 230–238
F.A. Mirza, D.L. Chen, D.J. Li, and X.Q. Zeng, Effect of Rare Earth Elements on Deformation Behavior of an Extruded Mg-10Gd-3Y-0.5Zr Alloy During Compression, Mater. Des., 2013, 46, p 411–418
J. Zhang, Z. Leng, S. Liu, J. Li, M.L. Zhang, and R.Z. Wu, Microstructure and Mechanical Properties of Mg-Gd-Dy-Zn Alloy with Long Period Stacking Ordered Structure or Stacking Faults, J. Alloys Compd., 2011, 509, p 7717–7722
X. Liu, Z.Q. Zhang, W.Y. Hu, Q.C. Le, L. Bao, and J.Z. Cui, Effects of Extrusion Speed on the Microstructure and Mechanical Properties of Mg-9Gd-3Y-1.5Zn-0.8Zr Alloy, J. Mater. Sci. Technol., 2016, 32, p 313–319
H.Y. Yu, H.G. Yan, J.H. Chen, B. Su, Y. Zheng, Y.J. Shen, and Z.J. Ma, Effects of Minor Gd Addition on Microstructures and Mechanical Properties of the High Strain-Rate Rolled Mg-Zn-Zr Alloys, J. Alloys Compd., 2014, 586, p 757–765
J. Gröbner, R. Schmid Fetzer, A. Pisch, C. Colinet, V.V. Pavlyuk, G.S. Dmytriv, D.G. Kevorkov, and O.I. Bodak, Phase Equilibria, Calorimetric Study and Thermodynamic Modeling of Mg-Li-Ca Alloys, Thermochim. Acta, 2002, 389, p 85–94
H.W. Dong, L.D. Wang, Y.M. Wu, and L.M. Wang, Effect of Y on Microstructure and Mechanical Properties of Duplex Mg-7Li Alloys, J. Alloys Compd., 2010, 506, p 468–474
K. Suresh, K.P. Rao, Y.V.R.K. Prasad, N. Hort, and K.U. Kainer, Microstructure and Mechanical Properties of As-Cast Mg-Sn-Ca Alloys and Effect of Alloying Elements, Trans. Nonferrous Met. Soc. China, 2013, 23, p 3604–3610
D.F. Zhang, X. Chen, F.H. Pan, L.Y. Jiang, G.S. Hu, and D.L. Yu, Research Progress of Effect of Rare Earth Elements on the Mechanical Properties of Magnesium Alloy, Funct. Mater., 2014, 5, p 5001–5007
G.S. Song and M.V. Kral, Characterization of Cast Mg-Li-Ca Alloys, Mater. Charact., 2005, 54, p 279–286
Y.Y. Zhou, L.P. Bian, G. Chen, L.P. Wang, and W. Liang, Influence of Ca Addition on Microstructural Evolution and Mechanical Properties of Near-Eutectic Mg-Li Alloys by Copper-Mold Suction Casting, J. Alloys Compd., 2016, 664, p 85–91
M.J. Mills and T. Neeraj, Dislocations in Metals and Metallic Alloys, Encyclopedia of Materials: Science and Technology, 2001, p 2278–2291
W. Zakulski, A. Debski, and W. Gasior, Enthalpy of Formation of the CaLi2 Phase, Intermetallics, 2012, 23, p 76–79
Acknowledgments
This paper was supported by National Natural Science Foundation (51671063), Research Fund for the Doctoral Program of Higher Education (20132304110006), the Fundamental Research Funds for the Central Universities (HEUCFG201703), Harbin City Application Technology Research and Development Project (2015AE4AE005, 2015RQXXJ001, 2016AB2AG013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miao, W., Che, C., Fu, K. et al. Microstructure and Mechanical Properties of Mg-8Li-(0, 1, 2)Ca-(0, 2)Gd Alloys. J. of Materi Eng and Perform 26, 4831–4837 (2017). https://doi.org/10.1007/s11665-017-2938-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-017-2938-6