Abstract
Vegetable oils, especially soybean oil, exhibit substantially poorer thermal-oxidative stability than commercially available petroleum oil quenchant formulations. Therefore, to achieve any commercially interesting performance, vegetable oils must be stabilized by the addition of antioxidant inhibitors. This work describes the ability of two commercially available antioxidants, Irganox L 57 and Irganox L 109, to stabilize soybean oil against thermal-oxidative degradation. In addition, the effect of antioxidant stabilization on quenching performance was evaluated by determining the profile of heat transfer coefficient variation throughout the quenching process at different times after being subjected to an accelerated thermal-oxidation aging test. The results of this work are discussed here.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Properties, such as hardness and strength, of carbon and alloy steels are dependent on the cooling time-temperature profile (cooling curve) exhibited by the quenching medium relative to the TTT (time-temperature-transformation) or CCT (continuous cooling transformation) curves for the steel of interest. Quenchants not only control properties but also, depending on the quenching process and quenchant selection, control the residual stress profile with the steel and are critically important to optimize distortion control and prevent cracking. Depending on the steel and hardening process, the most common quenchants include air, oil, water, brine, aqueous polymer solutions, and high-pressure gas quenching.
Of the vaporizable quenchants, petroleum oil-derived fluids continue to be the most commonly encountered ones throughout the industry globally. However, there is an ongoing effort to replace petroleum oil-derived industrial fluids because of the price and the environmental and toxicological property hazards exhibited by petroleum products. Various quenching media have been evaluated as alternatives to petroleum oil including water, especially utilizing water in conjunction with time quenching (Ref 1-3) or by intensive quenching (Ref 4), water-gas (Ref 5), aqueous polymers (Ref 6, 7), and high-pressure gas (Ref 8). However, petroleum oil-derived quenchants continue to dominate the marketplace.
Currently, there is increasing interest in the use of bio-based oils as replacements for petroleum oil base stocks for lubricant and quench oil formulation. Bio-based oils are defined as “industrial products including fuels, but not food or feed, made from renewable agricultural or forestry resources.” Seed oils are currently one of the most common sources of bio-based oils and among the various seeds from which seed oils are derived. Soybeans are in the greatest production worldwide and the United States has the highest soybean production rate followed by Brazil (Ref 9). Of the various seed oils produced in the USA in 2004, soybean oil has the largest production rate and is therefore one of the oils of the greatest interest for use in replacing petroleum oil-derived quenchants (Ref 10).
Tagaya and Tamura compared the quench severity of different vegetable oils including soybean, rapeseed, and castor oils with mineral oils and fish/animal oils with respect to fluid source and viscosity and oxidative stability for various naturally derived fluids (Ref 11). Fujimura and Sato followed this work in 1963 by examining the quenching performance of the vegetable oils reported by Tagaya and Tamura and different petroleum oil quenchants and concluded that the performance of soybean and rapeseed oil was essentially equivalent (Ref 12).
Currently, the most commonly cited vegetable oil base stocks used for quenchant formulation are canola oil (Ref 13) and soybean oil derivatives (Ref 14). Although vegetable oils exhibit a cooling performance different from the petroleum oil standard used, in none of the references was any systematic comparison of vegetable oil structure with oxidative stability and its corresponding impact on quenching performance demonstrated. The cooling curves reported were obtained according to the ISO 9950 (Ref 15), which is essentially equivalent to the ASTM D6200 “Standard Test Method for Determination of Cooling Characteristics of Quench Oils by Cooling Curve Analysis” (Ref 16). Both the ISO 9950 and ASTM D6200 utilize a 12.5-mm-dia × 60-mm INCONEL 600 cylindrical probe with a Type K thermocouple inserted into the geometric center.
Oxidative stability performance improvements of vegetable oils can be achieved by chemical or genetic modifications (Ref 17-19) or by process improvements such as winterization and partial hydrogenation (Ref 20, 21). Winterization (fractionation) is performed to remove crystallized fats and improve the pour point of the base oil. The performance objective is to reduce the linolenic and linoleic ester content of the vegetable oil to increase the oxidative stability, making the resulting vegetable oil more suitable for use in industrial applications (Ref 20). Honary demonstrated that the oxidative stability of soybean oil is substantially improved by partial hydrogenation. However, this also increases the level of saturation in the oil, which produces crystallized fats which may be removed by a winterization process (Ref 21).
Genetic engineering has produced soybean oil with >85% oleic acid ester contents (Ref 17, 18). There are, however, a number of substantial problems with genetic engineering to reduce polyunsaturation including costs associated with regulatory approval.
Totten et al. reported the results of cooling curve, hardenability, heat transfer, and wettability characterization studies conducted with crude and partially hydrogenated and winterized soybean oils provided by Honary (Ref 21, 22). The soybean oils investigated showed no significant differences in cooling behavior and the rewetting conditions on the test specimen surface, and the cooling rates were similar. Comparison of the cooling time-temperature and cooling rate curves showed that the vegetable oils exhibited faster cooling rates than the mineral oil used as a reference (Ref 22).
Although there are various references to the quenching performance of soybean oil and other vegetable oils such as the work reported by Prabhu (Ref 23, 24), with the exception of Tagaya and Tamura (Ref 25) and Canale et al. (Ref 26), no one has reported the effect of the oxidative degradation on quenching performance and the ability to stabilize cooling performance, as determined by cooling curve performance with the addition of antioxidants. The objective of this work was to study the degradation of quenching performance of soybean oil with and without the addition of antioxidants.
Experimental Methods and Materials
The soybean oil used for this work was purchased at the local market in Sao Carlos, Brazil, and was used in the “as-purchased” condition.Footnote 1 Quenching performance of these oils was compared to two commercially available petroleum quenching oils: MicroTemp 157—a conventional “slow” oil; MicroTemp 153B—an accelerated “fast” oil.
The two antioxidants used for this work are Irganox L57 and Irganox L109. Both were used as supplied by Ciba Geigy Corporation. The composition of the soybean oil blends prepared for this study is shown in Table 1.
Viscosity was measured at 40 and 100 °C according to ABNT NBR 10441—10/02 (Ref 28).
The oxidative stability of the vegetable oils and quenching oil was determined using an accelerated oxidation test previously reported by Bashford and Mills (Ref 29) and with equipment built for this purpose by FarahFootnote 2 (Ref 30). A schematic illustration is provided in Fig. 1. The oxidation test was conducted as follows: Into a 2300-mL glass vessel (12.5 cm diameter, 19 cm in height) was added 2000 mL of the vegetable oil which was heated to 150 ± 2 °C for 15 min with agitation. During this time, the fluid was aerated using a gas sparge tube at 4 L air/h. (The agitation was provided by an electrically driven propeller mixer with speed settings of 0-10, and a setting of 6 was used.) After 12 min, the electrical resistance immersion heater, aeration system, and agitation were automatically shut off for 15 min during which time the fluid was cooled to 125 ± 2 °C in 3 min. The fluid was reheated again with agitation and air sparging and these 15-min cycles were repeated over the test duration of 48 h.
Schematic illustration of the accelerated oxidation system: (a) heating, agitation, and blow air into the oil sample; (b) refrigeration system (Ref 30)
Cooling curves were obtained under unagitated conditions according to ASTM D6200-01—“Standard Test Method for Determination of Cooling Characteristics of Quench Oils by Cooling Curve Analysis”—at a bath temperature of 40 °C. This test method is based on the 12.5-mm-dia. × 60-mm cylindrical INCONEL 600 probe assembly. After heating the probe in a furnace to 850 °C (1562 °F), it was manually and rapidly immersed into 2000 mL of the oil to be tested which was contained in a tall-form stainless steel beaker. The probe temperature and cooling times are recorded at selected time intervals to establish a cooling temperature versus time curve.
Calculation of Heat Transfer Coefficients
Heat transfer processes are complex and the heat transfer coefficient is a complex function of variables describing this process. Generally, the heat transfer coefficient is a function of the fluid flow, component (probe) shape and dimensions, temperature, and physical properties of the liquid: thermal conductivity, specific heat capacity, density, and viscosity. The heat transfer coefficient can be defined as the quantity of heat transferred per unit time per unit area of a surface when the difference of temperatures between the surface and liquid equals one degree absolute. Since quenching processes are actually heat transfer processes, the heat transfer coefficient is an excellent single parameter for quenchant characterization.
In this work, the commercial code HT-MODFootnote 3 (Heat Treating Modeling) was used to simulate the heat treatment process (Ref 32). This code is also used to calculate heat transfer coefficients as a function of time by solving an inverse heat transfer problem. The model is based on a numerical optimization algorithm which includes a finite element module for calculating, with respect to time and space, the temperature distribution and its coupled microstructural evolution. In this case, since an Inconel 600 probe was used that does not undergo microstructural phase transformation, the differential problem solved is only the heat conduction (Eq 1) in a cylindrical probe:
where\( k\left( {\vec{r},T} \right),c\left( {\vec{r},T} \right) \) , and \( \rho \left( {\vec{r},T} \right) \) denote the thermal conductivity, the specific heat, and the density of the material, respectively, as dependent of the position \( \left( {\vec{r}} \right) \) and temperature T. \( T\left( {\vec{r},t} \right) \) is subjected to the initial condition
and the following boundary conditions at the surface of the steel probe:
where \( h_{i} \left( T \right) \) is the heat transfer coefficient corresponding to different portions of the boundary Γ and T qu is the quenchant temperature. Each one of these p cooling zones has a time-dependent heat transfer coefficient that varies strongly along each partial boundary, depending on the heat transfer mechanism (vapor blanket, nucleate boiling, convective cooling) that governs the energy flow.
Provided that the temperature change inside the component and on its surface is measured, it is possible to solve the inverse heat transfer problem to determine the time variation of the heat transfer coefficients which best satisfies production demands. The time dependence of the heat transfer coefficient can be approximated by polygonal functions, each one defined by a set of parameters\( h_{i}^{\left( r \right)} \left( {r = 1, \ldots ,p;\quad i = 1, \ldots ,q} \right) \).
On calling T m k , the measured temperature, and T c k , the numerically calculated temperature at those points, one can pose the problem of obtaining the values of the heat transfer coefficients h i that minimize the function:
n being the total number of measured temperatures, i.e., the number of points times the number of measurements at each point.
The selection of the initial values for these coefficients and of the quantity and length of the time intervals was sample-dependent. The mean square difference between the measured and calculated temperatures obtained after optimization of the heat transfer coefficients was about 1 °C. Table 2 summarizes the thermo-physical properties of INCONEL 600 used for this work (Ref 33).
Discussion
Thermal-Oxidation Test Results
In view of the relatively poor inherent oxidative stability of soybean oil and the commercial interest in using this commonly available base stock for industrial fluid formulation, it was selected for a subsequent preliminary screening test using two antioxidants, Irganox L 57 and Irganox L 109. The thermal-oxidation test described in the EXPERIMENTAL Section was used and the degree of oil oxidation was followed throughout the duration of the test by measuring the resulting fluid kinematic viscosity with respect to oxidation time as shown in Fig. 2. For hydrocarbon-based fluids, thermal-oxidative degradation will result in an overall viscosity increase with respect to time, although in some cases, this increase may be preceded by a small viscosity decrease due to initial hydrocarbon chain cleavage before an exponential increase in viscosity.
Although there was some variation of the fluid viscosity upon addition of the antioxidants, the differences were relatively minimal. Throughout the aging test, the results obtained show that both Irganox L 109 and Irganox L 57 exhibited better stabilization than their blends. For most of the test, Irganox L 109 exhibited somewhat better performance than Irganox L 57. At the conclusion of the test, however, there was an inversion between 60 and 70 h where Irganox L57 seemed to stabilize with little additional increase in viscosity, whereas the Irganox L 109 continued on its exponential viscosity increase. This is an important observation and unfortunately the test was not continued beyond 70 h. To draw a definitive conclusion, longer test durations would be required.
One of the reasons for evaluating blends of Irganox L 57 and Irganox L 109 was to search for potential synergies. Based on these limited studies, no positive synergies by blending both antioxidants were observed.
Figure 2 also shows that the performance of neither Irganox L 57 nor Irganox L 109 exhibited the much better performance obtained with both commercial petroleum oil quenchants: Microtemp 157 or Microtemp 153B. Clearly, substantially greater stabilization of the soybean oil candidates is necessary if oxidative stability that is comparable to that exhibited by petroleum oils is to be achieved.
Effect of Aging on Heat Transfer Coefficients during Quenching
Any hyrdrocarbon-containing quenchant will exhibit some finite propensity to oxidize over time when subjected to high interfacial temperatures such as those encountered upon immersion of austenitized steel (≈ 850 °C) and oxygen (air). The degree and rate of oxidation will vary with respect to the chemical structure of the hydrocarbon moiety; the presence, structure, and concentration of antioxidants; the presence of contaminants such as water and metals including copper and iron; and total load size and frequency of use of the quench bath among other variables (Ref 35-37). Therefore, some net increase in quenchant viscosity over time is expected and since viscosity is an important variable in heat transfer, some variation in heat transfer rates is also expected (Ref 34). The question is how great is the variation and what would be the relative variation when different quench media are compared.
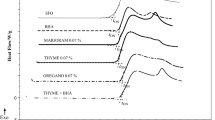
For this work, cooling curve analysis was performed according to the ASTM D6200 test procedure at a quenchant bath temperature of 40 °C with the different soybean oil formulations and petroleum oil-based quenchants shown in Table 1. The heat transfer coefficients as a function of time and surface temperatures data are shown in Fig. 3-6.
As expected, the heat transfer coefficient versus time data showed that the conventional, unaccelerated MicroTemp157 petroleum oil-based quenchant exhibited significantly longer film boiling behavior compared to the fast, accelerated MicroTemp 153B quench oil. This behavior is due to the addition of additives that rupture the vapor blanket that forms around the hot-metal surface upon immersion of the metal into the quenchant. Significantly, this work confirm an earlier reported cooling curve behavior showing that vegetable oils do not exhibit this behavior (Ref 38).
Table 3 summarizes the maximum heat transfer coefficient, surface temperature, and cooling time where this occurs. The maximum heat transfer coefficient for fresh, unoxidized soybean oil is significant less than soybean oil containing antioxidants and is comparable to the unaccelerated MicroTemp 157 quench oil which interestingly is less than the accelerated petroleum oil, MicroTemp 153B. Also, while the addition of an antioxidant or antioxidant mixture does significantly increase the maximum heat transfer coefficient relative to fresh, unstabilized soybean oil, the amount of increase with respect to different antioxidant additions is not very large.
The effect of antioxidant addition on surface temperature and time where the maximum heat transfer coefficient occurs does not vary greatly for soybean oil. However, the surface temperature where the maximum heat transfer coefficient occurs is similar for both MicroTemp 153B and MicroTemp 157, but the time where this event occurs is somewhat greater for both oils than the soybean oil formulations with the unaccelerated MicroTemp 157 exhibiting the longest time—as expected.
Aging of the different oils showed a general decrease in the maximum heat transfer coefficient with respect to time as would be expected with the overall fluid viscosity increases illustrated in Fig. 2. Generally, the amount of decrease was the greatest for the least stable oils. There was no clear correlation in the source temperature and cooling time where the maximum heat transfer coefficient occurred, which may be due to the overall reported variability of the ASTM D6200 method.
Compared to the variation of the maximum heat transfer coefficients observed for the soybean oil fluids with increasing aging time, neither petroleum oil-based quenchant—MicroTemp 153B or MicroTemp 157—exhibited any significant difference in cooling behavior throughout the test, which is reasonable in view of the minimal viscosity change observed throughout the aging test for both oils as shown in Fig. 2.
Conclusions
The effect of the addition of two commercial antioxidants, Irganox L57 and Irganox L109, on the thermal-oxidative stability of soybean oil was studied. The results showed that both L57 and L109 did inhibit the thermal-oxidative degradation process compared to uninhibited soybean oil. However, even though the concentration of these antioxidants was not optimized, neither produced a stabilized soybean oil that even roughly approximated the stability of a commercial, fully formulated petroleum-based quenchant.
Cooling curve analysis showed that soybean oil, with or without the addition of an antioxidant, exhibited any significant full-film boiling behavior, whereas the petroleum oil quenchants did exhibit such behavior and, as expected, the accelerated petroleum oil (MicroTemp 153B) exhibited the less stable vapor blanker cooling behavior compared to the conventional MicroTemp 157 quench oil.
The cooling curves study also showed that the maximum heat transfer coefficient was greater (faster) for soybean oil containing antioxidants compared to uninhibited soybean oil. In addition, the maximum heat transfer coefficient decreased faster (due to fluid viscosity increase attributable to the thermal-oxidative degradation process) than the inhibited soybean oil fluids, with Irganox L109 and Irganox L57 being the most effective. However, this performance fell considerably short of that observed for both fully formulated petroleum oil quenchants, the maximum heat transfer coefficients of which did not change significantly throughout the test.
As a result of this work, it is concluded that although the addition of antioxidants does stabilize soybean oil, substantially better performance is required if vegetable oils are to be effective functional equivalents to petroleum oil formulations. This may be done by selecting different vegetable oil compositions with less unsaturation, by genetic modification of soybean seed oils or by chemically modifying and stabilizing the vegetable oil structure.
Notes
Crude soybean oil is refined by a series of processes to remove impurities that affect the taste, smell, appearance, and stability of the oil. The refining processes involve degumming, alkali refining, bleaching, and deodorization. However, it is important to note that it is possible that the thermal treatment during the purification process may actually lead to peroxide formation and result in poorer oxidative stability when subjected to elevated temperatures during use (Ref 27).
3 HT-MOD is a commercial code which is available from KB Engineering S.R.L.; Florida 274, Piso 3, Of. 35 (1005) Buenos Aires, Argentina; Tel: 54-11 4326-7542; Fax: 54-11 4326-2424; Internet: http://www.kbeng.com.ar.
References
J.L. Burns and V. Brown, The How and Why of Time Quenching, Am. Mach., 1940, 84(15), p 523–526
M.S.P. Murthy, B.R. Ghosh, P.P. Sinha, M.C. Mittal, and B.K. Sarkar, Studies on the Effects of Quenching Media and Quench Delays on the Properties of 12 mm Thick 15CDV6 Steel Plates, Trans. Indian Inst. Metals, 1982, 35(1), p 33–42
C.W. Finkl and N. Cerwin, Method of Controlled Fluid Quenching of Steel, U.S. Patent 5,180,444, 19 Jan 1993
N.I. Kobasko, M.A. Aronov, J.A. Powell, L.C.F. Canale, and G.E. Totten, Intensive Quenching Process Classification and Applications, Heat Treat. Metals, 2004, 31(3), p 51–58
H. Yu, J.A. Nicol, R.A. Ramser and D.E. Hunter, Method of Heat Treating Metal with a Liquid Coolant Containing Dissolved Gas, US Patent 5,681,407, 28 Oct 1997
G.E. Totten, Polymer Quenchants: The Basics, Adv. Mat. Proc., 1990, 137(3), p 51–53
G.E. Totten, G.M. Webster, S.W. Han and S.H. Kang, Immersion Time Quenching Technology to Facilitate Replacement of Quench Oils with Polymer Quenchants for Production of Automotive Parts, The 1st International Automotive Heat Treating Conference, R. Colas, K. Funatani and C.A. Stickels, Ed., (Materials Park, OH), ASM International, 1998, p 449–455
J. Pritchard and S. Rush, Vacuum Hardening of High-Strength Steels: Oil Versus Gas Quenching, Heat Treat. Progr., 2007, May/June, p 19–23
S. Serhan, “The Use of Vegetable Oils in Bio-Based Products”, Presentation available from National Center for Agricultural Utilization Research, USDA/ARS, 1815 N. University St., Peoria, IL, USA
Anonymous, “Corn Oil”, Brochure Published by Corn Refiners Association, 1701 Pennsylvania Ave. N.W., Washington, DC., 20006-5805 (www.corn.org)
M. Tagaya and I. Tamura, “Studies on the Quenching Media 3rd Report. The Cooling Ability of Oils,” Technology Report, Osaka University, Vol 4, 1954, p 305–319
Y. Fujimura and T. Sato, The Composition of Quenching Oil and Quenching Effects, Iron Steel Inst. Jpn., 1963, 49, p 1008–1015
R.J. Brennan, and C.H. Faulkner, A New Quenching Alternative, Conf. Proceed. 2nd International Conference on Quenching and Control of Distortion, G.E. Totten, K. Funatani, M.A.H. Howes, S. Sjostrom, Eds., (Materials Park, OH), ASM International, 1996, p 423–428
L.A.T. Honary, Performance of Vegetable Oils as a Heat Treat Quenchant, Conf. Proceed. 2nd International Conference on Quenching and Control of Distortion, G.E. Totten, K. Funatani, M.A.H. Howes, S. Sjostrom, Eds., (Materials Park, OH), ASM International, 1996, p 595–605
ISO 9950-1995, “Industrial quenching oils—determination of cooling characteristics—nickel-alloy probe test method”
ASTM D6200-07 “Standard Test Method for Determination of Cooling Characteristics of Quench Oils by Cooling Curve Analysis”, ASTM International, 100 Barr Harbor Drive, West Conshohocken, PA 19428 USA
W. Castro, J.M. Perez, S.Z. Erhan, and F. Caputo, A Study of the Oxidation and Wear Properties of Vegetable Oils: Soybean Oil Without Additives, J. Am. Oil Chem. Soc., 2006, 83(1), p 47–52
S. Knowlton, Soybean Oil Having High Oxidative Stability, U.S. Patent 5,981,781, 9 Nov 1999
E.B. Cahoon, Genetic Enhancement of Soybean Oil for Industrial Uses: Prospects and Challenges, AgBioForum, 2003, 6(1&2), p 11–13
C. Tompkins and E.G. Perkins, Frying Performance of Low-Linolenic Acid Soybean Oil, J. Am. Oil Chem. Soc., 2000, 77(3), p 223–229
L.A.T. Honary, Soybean Based Hydraulic Fluid, U.S. Patent 5,972,855, 26 Oct 1999
G.E. Totten, H.M. Tensi, and K. Lanier, Performance of Vegetable Oils as a Cooling Medium in Comparison to a Standard Mineral Oil, J. Mat. Eng. Perf., 1999, 8(4), p 409–416
K.N. Prabhu and P. Fernandes, Determination of Wetting Behavior, Spread Activation Energy, and Quench Severity of Bioquenchants, Metall. Mater. Trans. B, 2007, 38(4), p 631–640
P. Fernandes and K.N. Prabhu, Comparative Study of Heat Transfer and Wetting Behaviour of Conventional and Bioquenchants for Industrial Heat Treatment, Int. J. Heat Mass Transf., 2008, 51(3-4), p 526–538
M. Tagaya and I. Tamura, “On the Deterioration of Quenching Oils,” Technology Report, Osaka University, Vol 7, 1957, p 403–424
L.C.F. Canale, M.R. Fernandes, S.C.M. Agustinho, G.E. Totten, and A.F. Farah, Oxidation of Vegetable Oils and Its Impact on Quenching Performance, Int. J. Mater. Prod. Technol., 2005, 24(1-4), p 101–125
T. Kinami, N. Horii, B. Narayan, S. Arato, M. Hosokawa, K. Miyashita, H. Negishi, J. Ikuina, R. Noda, and S. Shirasawa, Occurrence of Conjugated Linolenic Acids in Purified Soybean Oil, J. Am. Oil Chem. Soc., 2007, 84(1), p 23–29
ABNT NBR 10441—10/02. Produtos de petróleo—Líquidos transparentes e opacos—Determinação da viscosidade cinemática e cálculo da viscosidade dinâmica
A. Bashford and A.J. Mills, The Development of Improved Additives for Quenching Oils using Laboratory Simulations, Heat Treat. Metals, 1984, 11(1), p 9–14
A.F. Farah, Caracterização de óleos vegetais como alternativa para meios de resfriamento utilizados no tratamento térmico de têmpera. Dissertação (Doutorado)—Interunidades em Ciência e Engenharia de Materiais, Universidade de São Paulo, São Carlos, 2002
G.G. Lamb, C.M. Loane, and J.W. Gaynor, Indiana Stirring Oxidation Test for Lubricating Oils, Ind. Eng. Chem. Anal. Ed., 1941, 13(5), p 317–321
G. S. Sarmiento, A. Gastón, and J. Vega, Inverse Heat Conduction Coupled with Phase Transformation Problems in Heat Treating Process, Computational Mechanics—New Trends and Applications, E. Oñate, S.R. Idelsohn, Eds., CIMNE, Barcelona, 1998, CD Book. Part VI, Section 1, Paper 16
J. Clark, and R. Tye, Thermophysical Properties Reference Data for Some Key Engineering Alloys, High Temp. High Press., 2003/2004, 35/36, p 1–14
S. Blaine and P.E. Savage, Reaction Pathways in Lubricant Degradation. 2. N-Hexadecane Autoxidation, Eng. Chem. Res., 1991, 30, p 218502191
I. Debruyne, Soybean Oil Processing: Quality Criteria and Flavor Reversion, Oil Mill Gaz., 2004, 110, p 10–11
I. Paz and M. Molero, Catalytic Effect of Solid Metals on Thermal Stability of Olive Oils, J. Am. Oil Chem. Soc., 2000, 77(2), p 127–130
W.F. Bowman and G.W. Stochowiak, Application of Sealed Capsule Differential Scanning Calorimetry—Part II: Assessing the Performance of Antioxidants in Base Oils, Lubr. Eng., 1999, 55(5), p 22–29
D. Komatsu, E.C. Souza, E. Carvalho de Souza, L.F.C. Canale, G.E. Totten, Effect of Antioxidants and Corrosion Inhibitor Additives on the Quenching Performance of SoybeanOil, Strojniški vestnik (J. Mech. Eng. Slovenia), 2010, 56(2), p 121–130
Acknowledgments
The authors acknowledge CAPES for the financial support and MSc Renata Leal for physical chemistry analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is an invited paper selected from presentations at the 26th ASM Heat Treating Society Conference, held October 31 through November 2, 2011, in Cincinnati, Ohio, and has been expanded from the original presentation.
Rights and permissions
About this article
Cite this article
de Souza, E.C., Canale, L.C.F., Sarmiento, G.S. et al. Heat Transfer Properties of a Series of Oxidized and Unoxidized Vegetable Oils in Comparison with Petroleum Oil-Based Quenchants. J. of Materi Eng and Perform 22, 1871–1878 (2013). https://doi.org/10.1007/s11665-013-0514-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-013-0514-2