Abstract
A three-dimensional transient multi-physical field model with comprehensive reduction reaction consideration has been developed to capture the complex processes of a three-phase submerged arc furnace. The multi-physics model integrates electromagnetic, fluid flow, reduction reaction, and thermodynamics phenomenon in unison computational framework. The furnace internal structure consists of arc and furnace charge, for which the physical properties include temperature dependence. Aiming to search the optimum design for more efficient industrial operation, the electrode insertion depth is investigated. The predicted temperature distribution of high temperature is in agreement with the measurement and simulation results. The result shows that the temperature of arc zone is maximum, which is 5897.17 K. The high-temperature area of furnace charge is near the arc zone. The temperature distribution is similar with current density. With the increase of electrode insertion depth, the average voltage drop of three arc zones is 11.43, 11.12, 10.83, and 10.35 V, respectively. When the electrode insertion depth is 1.99 and 1.79 m, the maximum magnetic field intensity around the electrode bottom is of 0.068 and 0.063 T. Cr2O3 mainly reacts under the arc bottom, while iron oxides are reduced less in the same location. An optimum electrode insertion depth exists according to the simulation results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
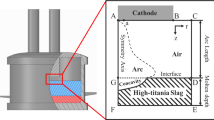
The submerged arc furnace has been widely used in the ferroalloy industry and has great energy saving potential.[1] In the ferrochrome production, ore is smelted by a three-phase submerged arc furnace.[2] Friedrich et al.[3] indicated that the inner part of the submerged arc furnace consists of the self-baked Soderberg electrodes, arc, furnace charge, slag, alloy, and a gas phase composed of CO and CO2. The schematic of submerged arc furnace is shown in Figure 1. The electric current is introduced through the three electrodes which are submerged in the furnace charge. The arc heat and Joule heat incurred by the electric power provide heat for the smelting process.
High-quality research has been carried out to understand the process and the control parameters of the submerged arc furnace. The benefits of using alternating current (AC) arc in the submerged arc furnace were firstly studied in the literature. Bowman and Edels[4] used the shock-wave technique to measure the free-burning AC arc. The AC arc was found to have a lower temperature, lower conductance, and greater power dissipation characteristics compared to the direct current arc. Larsen et al.[5] carried out a time-dependent numerical simulation for the AC arc in a silicon metal furnace where the crater gas consists of Si–O–C compounds. Saearsdottir et al.[6,7] simulated the AC arc at industry scale of which the arc current is of 100 kA and phase voltage is of 100 V. Barker et al.[8] studied the arcing measurements that can be used online in controlling the furnace. Moghadam et al.[9] studied the fluid flow and heat transfer process of the AC arc in ferrosilicon submerged arc furnace. Rehmet et al.[10,11] explored the arc behavior using three-phase AC plasma technology. The electrode jets and Lorentz force were found to have strong influence on the arc motion. Fulcheri et al.[12] presented a comprehensive review of three-phase AC arc plasma systems in the past 50 years. The review shows that the AC arc plasma has advantages in terms of efficiency, cost, and reliability.
Some numerical models were proposed to study the smelting state in the submerged arc furnace. Ranganathan and Godiwalla[13,14] analyzed the temperature profile and reduction reactions of submerged arc furnace for ferrochromium production. The Joule heating was considered, but the electromagnetic distribution was not presented in the study. Dhainaut[15] analyzed the electric field inside a submerged arc furnace, particular attentions were focused on the contact between two coke particles. Yang et al.[16,17] analyzed the physical and chemical phenomenon in the submerged arc furnace of ferrochrome production. The fluid flow, heat transfer, and reduction kinetics were simulated aiming to allocate an optimal operation condition for the smelting process. Scheepers et al.[7,18,19] developed a three-phase submerged arc furnace model using computational fluid dynamics (CFD) technique to simulate the process of phosphorus production. The study investigated the influence of various operating conditions on energy distribution within the burden and reaction characteristics. Bezuidenhout et al.[20] performed a numerical study on a circular, three-phase electrical furnace for the smelting of platinum group metal where the multiphase interactions and CO gas bubble were analyzed. Kadkhodabeigi et al.[21] presented a numerical model for the submerged arc furnace to investigate the effect of furnace crater pressure, metal height, and permeability of different internal zones. Barba et al.[22] used a three-dimensional finite element model to describe the furnace operation and discussed the control methods for the ferroalloy production. Karalis et al.[23] presented a steady-state computational fluid dynamic analysis of an industrial submerged arc furnace. The study showed that the furnace was divided into four different regions based on the governing equations of electric potential, momentum, and heat transfer. Halvorsen et al.[24] developed a simplified numerical method to predict the current and power distribution in a three-phase electrical smelting furnace where contribution of the electromagnetic induction is neglected. Teshahunegn et al.[25,26,27] proposed a scalar and vector potentials method to describe the dynamic of current distribution in the electrodes of a three-phase submerged arc furnace. The study showed that the skin and proximity effects of current distributions were presented in electrodes. Meier et al.[28] described an approach for the radiation in the submerged arc furnace. Wang et al.[29] proposed a transient three-dimensional model aiming to quantify the power consumption in a MgO electric arc furnace. In their study, the arc zone was considered as an equivalent resistance.[30] Although extensive works have been carried out recently, to the best of our knowledge, there is no attempt that successfully incorporated all essential physical considerations in one coupled numerical framework to model the complex smelting processes.
In the present work, a transient multi-physical field model combined with reduction reaction has been developed. When the furnace operates stably, the multi-physics field of arc and furnace charge are discussed. The multi-physics model can provide more valuable information about the submerged arc furnace smelting process. The AC arc is simulated together with the submerged arc furnace of which the physical properties are consistent with arc plasma. Parametric study is also carried out to analyze the influence of electrode insertion depth. Strategies for improving the furnace smelting state are also discussed.
Mathematical Model
In the submerged arc furnace, multi-physical field is closely coupled with each other. The energy input to the system is modeled in the form of electric potential. In the most of practical systems, the exact condition inside the furnace is normally unknown during the smelting process. Only the voltage and current can be measured by the existing instruments. Although the pellets are not conductive, the coke added into furnace makes the furnace charge conductive. Under the condition of high-temperature melting, the electrical conductivity of furnace charge increases. The electrical conductivity is influenced by the temperature distribution. The energy is used to melt the furnace charge and support the reduction reaction. Thus, the model contains two sub-models, which are the arc sub-model and furnace charge sub-model.
Arc Sub-model
A transient electromagnetics–temperature–flow multi-physical coupled sub-model of arc is developed to describe the arc behavior in the submerged arc furnace. The arc has magnetic fluid characteristic.[5] The transient Reynolds-averaged Navier–Stokes equations are closed by standard k–epsilon turbulence model equations. The heat transfer includes convection, conduction, and arc heat transfer. The governing equations of electromagnetic, momentum, and energy are described in the following.
Electromagnetic equations
The electromagnetic field is created by the AC. The current density is solved by electric charge conservation and Ohm’s law:
where \(\vec{J}\) is the electric current density (A/m2), \(\sigma\) is the electrical conductivity (S/m), and \(\vec{E}\) is the electric field (V/m). The electric potential method is used to calculate electric potential, which is expressed as follows:
where \(\phi\) is the electric potential (V), \(\vec{A}\) is the magnetic potential vector (V s/m), and t is the physical time (s). The magnetic potential vector is used to calculate the magnetic field, which is expressed as follows:
where \(\vec{B}\) is the magnetic field (T).
Momentum equation
In transient state, the continuity and the momentum equations are expressed as follows:
where ρ is the density (kg/m3), \(\vec{u}\) is the velocity vector (m/s), ρ is the fluid density, (kg/m3), P is the pressure (Pa), μ is the dynamic viscosity (Pa s), g is the gravitational acceleration, \(\vec{F}_{{\text{e}}}\) is the electromagnetic force (N/m3), which is expressed as follows:
Energy equation
The temperature distribution is calculated by the energy conservation equation:
where T is the temperature (K), λ is the thermal conductivity [W/(m K)], cp is the heat capacity [J/(kg K)], QArc is the arc heat (W/m3), which is calculated as follows[5]:
where \(\sigma_{{{\text{Arc}}}}\) is the electrical conductivity of arc (S/m), KB is Boltzmann’s constant, e is the electronic charge (C). QAR is the arc radiation. P-1 radiation model[18] is adopted to calculate the arc radiation, \(Q_{{\text{AR}}} = aG - 4\alpha n^{2} \xi T^{4}\), where a is the absorption coefficient, G is the incident radiation, ξ is the Stefan–Boltzmann coefficient, n is the refractive index of the medium.
Furnace Charge Sub-model
Electromagnetic–temperature–flow multi-physics coupled reduction reaction sub-model is developed for describing the furnace charge smelting process. The electromagnetic equations of arc and furnace charge are consistent. The energy equation include convection, conduction, Joule heat, and reaction heat. The furnace charge contains ferrochrome pellets, coke, and silica. The pellets have an average diameter of 20 mm. The porosity of pellets from preheating shaft kiln is 0.37. Thus, the source term of porous media resistance is added into the momentum equation. The governing equations of momentum, mass fraction of metal oxides, and energy are described in the following.
Momentum equation
In transient state, the momentum equation is expressed as follows:
where Si is the momentum source term, which is shown as follows:
where i stands for x, y, and z direction, α is the permeability coefficient, and C2 is the inertial resistance coefficient.
Mass fraction of metal oxides equation
The mass fraction of metal oxides equation is defined as follows:
where c is the metal oxides mass fraction. The flux term is downward velocity of the furnace charge, which is determined by industrial operation, s. SC is the source term [kg/(m3 s)], which is related to the degree of metal oxides, the equation is defined as follows:
where j is the serial number of simplified reaction, and K is coefficient to determine whether reaction occurs. When the temperature reaches the reaction temperature, K = 1, otherwise K = 0. Xj is the mass fractional conversion rate (s−1). Chromium and iron mass fractional conversion rate are described as[14] follows:
where kCr and kFe are the reaction rate constant (s−1). It is related to the activation energy and pre-exponential term through the Arrhenius equation:
where A is the pre-exponential term (s−1), Ea is the activation energy (kJ/mol), R is ideal gas constant [8.314 J/(mol K)], T is the temperature (K), The reaction parameters are listed in Table I, according to Ranganathan and Godiwalla.[14] Software HSC is used to absorb heat data.
Energy equation
The temperature distribution is calculated by the energy conservation equation:
where QJ is Joule heat, which is calculated as follows:
where \(\sigma_{{{\text{Fur}}}}\) is the electrical conductivity of furnace charge (S/m), QMelt is the latent heat of melting, which is equivalently converted to specific heat capacity, and QRea reaction heat is given as follows:
where q is the heat absorbed by reactions (J/mol), Δc is variation of mass fraction, M is the molar mass of the reactant (mol), and Δt is the simulation time step (s).
Assumptions and Simplification of Mathematical Model
To appropriate simplified model, several assumptions and simplifications are summarized as follows:
-
(a)
The arc exists at the end of the electrode with its shape approximated as a cylinder.
-
(b)
The chemical reactions are simplified into two main reduction reactions.
-
(c)
The operation mode of submerged arc furnace is continuous feeding and intermittent tapping. The calculation domain is full of ore at the beginning. After the reactions occur, the ore will move downward. The downward velocity of the furnace charge, which is determined by industrial operation, is used to describe the reaction process. For the ferrochrome production, the tapping process takes place once an hour after smelting. Tapping is not considered in this model.
-
(d)
To reduce computational resources, only arc and furnace charge are considered in the model. For the ferrochrome smelting, the current is from 55 kA to 155 kA. The electrical conductivity of electrode is 225,000 S/m by Tesfahunegn et al.[27] The electric voltage of electrode is only from 1.57 to 4.44 V. Thus, the electrodes are ignored.
Simulation Strategy
Geometry
The structure of submerged arc furnace is shown in Figure 2. The purpose is to study the reduction reaction of metal oxides in a submerged arc furnace. Thus, the computational domain is the inner region of furnace, which consists of the arc zone and furnace charge zone. The arc zone is created between the electrode tip, which is treated as arc plasma.[31,32] The furnace parameters refer to the work by Ranganathan and Godiwalla.[13] The details are tabulated in Table II.
The structural grid is adopted to discretize the entire computational domain. The sufficient mesh is refined in areas with the higher temperature gradient. The boundary layer and the arc zone are also refined for computational accuracy and stability. The grid resolution not only affects the computational time and resource, but also relates to the reliability of simulation results. The sensitivity of different grid numbers is examined in the present furnace model to obtain the grid independent results. The grid includes around 0.4 million, 0.6 million, and 0.8 million cells, named N1, N2, and N3.
Physical Properties
For the smelting of ferrochrome, the furnace charge contains ferrochrome pellet, coke, and silica. The main reason is that the quality of chrome ore is reduced, and the proportion of chrome ore powder is relatively large.[1] Chrome ore powder is pelletized, and fed into submerged arc furnace for smelting. The uniform particle size of pellets is 20 mm. The porosity of pellet is 0.37.[18] Figure 3 shows more details of the furnace charge for smelting. The physical properties are temperature dependent, which are extracted from the references and HSC software. They are summarized in Tables III, IV, V, and VI, respectively. The furnace gas contains carbon monoxide gas produced by reduction (77 pct), carbon dioxide by the oxidation of carbon monoxide (15 pct), less hydrogen (9 pct), and other gases (3 pct).
Boundary Conditions
Furnace boundaries are given the electromagnetic, thermal, and flow field conditions, which are listed in Table VI. For the furnace operation, there are two ways. One is constant current operation and the other is constant voltage operation. For the current operation, the electric resistance in the furnace is constant.[27] Actually, the electric resistance of arc and furnace charge is temperature dependent.[15,31] Due to the electric resistance which is variable, it need to operate for constant voltage.[23] The voltage drop of the electrode can be measured according to the Sheng et al.[33] According to the industrial measurement, the phase voltage is of 70.72 V for stable smelting, which is applied to the electrode tips. The frequency of alternating circuit is of 50 Hz. The phase angle between the electrodes is 120 deg. The setting of magnetic potential vector is based on reference Tesfahunegn et al.[25] No slip conditions are applied to the boundaries. The temperature boundary condition is determined according to the process of the furnace charge temperature rise.[5,34,35] The inner boundaries are solved by coupling method.[34] Considering reduction reactions, the initial composition of furnace charge is of 43.0 pct Cr2O3 and 12.0 pct iron oxides.
Numerical Procedure
The submerged arc furnace model combines with the heat transfer, reduction reaction, fluid flow, and electromagnetic field. The governing equations are integrated numerically using the commercial software ANSYS-FLUENT. Pressure-velocity coupling is treated with widely used SIMPLE algorithm, and the equations are discretized by a second-order upwind scheme. Since Reynolds number of the arc is higher than 2300, standard k–epsilon model is adopted to describe the arc flow.[33] The P-1 radiation model is applied to track the radiative heat transfer. The scattering and absorption coefficients are both considered as 0.6.[18] The velocity of furnace charge is the equivalent value evaluated according to the Ergun's formula. The piecewise linear method is used to insert the physical properties, which change with temperature. The simulation time step is 0.1 seconds.
Results and Discussion
The three-dimensional transient multi-physical field model is used to test and describe the ferrochrome pellets smelting process during the quasi-steady smelting state, which the time of calculation results is 1100 seconds. The arc and furnace charge smelting state are investigated, respectively. Furthermore, the effect of electrode insertion depths on stable smelting is also studied. Four different electrode insertion depths of 1.69, 1.79, 1.89, and 1.99 m are compared. The electrode insertion depth is defined as the depth from the top surface of furnace charge to the electrode tip, H. In the Reference 14, the depth is 1.79 m for steady smelting.
Model Validation
Figure 4 shows the grid independence test where the feed temperature of furnace charge is at 673 K. The vertical line of three grid numbers is compared when the smelting reached a stable smelting, which line is from (1.645, 0, 1.3) to (1.645, 0, 1.4). The temperature of arc zone is discussed based on three grid numbers. It shows that the temperature on the line in N1 grid number is not consistent with the temperature in N2 and N3 grid number. It also indicates that a finer grid N3 has little impact on the numerical results. Thus, the present grid density N2 is considered to provide accurate results.
The experimental data are extracted from the work by Ranganathan and Godiwalla[14] for a steady smelting process. Figure 5 shows the comparison of the predicted and measured temperature distribution along the line from (− 0.804, − 3.872, 2.19) to (− 0.804, 3.872, 2.19) at the stable smelting process. In the area around the electrode, the trend of measured data is corresponding to the numerical temperature of furnace charge. As depicted, the predicted temperature distribution of high temperature is in agreement with the measurement and simulation results, while the difference of low-temperature region exists because of the boundary condition.
Multi-physical Field of Arc
Arc plays an important role in energy transfer and transformation in SAF. Thus, it is very meaningful to study the current density, Joule heat, voltage drop, and temperature of the arc zone.
Electromagnetic field distribution
To understand the distribution of electromagnetic fields, the horizontal cross-section of arc zone (z = 1.35 m) is used to present the results. The current density distribution and current pathway of arc zone on the cross-section are shown in Figure 6. As seen in the section, the current flows in from one arc and out from the other arcs. Meanwhile, the current density at the arc zone center is lower than that at the arc zone boundary. The current density distribution is uneven, which is the result of skin effect by AC.
Figure 7 shows the Joule heat distribution in the same cross-section (z = 1.35 m) of the arc zone. The Joule heat value of three arc zones is also different. A great amount of Joule heat is produced on the arc zone side surface. The Joule heat distribution of arc zone is consistent with the current density distribution, which is based on Eq. [9].
The voltage drop of arc zone is vital for the ferrochrome pellets smelting process. According to the simulation, the surface average electric voltage values at the electrode bottom and arc bottom can be obtained. The surface average electric voltage difference between the two parts is the arc voltage drop, named ΔU. As shown in Figure 8, the electric voltage value of three arc zones is not the same. The minimum voltage drop of arc zone1 is at the insertion depth of 1.99 m, whose value is of 15.49 V. The maximum voltage drop of arc zone2 is at the insertion depth is 1.89 m, which value is of 11 V. The voltage drop of arc zone3 decreases with the depth of electrode insertion. The average voltage drop of three arc zones is 11.43, 11.12, 10.83, and 10.35 V with the electrode insertion depth, respectively. The voltage drop of furnace charge can be obtained indirectly by the arc voltage drop.
Temperature distribution
The temperature distribution of whole arc zone is described in Figure 9. As seen, the maximum temperature is found at the interface of arc zone and furnace charge. The skin effect of AC also affects the temperature distribution of arc zone, which is similar with current density. The temperature of arc zone is above 4000 K, which can be used to smelt the furnace charge of higher melting point and higher reaction temperature. Table VII lists the temperature value of arc zone with electrode insertion depths. The maximum temperature of arc zone named TM, and the average temperature of arc wall and arc bottom is TAW and TAB, respectively. As listed in the table, the average temperature of arc wall is higher than arc bottom. When the electrode insertion depth is 1.79 m, the arc temperature is the maximum, which is 5897.17 K. Compared with other insertion depth, TAW and TAB value of H = 1.79 m is also the largest.
Multi-physical Field of Furnace Charge
Electromagnetic field distribution
To show the current flow and current density distribution, the horizontal cross-section A-Plane (z = 1.30 m) and vertical cross-section B-Plane (x = − 0.804 m) of furnace charge are selected to analyze the results. The current density and current pathway distribution are shown in Figure 10. The current vector shows the current pathway within the furnace. As seen in A-Plane, the maximum current density exists at the center of the arc zone. The current density near the arc zone is higher than the other place. As shown in B-Plane, the current pathways can be categorized into four different types, which are consisted with the insight of Karalis et al.[23] The first type of pathway is the current flows between the electrode surfaces and arc zone surfaces. The second one is the current flows among the arc bottoms. The third one is the current flows from the arc bottom, arc wall, and electrode surface to the furnace wall. The last one is the current flows vertically from the arc bottom to the furnace bottom.
Figure 11 shows the maximum magnetic field intensity of cross-sections along z-axis with the electrode insertion depths. The maximum magnetic field intensity value at different cross-section is named Bm. When the electrode insertion depth is 1.99 m, the maximum value of Bm on the cross-section (z = 1.30 m) is of 0.068 T. As depicted, the value of Bm is the largest at the electrode bottom. The value of Bm at the furnace bottom is higher than that at the upper surface. Increasing the electrode insertion depths, the value of Bm decreases on the cross-sections under the arc bottom with the increase of z-axis. On the contrary, the value of Bm increases on the cross-sections above the electrode bottom with the increase of z-axis.
Thermal field distribution
The temperature distribution could be more intuitive to understand the furnace smelting state. It is a critical factor affecting the smelting efficiency inside the submerged arc furnace. The melting profile of high-temperature area determines whether the smelting process is at the optimal operation. Due to the electric resistance, the arc heat and Joule heat are generated at the arc and furnace charge zones. Figure 12 shows the temperature distribution of furnace charge. As depicted, the temperature close to the arc is higher than that in the furnace charge. Driven by the temperature gradient, the heat is then transferred from the arc zone to the furnace charge. The position of high temperature is around the electrode tips. The area of high temperature is affected by the reduction reactions. The endothermic reaction causes abnormal temperature distribution below the arc zone. Compared with Figure 10, it can be found that there is a small value of current density in the pitch circle of electrode where the z-axis is less than 0.5 m. The current density is consistent with the temperature distribution at the same location.
Through the selection of four positions, the influence of different electrode insertion depth on furnace temperature is analyzed. As shown in Figure 13(a), the depths of 1.79, 1.89, and 1.99 m have the same temperature value from y = − 3 to 3 m. However, the temperature value of H = 1.69 m is higher than the other insertion depths at the same location. From y = − 3.81 to − 3.0 m and from y = 3.0 to 3.81 m in the y-axis, no significant differences can be observed among various insertion depths. In Figure 13(b), the maximum temperature appears at the depth of 1.79 m along the line of z = 1.3 m. But the temperature of H = 1.69 m is the maximum in the center of the furnace. The temperature of H = 1.99 m is minimum from y = − 2.5 to 2.5 m. In Figures 13(c) and (d), the deeper insertion depth would cause a larger temperature of furnace charge below the electrode tips. The variation of insertion depths has a profound effect on the furnace charge temperature. H = 1.89 and 1.99 m can be used to increase the temperature of furnace charge. For the steady smelting, H = 1.69 and 1.79 m is better.
Reduction reaction field distribution
The majority of heat consumption within the furnace is accounted by the chemical reductions. There are three types of chemical reactions occurring in the smelting process, including the oxidation of iron, reduction reactions, and slagging reactions. The process of slagging reactions is insignificant and negligible.[36] Figure 14 shows the mass fraction of Cr2O3 and iron oxides distribution along the B-Plane through the electrodes. Before the ore feeds into the furnace, the metal oxides mass fraction of Cr2O3 and iron oxides are both uniformly distributed around the proportioning station. There are two factors affecting the reactions, which are the temperature and the metal oxides mass fraction. According to the reaction kinetics, the reactions take place first at the high-temperature area, as shown in Figure 12. At the stable smelting process, one could notice that the iron oxides react rigorously and completely in the furnace. Cr2O3 reacts completely around the arc bottom. Under the electrode, the Cr2O3 mass fraction decreases with the z-axis.
The electrode insertion depth is a significant factor affecting reduction reactions. It influences the overall smelting process by directly changing the temperature distribution within the furnace. To further examine the degree of smelting at electrode insertion depths, the metal oxides mass fraction distribution along A-Line and B-Line is discussed in detail. Figures 15(a) and (b) are at A-Line from (0, 0, 0) to (0, 0, 3.19). As seen in Figure 15(a), the amount of iron oxides reduction reaction is less with the increase of z-axis. At the depths of 1.79, 1.89, and 1.99 m, iron oxides are completely reduced where A-Line is less than 0.5 m along z-axis. Nevertheless, the reaction reduces with the increase of the electrode insertion depth. At the depth of 1.99 m, the reduction effect of iron oxides is less than the other insertion depths. As shown in Figure 15(b), the mass fraction of Cr2O3 decreases from the furnace bottom to the arc bottom, named descending stage. It increases to the initial composition 43 pct from the electrode bottom to the upper surface of furnace charge, named rising stage. By comparing electrode insertion depths, Cr2O3 reaction increases with the insertion depths in the descending stage. In the rising stage, the electrode insertion depth is opposite to Cr2O3 reaction trend. The reaction is more thorough at the arc zone along the z-axis. Figures 15(c) and (d) are at B-Line from (− 0.804, 1.434, 0) to (− 0.804, 1.434, 1.5). As seen in Figure 15(c), the amount of iron oxides reacts less below the arc zone. For the depth of 1.99 m, the mass fraction of iron oxides is largest. It can be noticed that the reaction of iron oxides is less under the electrode. However, the reaction of Cr2O3 reacts completely under the electrode, which is shown in Figure 15(d). This phenomenon can also be observed in Figure 14(b).
It indicates that an optimal insertion depth could exist in regard to promote the smelting process. According to the numerical predictions, the depths of 1.69 and 1.79 m seem to yield the best production for the current study case. The deeper electrode insertion depth does not facilitate the reduction of metal oxides. From the perspective of rapid temperature rise in the furnace, the suitable electrode insertion depth could be of 1.99 m (Figures 13(c) and (d)).
Conclusion
A three-dimensional transient multi-physical field model has been developed to predict the complex smelting state in a submerged arc furnace. Physical phenomena including the electromagnetism, turbulence fluid flow structure, heat transfer, and reduction reaction are incorporated in a seamlessly coupled modeling framework. The numerical results are in agreement with the experimental measurements by Ranganathan and Godiwalla at high temperature, which verifies the creditability and reliability of the proposed numerical model.
-
(1)
The electromagnetic and temperature fields of arc zone have strong characteristics in ferrochrome pellets smelting process. The current flows in from one arc and out from the other arcs. The current density distribution is uneven, which is the result of skin effect by AC. When the electrode insertion depth is 1.79 m, the arc temperature is the maximum, which is 5897.17 K. At the phase voltage of 70.72 V, the average voltage drop of arc zone is 11.12 V, which is related to the furnace charge.
-
(2)
For the furnace charge, four different types of current pathways can be observed in the predicted results. The position of high-temperature area is near the arc zones. The high-temperature area is affected by the reduction reactions. Based on the present study, the reaction area of Cr2O3 mainly occurs at the arc bottom, while the reduction of iron oxides occurs at a larger area in SAF.
-
(3)
The electrode insertion depth exhibits a profound effect on the smelting temperature and reduction reactions of metal oxides. A parametric study has been carried out to search the optimum electrode insertion depth. Considering the rapid temperature rise in the furnace, the electrode insertion depth of 1.99 m appears to be an optimum choice. For the propose of effective smelting in the furnace, the electrode insertion depth is 1.69 and 1.79 m.
Abbreviations
- a :
-
Absorption coefficient
- A :
-
Pre-exponential term (s−1)
- \(\vec{A}\) :
-
Magnetic potential vector (V s/m)
- \(\vec{B}\) :
-
Magnetic field (T)
- B m :
-
Maximum magnetic field intensity (T)
- c :
-
Reactant mass fraction
- \(C_{{\text{Cr}_2}{\text{O}_3}}\) :
-
Mass fraction of Cr2O3
- c Iron oxides :
-
Mass fraction of iron oxides
- c p :
-
Heat capacity [J/(kg K)]
- C :
-
Linear-anisotropic phase function coefficient
- C 2 :
-
Inertial resistance coefficient
- e :
-
Electronic charge (C)
- \(\vec{E}\) :
-
Electric field (V/m)
- E a :
-
Activation energy (kJ/mol)
- \(\vec{F}_{{\text{e}}}\) :
-
Lorentz force (N/m3)
- G :
-
Incident radiation
- H :
-
Electrode insertion depth (m)
- i :
-
x, y, And z direction
- j :
-
Serial number of simplified reaction
- \(\vec{J}\) :
-
Electric current density (A/m2)
- k :
-
Reaction rate constant (s−1)
- K B :
-
Boltzmann’s constant
- n :
-
Refractive index of the medium
- N :
-
Grid number
- M :
-
Reactant amount (mol.)
- P :
-
Pressure (Pa)
- q :
-
Heat absorbed by reactions (J/mol)
- Q Arc :
-
Arc heat (W/m3)
- Q J :
-
Joule heat (W/m3)
- Q Melt :
-
Latent heat of melting (W/m3)
- Q R :
-
Radiant heat (W/m3)
- R :
-
Ideal gas constant [8.314 J/(mol K)]
- S C :
-
Variation of reactant [kg/(m3 s)]
- S i :
-
Momentum source term
- T :
-
Temperature (K)
- T M :
-
Maximum temperature of arc zone (K)
- T AW :
-
Average temperature of arc wall, (K)
- T AB :
-
Average temperature of arc bottom (K)
- \(\vec{u}\) :
-
Velocity vector (m/s)
- ΔU :
-
Arc voltage drop (V)
- X Fe :
-
Mass fractional conversion rate of iron reduction (s−1)
- X Cr :
-
Mass fractional conversion rate of chromium reduction (s−1)
- \(\alpha\) :
-
Permeability coefficient
- \(\xi\) :
-
Stefan–Boltzmann coefficient
- \(\xi_{{\text{s}}}\) :
-
Scattering coefficient
- λ :
-
Thermal conductivity [W/(m K)]
- μ :
-
Dynamic viscosity (Pa s)
- ρ :
-
Fluid density (kg/m3)
- σ :
-
Electrical conductivity (S/m)
- φ :
-
Electric potential (V)
References
Y. Yu, B. Li, C. Wang, Z. Fang, X. Yang, and F. Tsukihashi: Energy., 2019, vol. 179, pp. 792–804. .
J. Daavittila, M. Honkaniemi and P. Jokinen: Tenth Int. Ferroalloys Congr., Cape Town, 2004, pp. 432–43.
B. Friedrich, M. Kalisch, D. Friedmann, R. Degel, F. Kaußen, and J. Bohlke: J. Sustain. Metall., 2018, vol. 4, pp. 77–94. .
B. Bowman and H. Edels: J. Phys. D., 1969, vol. 2, pp. 53–63. .
H.L. Larsen, G. Liping, and J.A. Bakken: INFACON 7, Trondheim, 1995, pp. 517–27.
G.A. Saearsdottir, J.A. Bakken, V.G. Sevastyanenko, and L. Gu: Ironmak. Steelmak., 2001, vol. 28, pp. 51–7. .
G.A. Saearsdottir and J.A. Bakken: Twelfth Int. Ferroalloys Congr., Helsinki, 2010, pp. 717–28.
I.J. Barker, M.S. Rennie, C.J. Hockaday, and P.J. Brereton-Stiles: IFAPA XI, 2007, pp. 685–94.
M.M. Moghadam, S.H. Seyedein, and M.R. Aboutalebi: J. Iron Steel Res. Int., 2010, vol. 17, pp. 14–8. .
C. Rehmet, V. Rohani, F. Cauneau, and L. Fulcheri: Plasma Chem. Plasma Process., 2010, vol. 33, pp. 491–515. .
C. Rehmet, F. Fabry, V. Rohani, F. Cauneau, and L. Fulcheri: Plasma Chem. Plasma Process., 2014, vol. 34, pp. 975–96. .
L. Fulcheri, F. Fabry, S. Takali, and V. Rohani: Plasma Chem. Plasma Process., 2015, vol. 35, pp. 565–85. .
S. Ranganathan and K.M. Godiwalla: Ironmak. Steelmak., 2001, vol. 28, pp. 273–8. .
S. Ranganathan and K.M. Godiwalla: Can. Metall. Q., 2011, vol. 50, pp. 37–44. .
M. Dhainaut: Tenth Int. Ferroalloys Congr., Cape Town, 2004, pp. 605–13.
Y. Yang, Y. Xiao, and M.A. Reuter: Int. Ferroalloy Congr., Cape Town, 2004, pp. 15–25.
Y. Yang, B. Zhou, J.R. Post, E. Scheepers, M.A. Reuter, and R. Boom: Fifth Int. Conf. CFD Process Ind. CSIRO, Melbourne, 2006, pp. 11–15.
E. Scheepers, Y. Yang, M.A. Reuter, and A.T. Adema: Miner. Eng., 2006, vol. 19, pp. 309–17. .
E. Scheepers, Y. Yang, A.T. Adema, R. Boom, and M.A. Reuter: Metall. Mater. Trans. B., 2010, vol. 41B, pp. 990–1005. .
J.J. Bezuidenhout, J.J. Eksteen, and S.M. Bradshaw: Miner. Eng., 2009, vol. 22, pp. 995–1006. .
M. Kadkhodabeigi, H. Tveit, and J.S. Tore: Trans. Iron Steel Inst. Jpn., 2011, vol. 51, pp. 193–202. .
P.D. Barba, F. Dughiero, M. Dusi, M. Forzan, M.E. Mognaschi, M. Paioli, and E. Sieni: Int. J. Appl. Electromagn., 2012, vol. 39, pp. 555–61. .
K.T. Karalis, N. Karkalos, N. Cheimarios, G.S.E. Antipas, A. Xenidis, and A.G. Boudouvis: Appl. Math. Model., 2016, vol. 40, pp. 1–15. .
S.A. Halvorsen, H.A.H. Olsen, and M. Fromreide: IFAC 49-20, 2016, pp. 167–72.
Y. Shi et al. (Eds.): ICCS 2018, LNCS 10861, 2018, pp. 518–527.
Y.A. Tesfahunegn, T. Magnusson, M. Tangstad, and G. Saevarsdottir: IEEE MTT-S, Reykjavik, 2018.
Y.A. Tesfahunegn, T. Magnusson, M. Tangstad, and G. Saevarsdottir: Metall. Mater. Trans. B., 2020, vol. 51B, pp. 510–8. .
T. Meier, K. Gandt, T. Hay, and T. Echterhof: Steel Res. Int., 2018, vol. 89, pp. 1–14. .
Z. Wang, N.H. Wang, and T. Li: IEEE, 2010, pp. 433–36. https://doi.org/10.1109/iCECE.2010.112.
Z. Wang, N.H. Wang, and T. Li: Mater. Sci. Forum, 2011, pp. 995–98.
J. Aubreton, M.F. Elchinger, A. Hacala, and U. Michon: J. Phys. D., 2009, vol. 42, pp. 95206–18. .
B. Sourd, J. Aubreton, M.F. Elchinger, M. Labrot, and U. Michon: J. Phys. D., 2006, vol. 39, pp. 1105–19. .
Y.Y. Sheng, G.A. Irons, and D.G. Tisdale: Metall. Mater. Trans. B., 1998, vol. 29B, pp. 77–83. .
X. Zhang, Z. Tong, D. Li, X. Hu, and Y. He: Appl. Therm. Eng., 2021, vol. 185, p. 115980. .
L.H. Gunnewiek, L. Oshinowo, T. Plikas, and R. Haywood: Tenth Int. Ferroalloys Congr., Cape Town, 2004, pp. 555–64.
Y. Yu, B. Li, Z. Fang, and C. Wang: J. Clean. Prod., 2021, vol. 285, p. 124893. .
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 51934002).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted April 16, 2021; accepted August 13, 2021.
Rights and permissions
About this article
Cite this article
Yu, Y., Li, B., Yun, C. et al. Modeling on Reduction Reaction of Metal Oxides for Submerged Arc Furnace in Ferrochrome Pellets Smelting Process. Metall Mater Trans B 52, 3907–3919 (2021). https://doi.org/10.1007/s11663-021-02304-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02304-5