Abstract
A modified cast-on method has been developed to reinforce aluminum castings with steel insert. Defect-free bond between the steel insert and the aluminum casting has been consistently obtained. Data obtained from a push-out experiment indicated that the bond strength was much higher than that obtained using the Al-Fin approach. This paper introduces this modified method in four sections: the coating of the steel pins, the cast-on method, microstructure characterization, and the bond strength. The section on the coating of the steel pins contains coating material selection, electroplating technique for plating Cu and Ni on steel, and diffusion bonding of the coatings to the steel. The section on cast-on method deals with factors that affecting the quality of the metallurgical bond between the coated steel and the aluminum castings. The results of microstructure characteristics of the bonding are presented in the microstructure characterization section. A push-out experiment and the results obtained using this method is described in the section of bond strength/mechanical property.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lightweight metals and alloys such as aluminum and magnesium have found increased applications in replacing iron and steels in the defense and automotive industries for weight reduction of vehicles. Such substitutions, however, have often resulted in compromised performance and/or reliability. A well-known solution to some of the performance and reliability problems associated with the use of light weight casting materials as a substitute for cast irons and steels has been to provide high strength inserts at critical locations where severe wear or high stress is known to occur. Critical locations are defined as areas in a casting where the stresses or temperatures exceed the capabilities of the lightweight materials. Inserts of expensive material can also be used at critical areas where severe corrosion is known to occur so that inexpensive material can be used for making the rest of a component/casting.
The concept of joining dissimilar materials into a single component to take best advantage of the unique properties of each material is not new.[1–3] Over the years, it has been referred to as bimetal or bimetallic construction, composite design, duplex materials, and others.[4–6] Cast-on method is one of the most cost-effective methods for bonding iron or steel to lightweight materials using a metal casting process.[1–3] Al-Fin is one of such methods which involves dip coating the steel insert in molten metal with low melting temperatures, such as zinc or aluminum alloys, and casting-in the coated steel into aluminum castings. The method has found some applications but has not found general acceptance in applications of high performance, reliability, and durability requirements. One explanation for this is the difficulty of achieving an effective, durable metallurgical bond between the insert and the adjacent lightweight cast material. Beile and Lund[6] disclose a bonding technique for achieving metallurgical bonding requiring an absolutely clean surface on the inserts. Practical methods to prevent oxidation are to employ vacuum, insert atmospheres, or reducing atmospheres. It has been reported that the production of an intimate bond may be prevented by the presence of an oxide film on the outer surface of the aluminized coating on the insert.[7]
A few approaches have been undertaken in an attempt to achieve an acceptable metallurgical bond between inserts and cast metals.[7,8] These approaches utilize pre-coatings to protect the insert surface from oxidation and other contaminations. However, none of these methods has been entirely successful in producing consistent, high strength bonds between inserts and lightweight casting material that will meet the long-term demands for reliability required in certain applications such as the manufacture of heavy-duty diesel engine components. These processes are prone to producing defects caused by voids, gas porosity, and oxides. The range of bond strength, measured as shear strength using a typical push-out method, is between 0 and 60 MPa.[9,10] In many cases, the insert will simply drop off from the casting as the number of defects is so great that no metallurgical bond is formed whatsoever. Therefore, weight reduction through the broad application of lightweight casting materials remains an unmet, yet highly desirable, goal for many applications including vehicles, marine markets, and certain military applications.
This article describes a modified cast-on method that is slightly different to that of the Al-Fin method. Instead of dip coating the steel insert in molten metal with low melting temperatures, such as zinc or aluminum alloys, the steel insert is plated metals with higher melting temperatures, such as silver, nickel, copper, and chromium.[9,10] This article focuses on inserts plated with a layer of nickel and then another layer of copper. Diffusion bonding is used to bond the electrically plated layers to the steel insert. The insert with two layers of plating are then placed in a mold and cast in an aluminum alloy casting. With suitable process control, defect-free metallurgical bond between the steel insert and aluminum castings is obtained. This paper details the technology development and the characterization of the bonds in the following sections: coating development, cast-on method, and results and discussion.
Coating Development
Coating Material Selection
Sacrificial coatings were designed in order to protect the steel insert from contamination and, more importantly, to prevent the formation of unfavorable intermetallic phases at the steel-aluminum interface. In addition, the coatings were diffusion bonded to the steel insert to prevent them from spalling off during casting. This resulted in coatings that protected the steel prior to casting, partially melted during the casting process to provide a clean interface for bonding, and prevented direct contact between the aluminum and steel and the resulting formation of undesirable phases.
Initially, a single-layer coating using a number of materials was tested. Later, it was decided to use a dual-layer coating on the steel insert. Coating materials considered were copper, nickel, silver, and chromium. The thickness of each coating layer varied from 25 (1 mil) to 100 μm (4 mils). The steel inserts were made of 1144 steel. The inserts were approximately 50.8 mm long and 12.7 mm in diameter.
The selection criteria for the materials used in the dual-layer coating were as follows:
-
The material used for the outer layer should have a larger thermal expansion coefficient than that used as the inner layer. Both coating materials should have larger thermal expansion coefficients than steel. This is to ensure that the coatings physically shrink fit onto the steel on cooling.
-
The coating materials should have a large solubility range for each other and the material used as the inner coating should have a large solubility in iron. This is to ensure that the coatings can metallurgically bond to each other during diffusion bonding.
-
The outer layers act as a sacrificial coating to help bonding between the inner layer and the aluminum casting. During the casting process, the sacrificial layers partially or totally dissolve into molten aluminum, leaving behind a fresh and clean surface of the insert to encourage chemical reaction between the insert and the molten metal.
Based on the above three criteria, two steel coating systems were selected. They were steel/nickel/copper (SNC) and steel/nickel/silver. Both systems were tested. This article focuses on the SNC system.
Electroplating Technique
Electroplating techniques were used to plate the dual-layer coatings on steel inserts. Initially, standard commercial techniques of electroplating were used. After the nickel layer was plated at 350 K (77 °C), the hot steel inserts were immediately dipped in a solution at room temperatures for coating the copper layer. Optical microscopy revealed that a gap was formed at the nickel/copper interface as shown in Figure 1. After careful review of the processing steps, it was deduced that when the hot insert was dipped into the electrolytic liquid at room temperatures, a thin layer of copper likely formed immediately on top of the nickel layer. Subsequently, the nickel-coated steel insert cooled and contracted during cooling, causing the nickel to pull away from the copper and leave a gap at the nickel/copper interface.
Based on the proposed mechanism of gap formation, the electroplating technique was modified. After nickel was plated, the inserts were cooled to room temperature. The copper layer was then coated at 316 K (43 °C). Figure 2 shows the SNC interfaces using the modified plating technique. No gap was found between the SNC layers. In order to strengthen the steel-nickel interface, the plating technique was further modified. The steel surface was deep etched to make the surface rougher.
The final electroplating procedure was as follows: (1) Soak, clean, and degrease inserts, (2) Remove rust on steel surface using 30 pct HCl solution, (3) Deep etch the insert surface using 50 pct HCl solution at 313 K (40 °C), (4) Plate nickel at 350 K (77 °C), (5) Rinse in tap water at room temperature, (6) Dip in 30 pct HCl solution at room temperature, (7) Rinse in tap water at room temperature, (8) Plate copper at 316 K (43 °C), and (9) Rinse in tap water at room temperature.
Diffusion Bonding
The electroplated coating layers were then diffusion bonded. The coated inserts were loaded into a vacuum furnace at room temperatures, heated to 1173 K (900 °C), held at 1173 K (900 °C) for 4 hours, and cooled in the furnace to room temperatures. Figure 3 shows the composition profiles in the steel substrate and the two coating layers. Nickel diffuses into both the steel substrate and the copper layer. Under the conditions used, there is no diffusion of the copper into the steel or vice versa.
Cast-on Method
The Method
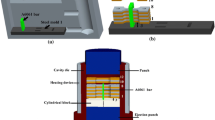
After diffusion bonding, the coated inserts were cast in place in a sand mold. The mold consisted of a pouring basin connected to a runner that subsequently fed three casting cavities. Each casting cavity contained two inserts that were vertically placed in the cavity and held in place in depressions in the sand mold. Figure 4 illustrates the locations of the steel insert and the casting configuration used.
The procedure for casting-in of steel inserts included the following: (1) Dry sand mold for 6 hours using heat lamps; (2) Pre-heat the coated inserts at 390 K (120 °C) for 15 minutes; (3) Melt and maintain aluminum alloy A354 at 993 K (720 °C); (4) Degas the melt at 993 K (720 °C) using argon + 4 pct chlorine gas; (5) Add 0.375 wt pct strontium and 0.5 wt pct grain refiner; (6) Place two preheated coated steel inserts in each mold cavity; (7) Pour the melt through a filter into the mold under argon protection; and (8) Allow melt to overflow casting cavities to ensure that the leading metal front (possibly containing oxides) does not freeze around the inserts.
Factors Affecting the Quality of the Bond
The quality of the bond between the steel insert and the casting was affected by many factors such as the melt quality, hydrogen content of the melt, pour temperature, and the temperature of the mold and the steel insert, the moisture content of the mold, the protective atmosphere of the molten metal during casting, and the quality of the melt flowing pass the inserts. However, the critical parameters that affected bond quality were hydrogen content and melt quality. In particular, entrained oxides at the leading edge of the metal front have to be removed from the cavity using overflow.
Figure 5 shows the influence of melt hydrogen on the quality of the bond. When degassing was insufficient, a substantial amount of porosity formed at the insert/casting interface. The bond quality improved with improved degassing. Ultimately, a defect-free bond was obtained. Experimental results indicated that defect-free bonds were obtained when degassing was sufficient such that no porosity could be observed with the naked eye at the center of a reduced pressure test (RPT) specimen sectioned in half. The use of an argon cover during casting noticeably improved the results.
During mold filling, the leading edge of the melt entrains oxides and loose sand particles as it flows over mold surfaces. The use of an over flow prevented the initial metal from freezing on the inserts. It is assumed that with sufficient overflow, even if part of the leading edge froze on the inserts, subsequent flow was sufficient to remelt and remove this material from the interface.
Figure 6 shows the steel/casting interface in three different castings (containing six steel inserts). No defects are observed at the steel/casting interface. Although some kinds of defects on the steel/casting were observed within a few millimeters near the surface of the casting, the defective regions can be easily removed by machining. The defective regions can also be moved out of the casting for easy machining operations.
The Strength of the Bond
Bond Strength Measurements
Samples illustrated in Figure 7 were cut for testing the mechanical properties of the steel/casting bond. A push-out test setup was designed to evaluate the interfacial strength of the bond. A punch of 9.5 mm (0.375 in.) diameter was used to push out a steel insert of 12.7 mm (0.5 in.) diameter out of the aluminum alloy. The specimen dimensions were 25.4 mm (1 in.) square and 6.35 mm (0.25 in.) thick with a 12.7-mm (0.5 in.)-diameter steel insert at the center. Five specimens were cut using a bench saw from one steel insert 38.1 mm (1.5 in.) height. The crosshead speed was 1.27 mm/min. The peak stress on the stress/displacement curve was defined as the interfacial strength of the bond. The strain at the peak stress was defined as the strain to fracture.
Bond Strength Under As-Cast Conditions
Push-out experiments were carried out to investigate the strength of steel/casting bond under various conditions. These conditions included the nominal thickness of nickel coating 25 to 100 µm (1 to 4 mils) with an outer layer copper coating of 100 µm (4 mils) and diffusion bonding times (1 to 4 hours at 900 °C). Figure 8 shows the interfacial strength as a function of measured thickness of nickel and diffusion bonding time under the as-cast conditions. The bond strength is between 55 MPa (8000 psi) to 82.7 MPa (12,000 psi), higher than 49.6 MPa (7200 psi) which is a typical bond strength using the Al-Fin process. The data suggest that the bond strength of samples using this modified process is not affected by the diffusion bonding time in the range between 1 and 4 hours. The bond strength is hardly affected by the nominal thickness of nickel coating in the range between 25 and 100 µm (1 to 4 mils). The measured thickness corresponding to the nominal thickness is 12.5 to 62.5 µm (0.5 to 2.5 mils), respectively.
Figure 9 shows the strain to fracture data for three types of samples, namely steel insert coated with Zn-5 pctAl (Al-Fin process), 1Ni/4Cu (25 µm Ni and then 100 µm copper), and 2Ni/4Cu (50 µm Ni and then 100 µm copper). The strain to fraction increases from an average of 14 pct for the samples made using the Al-Fin process to 17 pct for samples made using this new process, representing over 21 pct increase in the strain to fracture. Experimental data suggest that the bond produced using this modified method is tougher than that produced using the Al-Fin method.
Bond Strength After T5 or T6 Heat Treatment
Bond strength after heat treatment was also measured. Figure 10 depicts a comparison of the as-cast bond strength with that after T5 heat treatment (held at 423 K (150 °C) for 10 hours). The as-cast bond strength is not affected by T5 heat treatment.
The castings were then heat treated under the T6 conditions (held 799 K (526 °C) for 10 hours, quenched to 344 K (71 °C), and then held at 423 K (150 °C) for 10 hours). After T6 heat treatment, specimens were cut using a bench saw from the castings and tested for the bond strength. Figure 11 shows a comparison of the as-cast bond strength with that after T6 heat treatment. The interfacial strength measurement suggested that T6 heat treatment did not reduce the bond strength significantly. This indicated that castings with steel inserts can be safely T6 heat treated. Usually castings made using the Al-Fin process are not T6 heat treatable because of the defective steel/aluminum interface.
Microstructure Characterization
The microstructure near the steel/aluminum bond was carefully characterized. Figure 12 illustrates the microstructure of the steel/casting interface and the locations where the microhardness (I to III) and micro-compositions (1 to 7) were measured. Between steel and the aluminum casting, two layers of intermetallics, marked layer 1 and layer 2, respectively, were observed. The thickness of layer 2 increases with increasing thickness of the nickel coating.
The compositions at each of the locations (marked from 1 to 7) shown in Figure 12 are given in Table I under two conditions: 1Ni and 4Ni, representing the nominal thickness of nickel at 25 and 100 µm, respectively. The inserts were diffusion bonded at 1173 K (900 °C) for 4 hours. Note that locations marked with 1 to 4 correspond to that on the intermetallic layers. Data given in Table I suggest that the intermetallics of the 4Ni samples contain less iron and silicon but more nickel, copper, and aluminum than that of the 1Ni samples.
The microhardness of the phases near the bond was measured and is illustrated in Figure 13. The hardness of the intermetallic phases is much higher than that of aluminum alloy and steel. Also the hardness of the intermetallic phase increases with increasing content of Fe for the 4Ni samples. A large number of hardness measurements of the intermetallic phases were carried out to investigate the effect of the thickness of the nickel coating on the hardness of the intermetallics. The results indicated that the hardness was not affected by the thickness of the nickel coating.
The microhardness distribution across the steel/aluminum bond for the 4Ni sample. The locations of I, II, and III are illustrated in Fig. 12
The microstructure of the intermetallic phases near the bond is rather complicated. Figure 14 illustrates these intermetallic phases for samples with a layer of 25 and 100 µm coating of nickel, respectively. A few intermetallic phases can be found in both Layer 1 and Layer 2 defined in Figure 13. The general trends are (1) the thickness of layer 1 increases with increasing time for diffusion bonding and decreasing thickness of the nickel coating; (2) the thickness of layer 2 increases with increasing thickness of the nickel coating and decreasing time for diffusion bonding; and (3) the nickel content in the intermetallic phase increases with increasing thickness of the nickel coating. It is unclear what are these intermetallic phases containing nickel. It appears that the formation of nickel-containing intermetallic phases may account for the higher toughness of the bond using this modified cast-on method than that using the Al-Fin process. Much detailed research is needed to characterize the nature of these intermetallic phases.
Discussion
The key in obtaining defect-free metallurgical bond using the cast-on type of method is (1) obtaining a clean surface of the steel insert and (2) using clean molten metal.
It is difficult to bond steel insert directly with aluminum using the cast-on method because the steel surface is usually covered with a layer of oxides. This oxide layer can be formed in situ on the steel surface during the mold-filling process when the insert is heated up by radiation of heat from molten aluminum before it contacts the melt or by conduction of heat after it is partially submerged into the molten metal. The existence of oxides on the surface of the steel insert is a physical barrier that prevents the contact of the molten metal to steel, and thus the resultant metallurgical reaction between steel and aluminum. The Al-Fin process utilizes a low-melting-point alloy to coat the steel insert and to form the metallurgical bond during the dipping process. The coated alloy protects the metallurgical bond by serving as a sacrificial layer that partially dissolves into molten metal during the casting process, leaving a clean intermetallic front to react with molten aluminum alloy. Since a perfect bond can be produced during the dipping stage, the Al-Fin process should be able to yield good bond between steel insert and the aluminum casting. The failure of the Al-Fin process in producing a high-quality metallurgical bond is due to the contamination of the bond during the casting process because the molten metal contains entrained oxides, air bubbles, and dissolved hydrogen.
During the mold-filling process, the leading edge, or the flow front, of the melt entrains oxides, air bubbles, and loose sand particles as it flows over mold surfaces. These particles tend to adhere to interfaces such as the insert/melt interface. To make the case worse, the insert is usually at room temperatures and tends to freeze the flow front of the melt containing those particles at its surface, thus forming a defective bond. With sufficient overflow, or by placing the insert near the in-gates, even if part of the leading edge freezes on the inserts, subsequent flow of molten metal is capable of remelting and removing this frozen material from the interface. However, the use of overflow may not sufficient to dislodge bubbles and solid particles that adhere to the surface of the insert. Thus, the cast-on process is prone to defect formation at the insert/steel interface if particles that adhere to the insert are not removed.
Another important issue that leads to a defective bond is dissolved hydrogen in molten aluminum. Hydrogen has a much higher solubility in liquid than that in solid aluminum. On cooling from liquid to solid aluminum, the hydrogen atoms precipitate and form pores in the mushy zone in a casting. Recently, it has been found that pores in the mushy zone can travel at high speeds toward the regions of higher temperatures, or toward the region last to freeze, or hot spots, in a casting. As a result, a large number of hydrogen bubbles are collected at these regions. Inserts in a casting are usually placed in such regions. The use of overflow heats up the insert and makes the regions with insert places that are last to freeze.
Figure 15 illustrates the process of bubbles traveling from mushy zone to the regions of higher temperatures.[11] Figure 15(a) shows a small bubble escaping from the dendritic front. The bubble traveled to the right side of Figure 15(b) within 0.017 seconds. This translates into a speed around 14 cm/s. A larger bubble attached to the dendritic front may collect smaller bubbles escaping the mushy zone. Figure 15(a) shows a bubble at the freezing front. It becomes larger in Figure 15(b), dislodges from the freezing front during bubble eruption shown in Figure 15(c), and finally travels away from the dendritic front, shown at the right side of Figure 15(d). The bubbles escaped from the mushy zone are going to be collected at the regions that are last to freeze, or hot spots, in a casting. These regions are usually at the interfaces of steel insert which has been heated up by the molten aluminum during the mold-filling process.
Conclusions
A methodology has been developed to produce defect-free bonds between steel and aluminum alloys using a modified cast-on method, which is a cost-effective way of reinforcing aluminum parts with steel inserts. The steel insert is plated with a layer of nickel and then a layer of copper. The plated layers are bonded using a diffusion bonding process. The copper layer serves as a scarifying layer which dissolves into the molten aluminum and protects the nickel layer. The nickel layer is used to modify the intermetallic phases that form during a chemical reaction between the molten aluminum and the steel insert. The strength and ductility of the bond obtained using this modified method is better than those obtained with the conventional Al-Fin method. Microstructural characterization indicates that the intermetallic phases formed in at the Al/Steel interface contain nickel which is electroplated on the steel surface. The existence of these nickel-containing intermetallics may account for the fact that the aluminum/steel bond produced using this method is tougher than that using the Al-Fin process. Care must be taken to reduce oxides and hydrogen level in molten aluminum in order to minimize defect formation at the steel/aluminum interfaces.
References
M.G. Whitfield: Auto Aviat. Ind., 1944, vol. 90 (6), pp. 148, 212–13
M.G. Whitfield, and V. Sheshunoff: Materials & Methods, 1945, Vol. 22, No. 4, pp. 1090-6.
M.G. Whitfield, and V. Sheshunoff: Metal Treat., 1946, vol. 8, pp. 70-6.
T. Weissmann: Design Engineering, 1963, vol. 9, no. 8, pp. 42-3.
H.W. Crusey: Materials & Methods, 1955, vol. 41, no. 5, pp. 114-5.
Beile JH, Lund CH: Metals Eng. Q., 1966, 6 no.1, pp. 63-4.
M. Ohta: U.S. Patent No. 5,005,469, 1991.
J. Jorstad et al.: U.S. Patent No. 5,333,668, 1994.
M.R. Myers et al.: U.S. Patent No. 6,443,211 B1, 2002.
M.R. Myers et al.: U.S. Patent No. 6,484,790, 2002.
Q. Han: Scripta Met., 2006, vol. 55, p. 871-4.
Acknowledgments
This research was supported by the United States Department of Energy (DOE) and Cummins Inc. The author would like to thank Drs. S. Viswanasan, K. Moore, and E. Kenik for their participation in this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 5, 2015.
Rights and permissions
About this article
Cite this article
Han, Q. A Modified Cast-on Method for the Reinforcement of Aluminum Castings with Dissimilar Metals. Metall Mater Trans B 47, 3266–3273 (2016). https://doi.org/10.1007/s11663-016-0612-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0612-2