Abstract
Coke reaction behavior in the blast furnace hearth has yet to be fully understood due to limited access to the high temperature zone. The graphitization of coke and its interaction with slag in the hearth of blast furnace were investigated with samples obtained from the center of the deadman of a blast furnace during its overhaul period. All hearth coke samples from fines to lumps were confirmed to be highly graphitized, and the graphitization of coke in the high temperature zone was convinced to start from the coke surface and lead to the formation of coke fines. It will be essential to perform further comprehensive investigations on graphite formation and its evolution in a coke as well as its multi-effect on blast furnace performance. The porous hearth cokes were found to be filled up with final slag. Further research is required about the capability of coke to fill final slag and the attack of final slag on the hearth bottom refractories since this might be a new degradation mechanism of refractories located in the hearth bottom.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Coke is the only solid material remaining throughout the lower zones of a blast furnace, especially in the hearth level. It provides the mechanical support for the burden above it and ensures the permeability of the materials column.[1] Those irreplaceable functions make coke behavior in the high temperature zones a key factor that influences the operational efficiency as well as campaign life of blast furnace.[2,3] An in-depth understanding about coke reaction behavior in the high temperature zone of a blast furnace is critical to optimize both the ironmaking blast furnace and many other metallurgical processes based on carbothermic reduction if these processes are to be optimized with respect to their carbon/coke consumption.[3]
Due to the harsh environment and limited access to the lower zone of the blast furnace, direct observation of the coke behavior inside this high temperature zone cannot be achieved. Samples extracted from an operating blast furnace using tuyere drilling technique have provided a source of potentially useful information about various important inner phenomena in the tuyere level.[4,5] Using this method, the changes of coke characteristics in the raceway zone (a cavity located right in front of a tuyere, in which coarse coke particles are often found loosely packed[3]) and tuyere level,[6] i.e., carbon structural order,[4] mineral transformation,[7] reactivity and strength,[5] and formation/accumulation of new phases[8–11] are somewhat understood. In addition, the interfaces between coke, slag, and metal have been characterized using scanning electronic microscope with samples obtained from the tuyere level of a blast furnace.[12] However, for the zones below the tuyere level, it is impossible to extract samples from an operating blast furnace. Previous dissection attempts on blast furnace hearth focused mainly on the erosion profile of hearth refractory and the inner hearth condition,[13,14] while the coke samples obtained from this zone have not yet been analyzed in detail. Except for dissection study, coke samples located in the hearth can be obtained during the overhaul of a blast furnace. The overhaul involves rebuilding the hearth of blast furnace during which coke sample can be extracted. Samples obtained in this area could provide meaningful information about the transformation of coke structures and its interaction with other phases. In the present study, the graphitization of coke and its interaction with slag were postulated with samples obtained from the deadman (a stagnant coke cone in the lower central part of the furnace[3]) of a blast furnace during its overhaul period.
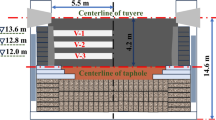
The typical overhaul process with detailed procedures has been described in earlier publications.[14,15] In the present study, the blast furnace from which the samples were extracted was a medium size furnace (2850 m3) with 3 tap holes and 30 tuyeres. The blast furnace was put into operation in Dec. 2006 and operated smoothly at approximately 2.56 t. HM/(m3 d). The coke and coal consumption rates were maintained at average of 317 kg/t. HM and 160 kg/t. HM, respectively. The average blast temperature was 1385 K (1111.8 °C). The average Micum index[16] M40 (cracking resistance index) and M10 (abrasive resistance index) of coke charged into the furnace were 87.5 and 5.6, respectively, which ensure the required coke quality for the blast furnace. After the blow-out and cool-down of the furnace, the hearth was dissected and some coke samples collected manually. The horizontal position of sample location was in the center of furnace, while the vertical position of sample location was at the center line of taphole of blast furnace. A number of particles (less than 1 mm) were also selected from the sample surface for the preparation of powder samples (less than 74 μm) for XRD examination. The analysis was conducted using a Rigaku diffractometer (DMAX-RB 12 kW; Rigaku Corporation, Tokyo, Japan) using Cu Ka radiation; the scanning angles were in the range from 10 to 90 deg (2θ) at a scan rate of 10 deg/minutes. Pieces about 20 mm × 20 mm × 6 mm were cut from the selected samples under dry conditions and then placed in a rounded plastic container with 25 mm diameter which was filled with resin. The material was ground and polished similar to the previous study.[8,12] The samples were coated with carbon and then examined with a Quanta 250 Environmental scanning electron microscope (SEM) equipped with energy-dispersive X-ray spectrometer (EDS) for chemical analysis and element mapping. After the SEM examination, mounted samples were ground and polished again to conduct optical detection. Samples were analyzed using a DAS microscope (Leica DMRP RXP). The images were generated using a reflected, white-light source fitted with an X–Y stage. The images were taken using a 40× oil immersion objective.
A large number of graphite crystals (gray phases in Figure 1(a)) as well as slag particles (white phases in Figure 1(a)) were observed on the surface of coke samples. The graphite crystals were present in various morphologies including flake (Figure 1(b)), plate (Figure 1(c)), columnar (Figures 1(d), (e)), which are similar to the forms of natural graphite crystals.[17–19] Those graphite crystals were also observed on the porous surface of tuyere coke samples.[11] The crystals of flake-like morphology with undeveloped hexagonal prism (Figures 1(b) through (d)), which have larger surface area than the columnar crystals (Figures 1(e) through (f)), can form a graphitic cover (‘‘shield’’) on a surface of BF coke.[11] Stanislav Gornostayev et al.[11] proposed that the graphitic shield can cover larger area of surface of BF coke thus preventing it from reactions with gases circulating in a BF in the case of favorable conditions for graphite crystallization because highly ordered coke displayed lower reactivity.[20] Considering the samples were obtained from the deadman where both liquid slag and iron have a close contact with coke, the graphite crystals on the coke surface may promote the carburization of iron since it has been reported that the dissolution rates of cokes were less than those of pure graphite.[21] The crystalline degree of slag phases cannot be determined from their appearances in the SEM images.
The XRD analysis of the particles selected from the coke surface, hearth coke lump as well as the original coke lump confirmed the high degree of crystallinity of coke samples from the deadman, as shown in Figure 2. The sharp graphite (002) peak can be clearly observed in the diffraction patterns of deadman coke samples, while the (002) peak in the diffraction pattern of original coke is much wider (Figure 2(a)). Even the deadman coke lump (>40 mm) possess a sharp (002) peak which is comparable with the (002) peak of pure graphite. Further magnification of the graphite peak area distinguished the different graphitization degree of samples with different sizes. As shown in (Figure 2(b)), the angles of graphite peaks of the deadman coke are all larger than that of pure commercial graphite, indicating graphite crystals of deadman coke possess a lower d 002 than pure graphite. The d 002 values of deadman coke and pure graphite were estimated to be 3.36 and 3.38 Å, respectively, from the Bragg’s law,[22] while the L c and L a values of selected samples estimated with Scherrer equation[22,23] were significantly different; the L c values of coke fine (<74 μm), coke particle (74 μm~1 mm), and coke lump (>40 mm) were estimated to be 510, 461, 194 Å, respectively. Figure 2(c) shows the unit cell of graphite crystal and co-relation between d 002, L c and L a, which indicates that the average number of planes (n ave) can be calculated using d 002 and L c. The value of n ave of coke fine, coke particle, and coke lump were calculated to be 153, 139, and 59, respectively, indicating the higher graphitization degree of coke fines and particles than that of coke lump in the high temperature zone of a blast furnace.
In the detection of coke carbon structural order in the tuyere level of a blast furnace, Gupta et al.[4] also found that the smallest size fraction of coke fines (−0.45 mm) indicated the highest ordering of carbon structure with a large proportion of graphite crystals, which was attributed to surface graphitization of +19 mm cokes particularly in the raceway and birds nest (located between the raceway and the deadman, in which coke is usually associated with the accumulation of hot metal[4]) regions. Their findings together with the present results suggest that the graphitization of coke in the high temperature zone start from the coke surface and the graphitization process leads to the formation of coke fines. Those coke fines from the regular flat shape graphite crystals (Figure 3(a) (A)) will be surrounded by liquid slag and iron, as shown in Figure 3. These coke-slag-iron composites are detrimental to the permeability of coke bed.
Gupta et al.[4] also found an inverse relationship between coke L c values and its total potassium such that total adsorbed potassium in coke decreases with increasing graphitization.[4] Therefore, graphitization of coke presents both positive (promoting carburization and resisting alkalis vapor attack) and negative (leading to the coke fine formation) effects, warranting a more detailed study to understand graphite formation and its evolution in coke and relation to the blast furnace performance.
The absence of quartz peak in the diffraction pattern of hearth coke was also confirmed in the XRD analysis, while the minerals in hearth coke were determined to be akermanite (Ca2MgSi2O7) and gehlenite (Ca2Al2SiO7) system which are the common minerals in the continuously cooled crystalline blast furnace slag,[24] as shown in Figure 2(a). The distribution and morphology of this slag phase in the coke pores are shown in Figure 4. It can be seen that all the macro coke pores are filled up with slag phase (Figure 4(a) (A) and (D)). Most macro pores interconnect with each other through a channel (Figure 4(a) (B) and (E)). The EDS results confirmed the slag composition to be CaO-Al2O3-SiO2-MgO which is in accordance with the X-ray diffraction results (Figure 2(a)). The optical micrographs in (Figure 4(b)) show the crystalline state of slag in hearth coke pores, which confirms that slags in all coke pores crystallize well and forms melilite mineral. This is also in agreement with the XRD results (Figure 2(a)) which confirmed the slag phase of hearth coke being akermanite (Ca2MgSi2O7)-gehlenite (Ca2Al2SiO7). Akermanite and gehlenite are the important end member of common melilite which is a solid solution with minimum melting point of 1661 K (1388 °C) and one of the major constituents of the Ca-Al-rich minerals representing the oldest material of the solar system.[25] The EDS maps showing the distribution of Ca, Si, Al, Mg, and O in slags located in hearth coke pores are presented in Figure 5, showing uniform distribution of these elements in various slag particles. Ten EDS points were selected to calculated the average chemical compositions of slag in coke which was compared with the final slag compositions of the dissected blast furnace, as shown in Table I. The compositions of slag in heath coke are quite similar with that of final slag, indicating that they are in the same chemical state in the blast furnace. Thus, the excavated coke samples were believed to be located in the slag layer of the blast furnace. The original coke minerals which consisted of primarily SiO2 (about 50 pct) and Al2O3[6] could not be identified in this area (Figures 3(a) (C) and 5), indicating that all coke minerals have melted with the gangue of ore to form the final slag of blast furnace.
Interconnected coke pores filled with blast furnaces slag. (a) SEM images showing the distribution slag phase in coke pores; (b) Optical micrographs showing the melilite mineral formed by slag. See text for details. C2A2S represents gehlenite (Ca2Al2SiO7), while C2MS2 represents akermanite (Ca2MgSi2O7)
Samples obtained in the tuyere level confirmed that tuyere coke have reacted greatly with gas or liquid to form a porous structure of coke matrix with many large pores greater than 50 μm.[12] Most coke pores that were originally closed will turn open after the consumption of coke walls and connect with each other. This provides the channels for liquid slag to flow from the surface to the interior of the coke in the hearth zone. Figure 6 shows the SEM micrographs that indicate slag flowing between coke pores through the channels. Those interconnected macro pores will be filled with final slag in the slag layer of the hearth. Coke soaked by slag will enter the iron layer of the hearth, which might effect coke dissolution into hot meal as well as the refractory of the hearth bottom. In a recent study about coke dissolution into hot metal, a layer of mineral were found to form at the coke-iron interfaces.[26] The mineral layer had a thin ribbon-like appearance interspersed with large alumina or calcium aluminate agglomerates,[26] while calcium enrichment of the mineral layer dictated the predominant phase and thus morphology of the mineral layer.[27] The mineral (product) layer formed at the coke-iron interface has been shown to affect the dissolution rate of coke in iron.[27,28] Since the calcium and alumina contents of final slag are much higher than that of original charge coke, mineral layer on the surface hearth coke saturated with final slag will form easier, as it moves from the slag layer to the iron layer.
In addition, hearth cokes carrying final slag may come in contact with the bottom of the furnace hearth, degrading the refractories. In a recent study about the reactions between selected calcium aluminates of synthetic coke ash and an aluminosilicate blast furnace hearth refractory,[29] the rate of reaction was observed to be dependent on temperature and the CaO composition of the synthetic coke ash. The combined effects of the volume change during the reaction and thermal expansion of the products are likely to increase the susceptibility of the blast furnace hearth refractory to spalling during furnace operation, and the new formed phases will promote further reaction-degradation with the coke ash.[29] The pervious dissections of the blast furnace revealed that the coke bed either sat on the hearth bottom or floated in the iron bath at different periods, and the bed penetrated deeper into the iron bath at the center than at the periphery.[3,14] Analysis based on a balance of forces indicates that the coke bed is more likely to sit on the hearth bottom in the case of large furnaces, and more likely to float in the case of smaller furnaces.[3] Therefore, resting of coke bed on the furnace bottom is a likely scenario for different blast furnaces even though the duration and frequency may vary with blast furnace size and operation conditions. If those porous coke pieces saturated with final slag penetrate through the bath of molten metal and reach the hearth bottom, serious degradation may occur to the refractories of hearth bottom. This is a subjected of interest that required further investigation.
In summary, all hearth coke samples from fines to lumps were confirmed to be highly graphitized. The graphitization extent of coke fines and particles were found to be greater than that of coke lumps, indicating that coke graphitization in the high temperature zone starts from the coke surface and lead to the formation of coke fines. The coke fines were readily surrounded by liquid slag and iron. Because both positive and negative effects exist with the graphitization of coke, it will be essential to perform detailed investigations about the multiple effects of coke graphitization on coke behavior such as rate of reaction with gas, dissolution rate in hot metal, fine generation rate, etc. The porous hearth cokes were also found to be soaked in final slag, while the original coke minerals were melted into the slag. Detailed research is required about the capability of coke pores to absorb slag (wettability between graphitized coke and liquid slag, influence of coke pore size on slag content absorbed by coke, etc.) and slag attack on the hearth bottom refractories (reaction kinetics between slag and refractories, influence of slag compositions on erosion degree, etc.) since this might be a newly discovered degradation mechanism of refractories located in the hearth bottom.
Reference
K. Li, J. Zhang, Z. Liu and X. Jiang: Chin. J. Proc. Eng., 2014, vol. 14, pp. 162–72.
T. Ariyama, R. Murai, J. Ishii and M. Sato: ISIJ Int., 2005, vol. 45, pp. 1371–78.
Yasuo O, Yasuhito S (1987) Blast furnace phenomena and modeling. Kluwer Academic Pub, Boston.
S. Gupta, Z. Ye, R. Kanniala, O. Kerkkonen and V. Sahajwalla: Fuel, 2013, vol. 113, pp. 77–85.
S. Dong, N. Paterson, S. Kazarian, D. Dugwell and R. Kandiyoti: Energy Fuels, 2007, vol. 21, pp. 3446–54.
S. Gupta, D. French, R. Sakurovs, M. Grigore, H. Sun, T. Cham, T. Hilding, M. Hallin, B. Lindblom and V. Sahajwalla: Prog. Energy Combust. Sci., 2008, vol. 34, pp. 155–97.
S. Gupta, Z. Ye, B.-c. Kim, O. Kerkkonen, R. Kanniala and V. Sahajwalla: Fuel Process. Technol., 2014, vol. 117, pp. 30–37.
K. Li, J. Zhang, Z. Liu, T. Wang, X. Ning, J. Zhong, R. Xu, G. Wang, S. Ren and T. Yang: Metall. Mater. Trans. B, 2014, vol. 45, pp. 1581–88.
Z. Ye, S. Gupta, O. Kerkkonen, R. Kanniala and V. Sahajwalla: ISIJ Int., 2013, vol. 53, pp. 181–83.
S. Gornostayev and J. Harkki: Metall. Mater. Trans. B, 2005, vol. 36, pp. 303–05.
S. S. Gornostayev and J. J. Härkki: Carbon, 2007, vol. 45, pp. 1145–51.
K. Li, J. Zhang, Z. Liu, M. Barati, J. Zhong, M. Wei, G. Wang, K. Jiao and T. Yang: Metall. Mater. Trans. B, 2015, vol. 46, pp. 1104–11.
D. Luo, J. Zhang, H. Guo, H. Zuo and H. Zeng, In The 5th International Congress on the Science and Technology of Ironmaking, Shanghai, China, 2009, pp. 1154–59.
T. Inada, A. Kasai, K. Nakano, S. Komatsu and A. Ogawa: ISIJ Int., 2009, vol. 49, pp. 470–78.
V. Andreyev, G. Gorbunov, A. Denisov, A. Kochergin and N. Gorshkov: Metallurgist, 1995, vol. 39, pp. 219–19.
R. Loison, P. Foch and A. Boyer: Coke: quality and production. Butterworth-Heinemann, London, 1989, pp. 170–180.
J. A. Jaszczak, G. W. Robinson, S. Dimovski and Y. Gogotsi: Carbon, 2003, vol. 41, pp. 2085–92.
G. Chen, D. Wu, W. Weng and C. Wu: Carbon, 2003, vol. 41, pp. 619–21.
Y. Gogotsi, S. Dimovski and J. Libera: Carbon, 2002, vol. 40, pp. 2263–67.
S. Gupta, V. Sahajwalla, P. Chaubal and T. Youmans: Metall. Mater. Trans. B, 2005, vol. 36, pp. 385–94.
M. B. Mourao, G. K. Murthy and J. F. Elliott: Metal. Trans. B, 1993, vol. 24, pp. 629–37.
H. P. Klug and L. E. Alexander: X-ray diffraction procedures: for polycrystalline and amorphous materials. 2nd ed, Wiley-VCH, New York, 1974, pp. 130–35.
L. Lu, V. Sahajwalla, C. Kong and D. Harris: Carbon, 2001, vol. 39, pp. 1821–33.
L. Gan, C. Zhang, J. Zhou and F. Shangguan: J. Non-Cryst. Solids, 2012, vol. 358, pp. 20–24.
R. Mendybaev, F. Richter and A. Davis: 37th Annual Lunar and Planetary Science Conference, Texas, 2006, pp. 2268.
M. W. Chapman, B. J. Monaghan, S. A. Nightingale, J. G. Mathieson and R. J. Nightingale: ISIJ Int., 2007, vol. 47, pp. 973–81.
M. W. Chapman, B. J. Monaghan, S. A. Nightingale, J. G. Mathieson and R. J. Nightingale: Metall. Mater. Trans. B, 2008, vol. 39, pp. 418–30.
M. W. Chapman: University of Wollongong, PhD Thesis, 2009, p. 404.
B. J. Monaghan, P. B. Drain, M. W. Chapman and R. J. Nightingale: ISIJ Int., 2014, vol. 54, pp. 810–19.
This work was financially supported by the Open Foundation of the State Key Laboratory of Advanced Metallurgy (41603007), the National Natural Science Foundation of China and Baosteel Group Co., LTD of Shanghai for the Key Joint Project (U1260202), and the National Science Foundation for Young Scientists of China (51304014). The authors gratefully acknowledge financial support from China Scholarship Council (award to Kejiang Li for one year’s study abroad at the University of Toronto).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted June 1, 2015.
Rights and permissions
About this article
Cite this article
Li, K., Zhang, J., Liu, Y. et al. Graphitization of Coke and Its Interaction with Slag in the Hearth of a Blast Furnace. Metall Mater Trans B 47, 811–818 (2016). https://doi.org/10.1007/s11663-015-0574-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-015-0574-9