Abstract
Aiming to fill the thermodynamic blank in CaO-FeO-Fe2O3 system, the determination of the Gibbs free energy of formation from elements for ternary Ca4Fe9O17 was carried out using a solid-state galvanic cell with air and calcium zirconate material, respectively, as the reference electrode and electrolyte. The ternary system Ca2Fe2O5-CaFe2O4-Ca4Fe9O17 was selected as the measuring electrode and its equilibrium was confirmed. The essential thermodynamic data of Ca2Fe2O5 and CaFe2O4 were cited from the reassessed data from a previous investigation. The reversible electromotive forces of the cell were determined from 1273 K to 1473 K (1000 °C to 1200 °C). The Gibbs free energy of formation from elements for Ca4Fe9O17 was derived and given by:
The increment of enthalpy and entropy of formation from elements for Ca4Fe9O17 at 298 K (25 °C) are calculated to be \( \Delta_{\text{f}} H_{{{\text{m}},298}}^{ \circ } = -6209.529 \times 10^{3} \;({\text{J}}\,{\text{mol}}^{-1} ) \) and \( \Delta_{\text{f}} S_{{{\text{m}},298}}^{ \circ } = -1038.009\;({\text{J}}\,{\text{mol}}^{-1} \,{\text{K}}^{-1} ) \). The Ellingham diagram was developed in temperature range 1273 K to 1473 K (1000 °C to 1200 °C). The oxygen potential of Ca4Fe9O17 was found to be slightly higher than CaFe2O4 and much higher than Ca2Fe2O5.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because of the outstanding applications in iron-making industrial process, the Fe-Ca-O ternary system including various compounds has been focused on for decades. As the basic binders of iron ore, the amount and status of calcium ferrite compounds are viewed to be of central importance for iron ore sintering. The basic thermodynamic data play a key role in the estimation and evaluation of calcium ferrite compounds in iron ore. Unfortunately, the serious insufficiency of basic data on calcium ferrite compounds makes these works difficult to carry out. For three known binary compounds, the thermodynamic data of Ca2Fe2O5 (C2F) and CaFe2O4 (CF) have been reported repeatedly and can be identified more precisely, whereas the data of CaFe4O7 (CF2) is unknown.[1–8] With respect to the large group of ternary calcium ferrite compounds in a CaO-FeO-Fe2O3 system, only four of them, CaFe3O5 (CWF), CaFe5O7 (CW3F), Ca4Fe9O17 (C4WF4), and Ca4Fe17O29 (C4WF8), are generally under consideration because of their well-documented existence.[9–14] The Gibbs free energy of reaction from oxides for CaFe3O5 has been determined separately by Björkman[6] and Lykasov and Popova.[15] Björkman provided the only recorded Gibbs free energy of formation from elements for CaFe5O7.[6] Except for the measurements mentioned above, no specific investigation concerning the Gibbs free energy of formation for ternary calcium ferrites can be found. Hence, much basic thermodynamic data is unknown and an urgent solution is required.
Two types of solid-state electrolytes of CaF2 and ZrO2-based materials have been frequently employed to determine the Gibbs free energy of calcium ferrite compounds. However, the mostly used CaF2 cannot afford a high-temperature environment,[8] whereas the ZrO2-based material is vulnerable to the CaO even below 1273 K (1000 °C).[16–18] The nonstoichiometric Ca1+x Zr1−x O3−x material was reported as an excellent oxygen ionic conductor with almost pure ionic conduction and great chemical and thermal stability from the investigation provided by Janke.[19] It has been used as an electrolyte in a solid-state galvanic cell for thermodynamic determination by some investigations.[20–22]
In the current work, efforts were made to determine the Gibbs free energy of formation for a ternary calcium ferrite Ca4Fe9O17 (C4WF4) using a solid-state galvanic cell embracing nonstoichiometric calcium zirconate (CZO) electrolyte. Measurements were performed by the aid of the electromotive force (emf) method through a relative high temperature range of 1273 K to 1473 K (1000 °C to 1200 °C).
Experimental
Materials
Analytical reagent-grade chemicals CaCO3, α-Fe2O3, Al2O3, and electrolytic Fe supplied by the Sinopharm Chemical Reagent Company were served as starting materials.
The essential CaFe2O4, Ca2Fe2O5, and wütite were synthesized by a simple solid-state reaction. The dried CaCO3 and α-Fe2O3 were completely mixed in a stoichiometric ratio of Ca2Fe2O5 and CaFe2O4. The mixtures were pressed into cylindrical specimens under 5 MPa pressure and then sintered at 1473 K (1200 °C) for 8 hours in air. The wütite was selected as another basic material for the preparation of electrode mixture. To obtain wütite, calculated amounts of α-Fe2O3 and electrolytic iron powder in 1.1:1 molar ratio were fully mixed and then compacted into cylindrical specimens under 5 MPa pressure. The wütite was synthesized by heating the mixture at 1173 K (900 °C) for 5 hours under Ar inert condition in a steel crucible and followed by quenching operation in liquid nitrogen. The compositions of resultant specimen were identified by X-ray diffraction (XRD) determination. Single-phase samples were qualified.
For further synthesis, the obtained high pure Ca2Fe2O5, CaFe2O4 and wütite were toughly ground to pass 300 meshes. Besides, adopted as filler material, amounts of high pure Al2O3 agent powder was calcined at 1473 K (1200 °C) for 3 hours in air and then ground to pass 300 meshes as well.
Phase Exploration
A thermodynamically stable system performing a reversible oxidation–reduction reaction and providing a constant oxygen partial pressure should be adequate for working as measuring electrode in electrochemical determination. According to the ACerS-NIST Phase Equilibrium Diagrams (No. 05038 and No. 02110), a ternary phase system of Ca2Fe2O5-CaFe2O4-Ca4Fe9O17 came into our sight, which was theoretically confirmed to be stable within 1273 K to 1473 K (1000 °C to 1200 °C) by Imlach and Glasser[23] and by Phillips and Muan.[10] The composition relations of all possible compounds on the section of Fe2O3-CaO-FeO system are depicted in Figure 1. No possible fourth substance exists in the field of the selected Ca2Fe2O5-CaFe2O4-Ca4Fe9O17 system. Hence, the Ca2Fe2O5, CaFe2O4, and Ca4Fe9O17 are suggested to perform at thermodynamic equilibrium.
To examine and synthesize in situ the expected equilibrium ternary phase system, the previously prepared CaFe2O4, Ca2Fe2O5, and wütite were completely mixed and poured into an alumina crucible then tamped toughly until it was difficult to insert a thin needle. For filling, the previously calcined Al2O3 powder was continuously poured and tamped into crucible until it was filled up and the inner powder was hard. At last, the crucible was sealed using cement seal. After drying for 2 days at room temperature in air, the sealed sample was heated at 1473 K (1200 °C) for 2 days then followed by quenching in liquid nitrogen. The resulting composition of mixture was identified by the XRD method. It would be qualified if the products are identical to the ideal system Ca2Fe2O5-CaFe2O4-Ca4Fe9O17 with no other phase detected. Ascribed to the further formation of CaFe2O4 and inevitable oxidation of wütite caused by the introduced oxygen during sample assembling, the mixture with a molar ratio Ca2Fe2O5:CaFe2O4:FeO = 2:1:7 was experimentally proved to be adequate.
Electrochemical Measurements
Based on the qualification of Ca2Fe2O5-CaFe2O4-Ca4Fe9O17 system, materials of Ca2Fe2O5, CaFe2O4, and FeO in identical molar ratio were repeatedly mixed to produce the measuring electrode. The Gibbs free energy of formation from elements for Ca4Fe9O17 was determined by the cell
The air electrode was adopted as positive electrode on the right-hand side. A gas electrode is said to hold more precise oxygen pressure than the metal–metal oxide electrode.[24,25]
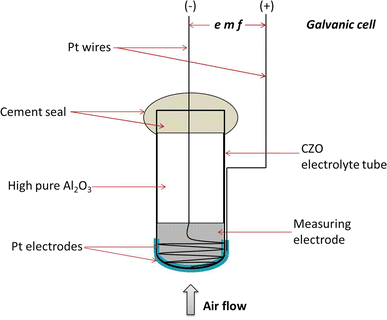
The apparatus for electrochemical measurements was depicted in Figure 2. A nonstoichiometric CZO electrolyte ceramic tube with one end closed and measuring 0.7 cm in diameter and 3.1 cm in length was used as the solid-state electrolyte. The employed CZO tube was synthesized via a co-precipitation method as described in our previous work[26] and shaped by injection molding process. The tube was tested for leaks and found to be impervious. The porous Pt electrode coats were fabricated by uniformly applying Pt paste over both the inner and outer bottom surfaces of CZO tube. Two Pt wires including one twining on the outer bottom and the other spirally adhering to the inner bottom were co-fired with the Pt paste at 1123 K (850 °C) for 3 hours in air. Consequently, uniform and porous Pt electrode films with Pt leads adhering on the inner and outer bottom surfaces were obtained simultaneously. The assembly of a galvanic cell is similar to the process described in a previous phase equilibrium exploration. The whole apparatus was placed inside a vertical furnace with Pt wires extending out and connecting to the CHI660B Electrochemical Workstation, which possesses the accuracy of ±0.1 mV. A Faraday cage made of stainless steel foil was placed between the furnace tube and the cell. The air reference electrode was served by a constant dry air flow at a rate of 300 mL min−1 over the outer Pt electrode.
As the cell was heated to a specific temperature, because of the different oxygen pressures of measuring electrode and reference electrode, an emf can be established on both Pt electrode sides of electrolyte and the signal can be continuously picked up by the CHI660 Electrochemical Workstation. Before measurement, the cell was heated for 1 day to equalize the measuring electrode system. The time taken for the cell to attain a nearly constant emf value is no longer than 50,000 seconds. The reversibility of cell was checked by microcoulometric titration method in both directions.[27,28] A small voltage was applied in both directions, and whether the emf returned to the value before titration was observed.
Results and Discussion
Phase Identification
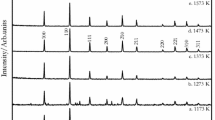
The XRD patterns of synthesized Ca2Fe2O5, CaFe2O4 and wütite are shown in Figure 3. Highly pure Ca2Fe2O5, CaFe2O4 and wütite can be identified without any trace of a possible second phase detected. They are qualified for subsequent phase exploration.
The XRD patterns of long-term-heated products from Ca2Fe2O5-CaFe2O4-FeO mixture are presented in Figure 4. A ternary phase system consisting of Ca2Fe2O5, CaFe2O4, and Ca4Fe9O17 can be confirmed. After being checked by the standard PDF (The Powder Diffraction File from The Joint Committee on Powder Diffraction Standards) data, no obvious shift in peak location was detected, which indicates a low possibility of forming a solid-solution system. Actually, the significant difference in lattice structure between Ca4Fe9O17 and binary calcium ferrites of CaFe2O4 and Ca2Fe2O5, suggests the Ca4Fe9O17-contained solid solution is hard to form in this system. In contrast, the phase diagram of CaO-Fe2O3 binary system from investigation by Phillips and Muan clearly shows no solid solution appearing between CaFe2O4 and Ca2Fe2O5 below 1489 K (1216 °C).[29] Therefore, the formation of a solid solution among this equilibrium phases is excluded.
In the transition process from initial Ca2Fe2O5-CaFe2O4-FeO mixture to the equilibrium Ca2Fe2O5-CaFe2O4-Ca4Fe9O17 system, the FeO in the starting components plays an important role as Figure 5 shows. Partial FeO combines with CaFe2O4 to produce the Ca4Fe9O17, whereas the other FeO is thought of being oxidized to Fe2O3 and subsequently consumed between Ca2Fe2O5 and CaFe2O4. Finally, the Ca2Fe2O5, CaFe2O4 and produced Ca4Fe9O17 form the equilibrium ternary phase system.
Essential Thermodynamic Data
In the light of phase exploration, the Ca2Fe2O5-CaFe2O4-Ca4Fe9O17 system is confirmed to be qualified for working as the measuring electrode. As a key to the determination of the unknown thermodynamic properties of Ca4Fe9O17, reliable standard thermodynamic data of CaFe2O4 and Ca2Fe2O5 are essential. The standard data of CaFe2O4 and Ca2Fe2O5 are rarely included in thermodynamic compilations except for the records by Knacke et al.[30] In contrast, their available experimental data have been frequently reported, for instance by Rezukhina and Baginska,[3] by Rajagopalan et al.,[4,5] by Björkman,[6] by Jacob et al.,[7] and the latest by Forsberg et al.[8] The Gibbs free energy of formation from elements for Ca2Fe2O5 was always given directly. Regarding the CaFe2O4, the Gibbs free energy of formation from oxides based on Reaction [2] was mostly provided.
A comparison of Gibbs free energy of formation from elements for Ca2Fe2O5 in different investigations is performed in Figure 6(a), whereas the Gibbs free energy of formation from oxides for CaFe2O4 is drawn in Figure 6(b). For Ca2Fe2O5, the agreement between the study by Björkman and by Forsberg et al. is excellent, whereas the result given by Rajagopalan et al. deviates somewhat. In contrast with the situation of Ca2Fe2O5, the relative larger deviation of reference data for CaFe2O4 can be clearly seen. The result provided by Jacob et al. shows a positive slope, which is entirely different to the negative slope values by Forsberg et al. and by Rezukhina and Baginska. Actually, there is controversy on the thermodynamic data of CaO in various compilations, which may give rise to the discrepancies in resultant data of Ca2Fe2O5 and CaFe2O4. Taking this into account and considering the suggestion provided by Jacob and Varghese,[31] Forsberg et al. cited the essential data from the more accurate JANAF Thermochemical Tables[32] and demonstrated a series experimental work of high quality. Hence, the data reported by Forsberg et al. are more reliable. However, the essential data of CaO dedicated by Forsberg et al. were derived via a nonlinear simulation in a narrow temperature range of 1000 K to 1400 K (727 °C to 1127 °C). Obviously, a fitting work through a wide temperature range is necessary to obtain more accurate essential data for subsequent citation. Thus, a nonlinear simulation was carried out by the aid of the least squares regression analysis from 300 K to 1700 K (27 °C to 1427 °C) for the extracted data from JANAF Thermochemical Tables.[32] The results are shown in Figure 7.
The wide-range nonlinear simulation by the current work exhibits higher accuracy especially at a lower temperature range. It is expected to be more accurate for the extrapolation of the resultant data in a low temperature range. Extracted from JANAF Thermochemical Tables,[32] the mathematical expressions of new simulated Gibbs free energy of formation from elements for CaO and Fe2O3 are respectively denoted as Eqs. [3] and [4], and the methods taken for reassessing thermodynamic data of CaFe2O4 and Ca2Fe2O5 are described in Figure 8.
The Gibbs free energy of Reactions [2] and [5] are formulated as Eqs. [6] and [7]. Equation [6] directly relates to the Gibbs free energy of formation from oxides for CaFe2O4, whereas that of Ca2Fe2O5 has to be deduced by a simple addition and expressed in Eq. [9]. The F represents the Faraday constant with a commended value of 96,485.3 C mol−1 advised by NIST.[33] The emfs of E 1 and E 2 dependent on temperature are determined as Eqs. [10] and [11] by Forsberg et al.[8] By introducing the raw determined data of Forsberg et al.,[8] the Gibbs free energy of formation from oxides for CaFe2O4 and Ca2Fe2O5 can be calculated as Eqs. [12] and [13]. In this way, the Gibbs free energy of formation from elements for CaFe2O4 and Ca2Fe2O5 can be obtained by taking our new simulation data from Eqs. [3] and [4] into [12] and [13]. The final results are presented as Eqs. [14] and [15].
Electrochemical Determination
For the measured cell, the oxygen pressure on the right-hand positive electrode depends on the following reaction:
On the left-hand negative electrode, the oxygen chemical potential is fixed by the equilibrium inside ternary phase system, which is described as
Then overall cell reaction is illustrated by
The electrochemical relation for Reaction [19] can be directly established as
The ∆ r G and E, respectively, represent the Gibbs free energy of Reaction [19] and the determined emfs of cell. Theoretically, by another transformation, the calculation for Gibbs free energy of formation from elements for Ca4Fe9O17 can be achieved as
Taking the determined equilibrium emfs and the prepared essential data \( \Delta_{\text{f}} G_{\text{m}}^{ \circ } ({\text{CaFe}}_{ 2} {\text{O}}_{4} ) \) and \( \Delta_{\text{f}} G_{\text{m}}^{ \circ } ({\text{Ca}}_{ 2} {\text{Fe}}_{ 2} {\text{O}}_{5} ) \) into Eq. [21], the expected \( \Delta_{\text{f}} G_{\text{m}}^{ \circ } ({\text{Ca}}_{ 4} {\text{Fe}}_{ 9} {\text{O}}_{ 1 7} ) \) can be obtained.
The varying lines of emfs during the measuring process at different temperatures are shown in Figure 9. More time is spent to attain steady value at a lower temperature. The emf values are found decrease with the rise of temperature. Generally, the equilibrium reaction in the measuring electrode results in higher oxygen pressure as temperature increases, whereas the oxygen pressure on air reference electrode is constant. Consequently, the difference of oxygen potential between electrodes is produced to be closer. It should be responsible for the reducing emf values.
The obtained emf values dependent on temperature were plotted in Figure 10. A highly linear relation can be clearly observed and its mathematical expression was obtained by the linear fitting method. According to the mathematical relation [21], the Gibbs free energy of formation from elements for Ca4Fe9O17 is obtained as
The uncertainty in \( \Delta_{\text{f}} G_{\text{m}}^{ \circ } ({\text{Ca}}_{ 4} {\text{Fe}}_{ 9} {\text{O}}_{17} ) \) should be attributed to the data taken from the references, especially for the introduced data of CaFe2O4, which possesses a significant high stoichiometric coefficient in Reaction [19]. In such a situation, even a tiny uncertainty is apt to be amplified several times.
Oxygen Potential
Developed from the results in the current work, the Ellingham diagram for substances with available thermodynamic data in FeO-CaO-Fe2O3 system from 1273 K to 1473 K (1000 °C to 1200 °C) is revealed in Figure 11. Obviously, the Ca2Fe2O5 exhibits the best thermodynamic stability, followed by CaFe2O4 and Ca4Fe9O17. The oxygen potential of CaFe2O4 and Ca4Fe9O17 seems close to each other with the CaFe2O4 showing slightly more stable.
The Enthalpy and Entropy of Ca4Fe9O17 at 298 K (25 °C)
Indicated by the mathematical expression (Eq. [22]), we cannot directly read the enthalpy \( \Delta_{\text{f}} H_{298}^{ \circ } \) and entropy \( \Delta_{\text{f}} S_{298}^{ \circ } \) of Ca4Fe9O17 by the constant term and coefficients of the temperature-dependent term because of the nonzero increment of heat capacity \( \Delta C_{\text{p}} \) at specific temperature for the formation reaction of Ca4Fe9O17. In this situation, according to the Kirchhoff relation, the increment of enthalpy \( \Delta_{\text{f}} H_{\text{T}}^{ \circ } \) can be obtained by
and further computed as
Taking Eq. [24] into the Gibbs–Helmholtz equation
one can obtain
Comparing Eq. [26] with the corresponding terms in Eq. [22], the increment of enthalpy and entropy of formation from elements for Ca4Fe9O17 at 298 K (25 °C) can be obtained as \( \Delta_{\text{f}} H_{{{\text{m}},298}}^{ \circ } = -6209.529 \times 10^{3} \;({\text{J}}\,{\text{mol}}^{-1} ) \) and \( \Delta_{\text{f}} S_{{{\text{m}},298}}^{ \circ } = -1038.009\;({\text{J}}\,{\text{mol}}^{-1} \,{\text{K}}^{-1} ) \).
Conclusions
The Gibbs free energy of formation from elements for a ternary calcium ferrite compound Ca4Fe9O17 was determined through a temperature range of 1273 K to 1473 K (1000 °C to 1200 °C). A solid-state galvanic cell was employed embracing an air reference electrode and calcium zirconate electrolyte. The ternary phase system CaFe2O4-Ca2Fe2O5-Ca4Fe9O17 was confirmed to be thermodynamically stable within the measuring temperature range and was selected for working as a measuring electrode. During the measuring process, the increase in oxygen pressure in ternary equilibrium phases can be clearly read by the decreasing emf value with the increase of temperature. Revealed by the determined thermodynamic data, the Ca4Fe9O17 possesses a slightly lower thermodynamic stability than that of CaFe2O4 and is remarkably unstable compared with Ca2Fe2O5.
References
E.T. Turkdogan: Trans. TMS-AIME, 1961, vol. 221, pp. 1090–95.
K.C. Sharma, R.D. Agrawal, and M.L. Kapoor: Indian J. Technol., 1986, vol. 24, pp. 751–53.
T.N. Rezukhina and J. Baginska: Elektrokhimiya, 1967, vol. 3, pp. 1146–49.
K.V. Rajagopalan, R. Kalanaraman, and M. Sundaresan: J. Electrochem. Soc. India, 1987, vol. 36, pp. 157–59.
K.V. Rajagopalan, R. Kalanaraman, and M. Sundaresan: J. Electrochem. Soc. India, 1990, vol. 39, pp. 224–26.
B. Björkman: Scand. J. Metall., 1984, vol. 13, pp. 193-200.
K.T. Jacob, N. Dasgupta, and Y. Waseda: Z. Metallkd., 1999, vol. 90, pp. 486–90.
S. Forsberg, P. Wikström, and E. Rosén: Metall. Mater. Trans. B, 2002, vol. 33B, pp. 385–92.
M. Hillert, M. Selleby, and B. Sundman: Metall. Trans. A, 1990, vol. 21A, pp. 2759–76.
B. Phillips and A. Muan: Trans. TMS-AIME, 1960, vol. 218, pp. 1112–18.
R. von Schenck, A. Laymann, and E. Jenckel: Z. Anorg. Allg. Chem., 1937, vol. 235, pp. 65–76.
A. Burdese: Metall. Ital., 1952, vol. 44, pp. 343–46.
D.A. Reeze and A.G. Gregory: Trans. Inst. Min. Metall. (Sect. C), 1967, vol. 76, pp. 273–77.
A.A. Lykasov and N.V. Kozheurova: Neorg. Mater., 1980, vol. 16, pp. 1079–82.
A.A. Lykasov and T.V. Popova: Neorg. Mater., 1995, vol. 31, pp. 84–87.
R.C. Garvie: J. Am. Ceram. Soc., 1968, vol. 51, pp. 553–56.
V.S. Stubican and J.R. Hellmann: Adv. Ceram., 1981, vol. 3, pp. 25–36.
P. Duran, P. Recio, and J.M. Rodriguez: J. Mater. Sci., 1987, vol. 22, pp. 4348–56.
D. Janke: Metall. Trans. B, 1982, vol. 13B, pp. 227–35.
. Róg, M. Dudek, A. Kozłowska-Róg, and M. Bućko: Electrochim. Acta, 2002, vol. 47, pp. 4523–29.
M. Dudek: Mater. Res. Bull., 2009, vol. 44, pp. 1879–88.
G. Róg, A. Kozłowska-Róg, and M. Dudek: J. Chem. Thermody., 2007, vol. 39, pp. 275–78.
J.A. Imlach and F.P. Glasser: Trans. J. Br. Ceram. Soc., 1971, vol. 70, pp. 227–34.
C. Mallika, O.M. Sreedharan, and R. Subasri: J. Eur. Ceram. Soc., 2000, vol. 20, pp. 2297–313.
R. Ganesan, T. Gnanasekaran, and R.S. Srinivasa: J. Nucl. Mater., 2003, vol. 320, pp. 258–64.
H. Li and X. Guo: Curr. Appl. Phys., 2013, vol. 13, pp. 500–04.
S.C. Parida, K.T. Jacob, and V. Venugopal: Solid State Sci., 2002, vol. 4, pp. 1245–55.
G. Róg, M.M. Bućko, and A. Kozłowska-Róg: J. Chem. Thermody., 2008, vol. 40, pp. 21–24.
B. Phillips and A. Muan: J. Am. Ceram. Soc., 1958, vol. 41, pp. 445–54.
O. Knacke, O. Kubaschewski, and K. Hesselmann: Thermochemical Properties of Inorganic Substances, 2nd ed., Springer-Verlag, Berlin, 1991.
K.T. Jacob and V. Varghese: Metall. Mater. Trans. B, 1996, vol. 27B, pp. 647–51.
M.W. Chase, C.A. Davies, J.R. Downey, D.J. Fruip, R.A. McDonald, and A.N. Syverud: JANAF Thermochemical Tables, 4th ed., J. Phys. Chem. Ref. Data, New York, 1998.
P.J. Mohr, B.N. Taylor, and D.B. Newell: J. Phys. Chem. Ref. Data, 2010, vol. 41, pp. 1–83.
Acknowledgments
The authors would like to thank the National Natural Science Foundation of China for the financial support (No. 50974012 and No. 51374017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted May 6, 2014.
Rights and permissions
About this article
Cite this article
Li, HY., Guo, XM. Determination of Gibbs Free Energy of Formation from Elements for Ca4Fe9O17 by Solid-state Galvanic Cell. Metall Mater Trans B 46, 278–285 (2015). https://doi.org/10.1007/s11663-014-0179-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0179-8