Abstract
The rotation cylinder method was used to investigate the dissolution kinetics of Al2O3 into molten CaO-Al2O3-Fe2O3 slag, and weight losses of the Al2O3 rod were selected as experimental variables. The final melt was milled into powders for XRF detection, and the Al2O3 rod with adhesive melt after experimental was examined by scanning electron microscopy and energy dispersive spectrometer. It is concluded that diffusion is the restricted step in Al2O3 dissolution into molten CaO-Al2O3-Fe2O3 slag; the chemical composition of the initial melts exerts a great influence on the activation energy for Al2O3 dissolution; the range of Al2O3 diffusivity is about 1.86 × 10−7 to 2.45 × 10−6 cm2/s; no apparent intermediate layer between the Al2O3 rod and melt is observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron ore sinter is the main raw material for iron-making using blast furnaces in the East Asian regions, especially China, Japan, and Korea. Good-quality iron ore sinters are critical for strengthening the smelting operation of blast furnaces.[1,2] As the main bonding phase of high-basicity sinter, the physical and chemical properties of calcium ferrite melt and the texture of the structure after solidification have great influences on the quality of the resultant product. As ore quality decreases, iron ores with higher gangue compositions are used in sintering, which results in chemical composition fluctuations in the raw material. Iron ores from Australia, India, and Brazil were recently observed to contain high Al2O3 contents.[2] Previous studies show that high Al2O3 contents have negative effects on the properties of the sinter and its sintering index because Al2O3 contents are directly related to silicon-ferrite of calcium and aluminum (SFCA) generation.[3–9] Several studies have focused on the influence of Al2O3 on the formation of SFCAs during sintering. Maeda et al. investigated the effects of Al2O3 on the generation rate of calcium ferrite melts; results showed that Al2O3 affects the calcium ferrite by increasing its fusion rate and decreasing its formation temperature. Al2O3 also promotes dissolution of solid Fe2O3 in the calcium ferrite melt.[10]
Iron ore sintering involves a solid–liquid coexistence reaction, and the heating times employed in this process at high temperature are usually very short. The solid–liquid reaction rate is critical for the formation of the final components of SFCAs. Thus, exploring the reaction mechanism and factors influencing iron ore sintering is an important endeavor. Several studies have focused on characterizing the formation ability of the liquid phase by analyzing the relationship between the chemical composition and amount of the liquid phase available. However, few studies on the kinetics of solid–liquid reaction processes, specifically the dissolution kinetics of Al2O3 into the calcium ferrite melt, are available. The present study will promote the current understanding on the formation kinetics of SFCA from calcium ferrite.
A large amount of literature[11–23] on the dissolution of Al2O3 in other melts is available. The methods used to investigate dissolution kinetics in these studies include in situ and ex situ investigation. However, the in situ investigation is not suited to study the dissolution kinetics of Al2O3 into molten CaO-Al2O3-Fe2O3 slag. Because the shrink of Al2O3 particle or ball cannot be real-time observed during the dissolving process of Al2O3 into molten CaO-Al2O3-Fe2O3 slag by high-temperature confocal laser scanning microscope (CLSM) due to the molten CaO-Al2O3-Fe2O3 slag is not transparent. Finally, the ex situ characterization is used in the present paper and the rotation cylinder method[11–17] is selected to investigate the dissolution kinetics of Al2O3 into molten CaO-Al2O3-Fe2O3 slag.
Experimental
Raw Materials
All of the raw materials used in this study were analytically pure (AP) chemical reagents (Kenong Chemical Co., Inc.). The size distributions of the raw materials are measured using the screen method and are shown in Table I.
Experimental Procedure
The rotation cylinder method was used to investigate the dissolution kinetics of Al2O3 in molten CaO-Al2O3-Fe2O3 slag. The experimental apparatus is shown in Figure 1.
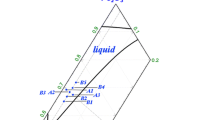
The experimental variables include initial chemical composition of the calcium ferrite melt, dissolution time, rotation speed, and temperature. The calcium ferrite melts were based on the CaO-Al2O3-Fe2O3 system and some additives, such as MgO and SiO2. The ratio of Fe2O3/CaO (in terms of percent mass) in the calcium ferrite melts was varied from 1.21 to 1.79 for a CaO-Al2O3-Fe2O3 system with a fixed MgO content of 4 pct mass. To investigate the Al2O3 concentration driving dissolution, the Al2O3 content in the calcium ferrite melts was varied from 14.7 to 22.5 pct mass. The influence of SiO2 on the dissolution rate was also investigated by varying the SiO2 content in the calcium ferrite melts from 0.99 to 3.85 pct mass with a fixed ratio of Fe2O3/CaO. The chemical compositions of the initial melts are shown in Table II and are plotted in the CaO-Al2O3-Fe2O3 ternary phase diagrams shown in Figure 2. The experimental scheme is shown in Table III.
All of the dissolution experiments in this study were carried out in an Ar atmosphere. All of the samples were first weighed according to Table II and then roasted at 1173 K (900 °C) for 1 h to remove the CO2 present in the calcium carbonate. After heating of the furnace to a defined temperature, the roasted samples were loaded into a MgO crucible that was then placed inside an Al2O3 crucible in the furnace. Finally, an Al2O3 rod was inserted into the calcium ferrite melt at a defined depth and rotated with a defined speed for a certain period of time based on the parameters indicated in Table III. The Al2O3 rod was then washed with HCl and weighed. The final melt was naturally cooled down to room temperature and then milled into powders (over 90 pct below 0.74 μm, determined via the screen method) for XRF detection. The accuracy of the method was determined by performing repeated tests. The Al2O3 rod with adhesive melt was examined by scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS).
Results and Discussion
Derivation of Dissolution Kinetics Parameters
Al2O3 dissolution rate
Weight losses of the Al2O3 rod during dissolution into the molten calcium ferrite are shown in Table IV. A good linear relationship between the weight loss of the Al2O3 rod in unit of immersion depth and immersion time at 1673 K (1400 °C) is illustrated in Figure 3. The change in solid–liquid contact area shows no apparent influence on Al2O3 dissolution into the calcium ferrite melt. This result is consistent with previous studies.[11–17] Hence, the mean dissolution rate can be calculated using Eq. [1]:
where R d, W 0, W t , A, and t respectively refer to the dissolution rate (g/cm2 s), initial mass of the Al2O3 rod (g), final mass of the Al2O3 rod (g), immersion area of the Al2O3 rod (cm2), and immersion time (s). Equation [1] illustrates that R d is proportional to the weight loss of the Al2O3 rod in unit of immersion depth for defined time.
Diffusion coefficient of Al2O3 dissolution
The existing literature indicates that mass transfer in the boundary layer is the restricting step for Al2O3 dissolution in continuous casting systems.[12] However, Maeda et al.[10] pointed out that rotation speed has little influence on mass transfer and that the dissolution reaction at the solid–liquid interface is the restricting step for Fe2O3 dissolution into calcium ferrite melts. Thus, exploring the relationship between dissolution rate and mass transfer is necessary to understand the restricting step of Al2O3 dissolution into the calcium ferrite system better. Cooper and Kingery,[11] and, more recently, Sandhage and Yurek,[14] showed that when rotating cylinders are used at shallow immersion depths, mass transfer is best described by the Levich–Cochran equation, Eq. [2],
which applies for \( \frac{v}{D} \gg 1 \) and \( 10 < r^{2} \frac{w}{v} < 10^{4} \).[24]
Here δ, D, v, and ω, respectively, represent the thickness of the concentration boundary layer (cm), diffusion coefficient (cm2/s) of the solute, kinematic viscosity of the melt (cm2/s), and angular rotation rate of the rod (rad/s).
In this study, the Reynolds number (\( Re = r^{2} {\omega \mathord{\left/ {\vphantom {\omega v}} \right. \kern-0pt} v} \)) was about 0.83 to 1.33 and the Schmidt number (\( S_{\text{C}} = {v \mathord{\left/ {\vphantom {v D}} \right. \kern-0pt} D} \)) was about 105; thus, the requirements for Eq. [2] are satisfied.
Equation [2] can be substituted into Eq. [3] to determine the rate of displacement of the dissolving oxide interface.
where ξ denotes the displacement rate of the dissolution interface (cm), ρ 1 represents the liquid density (g/cm3), ρ 0 stands for the solid density (g/cm3), W b refers to the mass percentage of the solute in the whole melt (pct), and W i denotes the mass percentage of Al2O3 in the boundary interface (pct).
Modifying Eq. [3] to express the rate of dissolution in g/cm2 s,
Substituting for δ from Eq. [2] and rearranging. Substitution of appropriate values of density and kinematic viscosity into Eq. [5] enables us to determine the effective diffusivity of Al2O3 in the calcium ferrite melt.
The melt viscosity can be measured by using thermodynamic calculation software (Factsage 6.2) and the melt density can be calculated using Eq. [6] proposed by Huang.[25]
where ω(SiO2), ω(CaO), ω(Fe2O3), ω(MgO), and ω(Al2O3), respectively, denote the percent masses of SiO2, CaO, Fe2O3, MgO, and Al2O3 in the melt.
Viscosities and densities calculated using these methods are given in Table V. Using these data, it is possible to calculate the effective diffusivity of alumina, \( D_{{{\text{Al}}_{2} {\text{O}}_{3} }} \), for each melt, also presented in Table V. Al2O3 diffusivity rates ranged from about 1.86 × 10−7 to 2.45 × 10−6 cm2/s. The results demonstrate that both the melt density and viscosity are related to the chemical composition of the melt. The diffusion coefficients of Al2O3 decrease initially and then increases with increasing Fe2O3/CaO mass ratio. The relationship between the diffusion coefficients of Al2O3 and the Al2O3 mass percentage of the initial melts is confused. The factors on the diffusion coefficients of Al2O3 are too many, such as the liquid viscosity, the thickness of the concentration boundary layer, and the properties of melt. So we do not do deeply research. The diffusion coefficients of Al2O3 decrease with increasing SiO2 mass percentage of the initial melts.
Rotation Speed in Al2O3 Dissolution
During dissolution, the mass balance for Al2O3 can be expressed by the following equation, Eq. [7]:[12]
where A, \( \rho {\text{Al}}_{2} {\text{O}}_{3} \), and r represent the surface area of the Al2O3 rod in contact with the melt (cm2), the density of the Al2O3 rod (g/cm3), and the radius of the Al2O3 rod (cm), respectively. R d can be calculated using Eq. [8]:
where k, \( C_{{{\text{Al}}_{2} {\text{O}}_{3} }}^{s} \), and \( C_{{{\text{Al}}_{2} {\text{O}}_{3} }}^{b} \) refer to the mass transfer coefficient of the solute (cm/s), the mass concentration of Al2O3 at the boundary layer (g/cm3), and the mass concentration of Al2O3 in the whole melt (g/cm3), respectively. k can also be expressed by the Levich–Cochran equation:
where D denotes the diffusion coefficient of Al2O3 in the melt (cm2/s), v represents the kinematic viscosity of the melt (cm2/s), and ω stands for the angular rotating rate (rad/s).
Equation [10] can be obtained by combining Eqs. [7], [8], and [9].[12]
These equations can be combined because D, v, and \( \left( {C_{{{\text{Al}}_{2} {\text{O}}_{3} }}^{s} - C_{{{\text{Al}}_{2} {\text{O}}_{3} }}^{b} } \right) \) are constants in the special melt, and R d is proportional to the square root of the angular velocity of the rod (\( R_{\text{d}} \propto w^{1/2} \)). The dissolution rate was plotted against the angular velocity in Figure 4, and the result shows that the dissolution rate increases with the rotation speed. Acknowledging that there is a measure of experimental uncertainty associated with this kind of work, Figure 4 supports the prediction from Eq. [5], i.e., diffusion is the rate-limiting step of alumina dissolution.
Chemical Composition of Calcium Ferrite Melt During Alumina Dissolution
Effect of chemical composition on Al2O3 dissolution
Figure 5 demonstrates that the weight loss of the Al2O3 rod at defined immersion depths varies with the Al2O3 percent mass in the initial melts at 1673 K (1400 °C). The weight loss of the Al2O3 rod in unit of immersion depth decreases with addition of Al2O3 percent mass because the Al2O3 concentration driving dissolution decreases.
The weight loss of the Al2O3 rod in unit of immersion depth varied with the Fe2O3/CaO mass ratio of the initial melts at 1673 K (1400 °C), as shown in Figure 6. Dissolution rates decreased initially and then increased with increasing Fe2O3/CaO mass ratio. Thermodynamic calculation results indicate that the melt viscosity calculated by Factsage 6.2 increases with increasing Fe2O3/CaO mass ratio. The diffusion coefficient of ions in the melt is inversely proportional to the melt viscosity, which is indicated by the Albert Einstein–Stokes equation. However, the diffusion rate is influenced by other factors because the dissolution rate increases with increasing Fe2O3/CaO mass ratio. The radii of Fe3+ and Al3+ are adjacent and can replace each other easily; as such, adding Fe3+ can promote mutual diffusion of Fe3+ and Al3+ in the solid–liquid phase and increase the dissolution rate. Ionic radii considered in this study are shown in Table VI.
Figure 7 demonstrates that the weight loss of the Al2O3 rod in unit of immersion depth varied with the SiO2 mass percentage in the initial melts at 1673 K (1400 °C). The weight loss of the Al2O3 rod in unit of immersion depth decreased with increasing SiO2 mass percentage, which indicates that the Al2O3 dissolution rate decreases. As the viscosity calculated by Factsage 6.2 increases with increasing SiO2 in the calcium ferrite melt, an increase in the dissolution drag coefficient of the melt may be observed.
The final melt was milled into powders (more than 90 pct below 0.74 μm, with screen method) for XRF detection. The results shown in Table VII demonstrate the content of MgO that has greatly changed; this is because that MgO crucible easily attacked at the experimental temperature 1673 K (1400 °C) and MgO powders between MgO crucible and Al2O3 crucible partly dissolve in melt; however, it is pointed out that the content of MgO has little influence on the Al2O3 dissolution rate, because that the dissolution rate of Al2O3 was not changed much like the fluctuation of MgO in the final melt.
Concentration driving force for alumina dissolution
Equation [10] can be rewritten by changing v to η and considering the relationship between mass fraction and concentration.[8]
where η, ρflux, \( ({\text{mass}}\;{\text{percent}}\;{\text{Al}}_{2} {\text{O}}_{3} )_{s} \), and \( ({\text{mass}}\;{\text{percent}}\;{\text{Al}}_{2} {\text{O}}_{3} )_{b} \) refer to the dynamic viscosity of the melt, the density of the melt calculated by Eq. [6], the mass fraction of Al2O3 at the interface (g/cm3), and the mass fraction of Al2O3 in the melt (g/cm3), respectively.
where k o denotes (\( 0.0062_{{\rho {\text{flux}}}}^{7/6} \omega^{{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-0pt} 2}}} \)), which is independent of the composition of the melt. Eq. [12] shows that k and \( \left[ {({\text{mass}}\;{\text{percent}}\;{\text{Al}}_{2} {\text{O}}_{3} )_{s} - ({\text{mass}}\;{\text{percent}}\;{\text{Al}}_{2} {\text{O}}_{3} )_{b} )} \right] \) greatly influence R d.
A good linear relationship between R d and \( \left[ {({\text{mass}}\;{\text{percent}}\;{\text{Al}}_{2} {\text{O}}_{3} )_{s} - ({\text{mass}}\;{\text{percent}}\;{\text{Al}}_{2} {\text{O}}_{3} )_{b} )} \right] \) is shown in Figure 8. However, extension of the linear fit deviates from the origin. This suggests that the mass transfer coefficient may not be independent of the flux chemical composition. The viscosity in this paper is calculated by thermodynamic calculation software (Factsage 6.2) and the diffusion coefficient is calculated by Stokes–Einstein Eq. [13]:[27]
where, η, r, k, and T refers to liquid viscosity (Pa s), the radius of spread particle (m), Boltzmann constant, and degree Kelvin (K).
It is thought that the chemical composition of calcium ferrite melt affects the mass transfer coefficient by affecting both viscosity and diffusivity of Al2O3 (or Al3+) in the calcium ferrite melt. Figure 9 shows the dependence of the viscosity of the melts and Al3+ diffusivity on melt composition. The viscosity of the melts increases and the diffusion rate of ions decreases with increasing Al2O3 content in the melt. The influences of alumina concentration and properties of the melts on the dissolution rate are shown in Figure 10. Better linear relations between the revised dissolution rate and the solid–liquid concentration difference of Al2O3 are observed, which demonstrates that the solute concentration difference in the solid–liquid phase exerts major effects on the dissolution rate, diffusion rate, and viscosity of the melt.
Temperature in Alumina Dissolution
A good linear relation between the weight loss of the Al2O3 rod in unit of immersion depth and temperature is indicated in Figure 11. The melt viscosity calculated by Factsage 6.2 decreases with increasing temperature; thus, temperature has a great influence on Al2O3 dissolution. This result is similar to that in a previous report by Liu et al.[13] who performed in situ observations of Al2O3 particle dissolution in CaO-Al2O3-SiO2 melts.
Activation Energy for Al2O3 Dissolution
Dissolution experiments were conducted at various temperatures to determine the activation energy for the alumina dissolution process. The relationship between dissolution rate and activation energy can be expressed by the following formula based on the Arrhenius equation.[12]
where E, R, and T refer to the activation energy (J/mol), gas constant (J/mol K), and adiabatic temperature (K), respectively. Figure 12 shows the activation energies determined for alumina dissolution. A good linear relationship between the dissolution rate and temperature is observed. The activation energies of Al2O3 dissolution of A1 and A3 are 245.35 and 350.94 kJ/mol, respectively; these values indicate that the chemical composition of the initial melts exerts great influences on the activation energy for Al2O3 dissolution.
Interface Observation of Solid–Liquid Dissolution Reaction
When the solid alumina is in contact with the liquid melt, formation of an intermediate solid phase at the interface is possible, and this phase may dissolve in the flux. Therefore, examining the interface between the Al2O3 rod and the melt at the microscopic level after contact for a defined period of time is useful. Microscopic analysis of the Al2O3 rod after the dissolution experiment was carried out using a scanning electron microscope and an energy dispersive spectrometer. BSE images of the cross section of samples after dissolution in different melt compositions and dissolution time at 1673 K (1400 °C) are shown in Figure 13. No apparent intermediate layer between the Al2O3 rod and melt is observed. The cooled melt is mainly composed of two embedded phases and its morphology varies with the initial melt composition and dissolution time.
The elemental distributions of the solid–liquid interface of sample A1 obtained after immersion times of 30 and 60 minutes are shown in Figures 14 and 15, respectively. The interface melt is mainly composed of two phases, one that mainly contains Ca, Al, and O and another that mainly contains Ca, Fe, Al, and O. The Al content in the latter phase is lower than that in the former. A third phase containing Mg, Fe, Al, and O may also be found in sample A1 after immersion for 30 minutes. Combining the SEM and XRD results, CaAl2O4, Ca2(Fe,Al)2O5, and MgFeAlO4 may be concluded to be the main phases in the final melt after dissolution.
The solid–liquid interface of sample A1 obtained after immersion for 60 minutes was examined by spot scanning to identify the phases in the final sample; relevant results are shown in Figure 16. The two phases identified roughly indicate the presence of CaAl2O4 and Ca(Fe,Al)2O4. Figure 17 shows a line-scanning image of the solid–liquid interface; here, the distribution of the main elements in each phase agrees with the above discussions.
The solid–liquid interface of sample S2 was also examined by surface scanning, spot scanning, and line scanning; relevant results are shown in Figures 18, 19, and 20, respectively. Surface scanning indicates that the light gray phase in the melt is composed of Fe, Mg, Si, Ca, and O. In this phase, Fe contents are high and Al contents are low. The gray phase in the melt is composed of Fe, Al, Ca, and O; in this phase, the Fe content is low.
Spot scanning results demonstrate that the two main phases in the melt are composed of CaAl2O4 and (Ca, Mg, Fe)2(Fe, Al)2O5. While the SiO2 content in the melt is too low for proper identification of an apparent phase in the samples, Si was uniformly distributed in the sample.
Line scanning shows that the Ca distributions in the two phases in the melt are uniform. Fe mainly gathered in the calcium ferrite phase, where the Al content is low. Mg and Si contents are also low in this phase, and their distributions are not obvious.
Conclusions
This paper reports the dissolution kinetics of Al2O3 in calcium ferrite melts. The following conclusions may be drawn:
-
1.
The dissolution rate of alumina shows good linear relations with temperature, dissolution time, and rotating speed. The chemical composition of the initial melt exerts a great influence on the dissolution rate. Diffusion is the rate-limiting step in Al2O3 dissolution in calcium ferrite systems.
-
2.
The Al2O3 diffusion coefficient rates in the present study range from about 1.86 × 10−7 to 2.45 × 10−6 cm2/s. The chemical composition of the initial melts exerts a great influence on the activation energy for Al2O3 dissolution. The activation energy of samples A1 and A3 in this study are 245.35 and 350.94 kJ/mol, respectively.
-
3.
Differences in Al2O3 concentration in the solid–liquid phase are the main factor influencing dissolution. No apparent intermediate layer between the Al2O3 rod and melt is observed.
References
K. Yi: Sinter. Pelleting, 2008, (4), p. 54.
X. Gao and W.Q. Liu: China Steel, 2009, (10), pp. 25–27.
X.M. Guo: The Formation and Mineralogy of Calcium Ferrite during Sintering Process, Metallurgical Industry Press, 1999, pp. 1–2.
L. Lu, R.J. Holmes, and J.R. Manuel: ISIJ Int., 2007, vol. 47(3), pp. 349–58.
M.I. Pownceby and J.M.F. Clout: Miner. Process. Extr. Metall., 2003, vol. 112(1), pp. 44–51.
M. S. Xue and X. M. Guo: J. Chin. Rare Earth Soc., 2008, vol. 26(08), pp. 205–09.
X. Y. Xie and Y. J. Liu: South. Met., 2011, vol. (5), pp. 30–38.
J.B. Liu and L.S. Li: J. Anhui Univ. Technol., 2009, vol. 26(4), p. 333–37.
S.K Zhao, Y. Huang, X X. Huang, and J.K. Guo: J. Ceram., 2002, vol. 23(3), pp. 149–55.
T. Maeda, K. Nishioka, K. Nakashima, and M. Shimizu: ISIJ Int., 2004, vol. 44(12), pp. 2046–51.
A.R. Cooper Jr. and W.D.K.J. Kingery: J. Am. Ceram. Soc., 1964, vol. 47(1), pp. 37–43
A.H. Bui, H.M. Ha, I.S. Chung, and H.G. Lee: ISIJ Int., 2005, vol. 45(12), pp. 1856–63.
J.H. Liu, F. Verhaeghe, M.X. Guo, B. Blanpain, and P. Wollants: J. Am. Ceram. Soc., 2007, vol. 90 (12), pp. 3818–24.
K.H. Sandhage and G.J. Yurek: J. Am. Ceram. Soc., 1990, vol. 73 (12), pp. 3633–42, 3643–49.
K. Yajima, H. Matsuura, and F. Tsukikashi: ISIJ Int., 2010, vol. 50(2), pp. 191–94.
M.I. Pownceby and J.M.F. Clout: Trans. Instn. Min. Metall., 2000, vol. 109, pp. 36–48.
M.I. Pownceby and T.R.C. Patrick: Eur. J. Mineral., 2000, vol. 12, pp. 455–68.
B J. Monaghan, L. Chen: Process Metall. Steelmak., 2005, vol. 5, pp. 346–52.
B.J. Monaghan, L. Chen, and J. Sorbe: Ironmak. Steelmak., 2005, vol. 32(3), pp. 258–64.
S.H. Lee, C. Tse, K.W. Yi, P. Misra, V. Chevrier, C. Orrling, S. Sridhar, and A.W. Cramb: J. Non Cryst. Solids, 2001, vol. 282(1), pp. 41–48.
R. Hagemann, L. Pettsold, P.R. Sheller: Steelmaking, 2010, vol. 2(4), pp. 262–66.
K. Sadrnezhaad, A. Gharavi, A. Namazi: IJE Trans. A Basics, 2003, vol. 16(1), pp. 59–70.
B.Hashemi, Z.A.Nemati, S.K.Sadrnezhaad, and Z.A.Moghimi, J. Mater. Sci. Technol., 2006, vol. 22(6), pp. 826–32.
V.G. Levich: Physicochemical Hydrodynamics, Prentice-Hall, Englewood Cliffs, NJ, 1962.
X.H. Huang: Iron and Steel Metallurgy Principle, Metallurgical Industry Press, 2008, p. 211.
W.M. Haynes and D.R. Lide: Natl. Inst. Stand. Technol., 2010, pp. 4–45, 12–12.
K. Sun: The Macroscopic Reaction Kinetics and its Analytical Method, Metallurgical Industry Press, 1998, p. 33.
Acknowledgments
The authors are especially grateful to National Natural Science Foundation of China (Grant No. 51104192).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 17, 2013.
Rights and permissions
About this article
Cite this article
Xiang, S., Lv, X., Yu, B. et al. The Dissolution Kinetics of Al2O3 into Molten CaO-Al2O3-Fe2O3 Slag. Metall Mater Trans B 45, 2106–2117 (2014). https://doi.org/10.1007/s11663-014-0159-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0159-z