Abstract
Calcium ferrite is the main binding phase for high-basicity sinter. The production and structure of calcium ferrite greatly influence the quality of the sinter. With the change in gangue composition, MgO becomes an important factor in the generation of calcium ferrite. In this study, the rotating cylinder method was used to study the dissolution kinetics of MgO into CaO-MgO-Fe2O3 melt. The experimental variables included the temperature, the initial composition of the melt, the Fe2O3/CaO mass ratio, the rotation time, and the rotation speed. The results indicate that the dissolution rate increases with increasing dissolution time, temperature, and rotation speed but decreases with increasing MgO content and Fe2O3/CaO mass ratio in the initial slag. The dissolution rate was observed to increase and then decrease with the addition of SiO2 in the initial slag. The activation energy and diffusion coefficient for MgO dissolution were found to range from 117.31 to 234.24 kJ mol−1 and from 1.03 × 10−6 to 1.18 × 10−5 cm2 s−1, respectively. The concentration difference between the solid and liquid phases is the main driving force for dissolution, but the viscosity and magnesium ion diffusivity of the melt also affect the process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sintering, an important method for iron ore agglomeration, plays a significant role in the ironmaking process. The quality of sinter has a crucial influence on the production and energy consumption of the blast furnace process. In recent years, the diversity of import channels for iron ores has led to the instability of raw materials. In addition, iron ores in China are typically of low iron grade and complex in gangue composition, resulting in difficulties during the agglomeration process.
The ore-forming process via sintering involves a series of complex physical and chemical processes, the most important of which are solid-phase reaction, liquid formation, and cooling and consolidation. For high-basicity sinter, the adhesive silico-ferrite of calcium, and aluminum (SFCA) is the main binding phase in the sintering process. The fine ore composition, particularly the Fe, SiO2, and Al2O3 levels, directly constrains the high-temperature bonding phases. Lower Fe-grade (<62 pct Fe) ores and concentrates will typically form SFCA as part of the final assemblage. Medium-grade (62 to 65 pct Fe) ores will form a mixture of SFCA and SFCA-I. SFCA-I is the most desirable bonding phase in iron ore sinter.[1] The crucial processes in iron ore sintering are the assimilation between the initial liquid phase of calcium ferrite and solid particles and the subsequent crystallization process. The initial liquid phase is generated from the fines adhering to the surfaces of large particles during sintering via reactions between iron ore and fluxes. This melt then assimilates the nuclei particles to produce more melt.[2] Therefore, the assimilation process involves solid particles, such as Al2O3, MgO, and SiO2, entering into the liquid phase by dissolution and wetting.
MgO has been shown to greatly influence the sintering index and the strength of the sinter. Sugiyama et al.[3] studied the crystal structures of Mg-rich SFCA (SFCAM) and showed that the structure of SFCAM was iso-structural with aenigmatite and exhibited alternating stacking of the tetrahedral and octahedral layers. The tetrahedral sites of oxygen are occupied by Fe, Al, and Si. The octahedral sites of oxygen are occupied by Fe, Mg, and Al, and this feature contrasts with that of the Mg-free SFCA phase, in which Al prefers tetrahedral sites. Guo et al.[4] found that the effect of MgO on the generation of calcium ferrite (CF) was related to the basicity and the existence of Al2O3 and also found that an increase in the MgO content can promote the generation of CF for high-basicity sinter without Al2O3. Oluwadare[5] investigated the roles of Al2O3 and MgO in the evolution of phases in iron ore sinters and found that Al2O3 is necessary for the formation of SFCA but that MgO does not favor the formation of SFCA when the sintering time is long and the temperature is high. On the other hand, Al2O3 may not be necessary for the formation of SFCA in sinters if the sintering temperature is below 1573 K and the sintering time is short. Kimura et al.[6] observed that the liquid area is increased by the addition of Al2O3 and decreased by the addition of MgO for CaO-SiO2-FexO-Al2O3 or -MgO systems at various oxygen partial pressures. Umakoshi et al.[7] reported that the dissolution rate of burnt dolomite in molten FetO-CaO-SiO2 slags is controlled by the mass transport of CaO in burnt dolomite for low FetO slag and that of MgO for other slags in the boundary layer of molten slag. The MgO content becomes an important factor in the quality of sinters because of the change in gangue composition of iron ores. The studies mentioned above have revealed the structure of SFCAM and described the influence of MgO on the formation of SFCA, thereby contributing to a full understanding of the sintering mechanism and of how to control the generation of calcium ferrite for multi-components.
However, most of these studies have focused mainly on the effect of MgO on the generation of SFCA or sinter properties[3–6]; they have not reported on the dissolution kinetics of MgO into calcium ferrite melt, which is important to better understanding of the assimilation process and improvement of the sintering process. Therefore, this study was conducted to examine the dissolution of MgO into CaO-MgO-Fe2O3 slag using the rotating cylinder method.
Experimental
Raw Materials
The CaO-MgO-Fe2O3 slags used in this study were synthesized by mixing an analytical reagent (AR). The purity and size distributions of the slag are shown in Table I.
Experimental Procedure
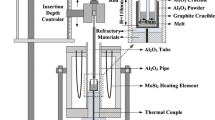
The rotating cylinder method was used to investigate the dissolution kinetics of MgO in molten CaO-MgO-Fe2O3 slag. The equipment used in this experiment has been described in detail in previous research.[8,9]
The initial chemical composition of calcium ferrite slag, the dissolution time, the rotation speed, and the temperature were the main experimental variables. The chemical compositions of the initial slags used for the dissolution experiments are shown in Table II and plotted in the CaO-MgO-Fe2O3 ternary phase diagram shown in Figure 1.
Table III shows the experimental scheme. A1-A3 were used to study the effect of the initial MgO content on the dissolution rate of MgO, and B1-B5 were used to investigate the effect of the Fe2O3/CaO mass ratio on the dissolution rate of MgO. C0-C3 were used to study the effect of the SiO2 content of the initial slag on the weight loss of an MgO rod during the dissolution process. The experiments were carried out at 1623 K, 1673 K, and 1723 K (1350 °C, 1400 °C, and 1450 °C).
The slag samples were prepared by mixing to produce the chemical compositions listed in Table II and were roasted at 1273 K (1000 °C) for 2 hours to remove the CO2 present in the calcium carbonate. The roasted samples were loaded into an Al2O3 crucible and heated to a selected temperature in the furnace in an Ar atmosphere. An MgO rod was then inserted into the CaO-MgO-Fe2O3 melt at a defined depth and rotated at a defined speed for a certain period of time, as shown in Table III. The mass loss of the MgO rod was calculated from the change in the MgO content of the slag before and after the dissolution experiment. The mass loss of the MgO rod could not be measured directly because of the difficulty of removing adhered slag. Thus, the final melt was cooled to room temperature and then milled into powder for chemical analysis to determine the mass loss of the MgO rod indirectly from the changes in the MgO and Al2O3 contents in the melt before and after the dissolution experiment.
Results and Discussion
Diffusion Coefficient for MgO Dissolution
Table IV shows the weight loss of the MgO rod during the dissolution experiment. As Figure 2 shows, the weight loss of the MgO rod per unit immersion depth was directly proportional to the immersion time at 1673 K (1400 °C). The results indicate that the solid–liquid contact area decreases with the dissolution of MgO and that the solid–liquid contact area had no apparent effect on the dissolution of MgO into the calcium ferrite melt. These results are consistent with those of previous studies.[10–16] Therefore, the dissolution rate can be calculated using Eq. [1]:
where R d is the dissolution rate; t is the immersion time; and W 0, W t , and S are the initial mass, final mass, and initial immersion area of the MgO rod, respectively.
According to the results of a previous study,[17] the diffusion in the boundary layer is the restricting step in Al2O3 dissolution in the continuous casting process. However, Maeda et al.[18] indicated that the dissolution reaction at the solid–liquid interface is the restricting step for Fe2O3 dissolution into calcium ferrite melts and that rotation speed has no obvious influence on mass transfer. Xiang et al.[8] noted that the rotation speed of rod samples greatly affects mass transfer and that the diffusion at the solid–liquid interface is the restricting step during the reaction. Therefore, to better understand the restricting step of MgO dissolution into the calcium ferrite melt, it is necessary to explore the relationship between the dissolution rate and the mass transfer.
Cooper et al.[15] and Sandhage et al.[14] showed that the mass transfer of rotating cylinders in shallow immersion depths could be described by the Levich–Cochran equation,[19] which applies for Schmidt numbers (υ/D) far greater than 1 and Reynolds numbers (Re=r 2 ω/υ) in the range of 10 to 104. The equation is as follows:
where δ is the thickness of the concentration boundary layer, D is the diffusion coefficient of the solute, \( \upsilon \) is the kinematic viscosity of the melt, and w is the angular rotation rate of the MgO rod. Schmidt numbers ranging from 8.1 × 103 to 8.8 × 104 were calculated from the data shown in Table V. The Reynolds numbers ranged from approximately 0.83 to 1.33. These values satisfy the aforementioned conditions for the applicability of Eq. [2].
Equation [2] can be substituted into Eq. [3] to determine the displacement of the dissolving oxide interface:
where \( \xi \) is the displacement rate of the dissolution interface, ρ l is the liquid density, ρ 0 is the solid density, W b is the mass percentage of the solute in the whole melt, and W i is the mass percentage of MgO at the boundary interface.
Equation [3] can be modified to express the dissolution rate in units of g cm−2 s−1, as shown by Eq. [4]:
Combining Eqs. [2] and [4], D can be described by Eq. [5][17]:
In this study, the melt viscosity was calculated using the viscosity module in the FactSage 6.2 software. According to Huang et al.,[20] the melt density ρ l can be calculated using Eq. [4] as follows:
Influencing Factors on Weight Loss
Figure 3 shows the relationship between rotation speed and weight loss during the dissolution process. The weight loss increases slightly with increasing rotation speed. The results indicate that the dissolution rate increased and the mass transfer between the solid and liquid phases improved with increasing rotation speed. The weight loss per unit immersion depth for different compositions varied linearly with temperature, as shown in Figure 4. According to Eq. [4], the dissolution rate is negatively correlated to the viscosity. Therefore, increasing the temperature increases the dissolution rate. The weight losses for the different chemical compositions considered were different, which might be attributable to differences in the viscosity of the melt and the concentrations between the solid and liquid phases, which result in different dissolution rates.
Figures 5 and 6 illustrate the relationships of the MgO mass percentage and the Fe2O3/CaO mass ratio in the initial slag to the weight loss of the MgO rod, respectively. Figure 5 shows that the weight loss of the MgO rod decreases with increasing mass percentage of MgO because a decrease in the concentration difference between the solid and liquid phases leads to a decrease in the driving force, which results in a decrease in the dissolution rate. Figure 6 shows that weight loss of the MgO rod decreases with increasing Fe2O3/CaO mass ratio. This is consistent with the dissolution behavior of SiO2 into CaO-SiO2-Fe2O3 slag[9] but is different from the dissolution behavior of Al2O3 into CaO-Al2O3-Fe2O3[8]: the dissolution rate of Al2O3 decreases initially and then increases with increasing Fe2O3/CaO mass ratio. The results calculated using the viscosity module of FactSage 6.2, summarized in Table V, demonstrate that viscosity increases with increasing Fe2O3/CaO ratio and that the Fe2O3/CaO mass ratio increases greatly from B1 to B5, whereas the dissolution rate of MgO exhibits no significant change. The diffusion coefficient of ions is inversely proportional to the melt viscosity, as expressed by the Einstein–Stokes equation,[19] shown as Eq. [7]:
where η, r i, and k are the dynamic viscosity of the melt, the radius of ion i, and the Boltzmann constant, respectively.
The diffusion coefficient decreases with increasing viscosity, as shown in Eq. [7]. Thus, increasing the Fe2O3/CaO mass ratio and decreasing the temperature may promote the viscosity of the melt and reduce the diffusion coefficient, thereby decreasing the dissolution rate of MgO.
As Figure 7 shows, the weight loss of the MgO rod increases and then decreases with the addition of SiO2 to the initial slag at 1673 K (1400 °C), which means that the dissolution rate of MgO also increases and then decreases. This occurs because Si4+ promotes the solution of Mg2+ into calcium ferrite melt to form SFCA containing magnesium. As the SiO2 content of the initial slag increases, more Mg2+ enters the calcium ferrite melt and increases the viscosity of melt. When the amount of SiO2 added is small, Mg2+ enters the calcium ferrite melt without an obvious change occurring in the melt viscosity, and thus the dissolution rate increases. As the amount of SiO2 added increases, the viscosity increases to a greater extent and plays an increasingly important role in the dissolution process, which decreases the melt diffusion and thereby decreases the dissolution rate.
Table VI shows the contents of MgO and Al2O3 in the final slag. The mass fraction of Al2O3 varied greatly with the dissolution of alumina crucible under different conditions. The dissolution rate of MgO, on the other hand, did not exhibit any obvious change. These results indicate that the Al2O3 content has little influence on the MgO dissolution rate.
Concentration Driven Force
The mass balance of MgO during the liquid–solid dissolution reaction can be described by the following equation[16]:
where ρ MgO and r are the density and radius of the MgO rod, respectively, and R d can be described as shown in Eq. [9]:
where \( k \), \( C_{\text{MgO}}^{\text{i}} \), and \( C_{\text{MgO}}^{\text{b}} \) are the mass transfer coefficient of the melt and the mass concentrations of MgO at the boundary layer and in the melt, respectively, and ρ flux is the density of the melt, which can be calculated from Eq. [6]. The terms (mass pct MgO)i and (mass pct MgO)b refer to the mass fraction of MgO at the liquid–solid interface and in the melt, respectively. According to previous studies,[14,15,17,21] the dissolution of solid particles into slag is rate limited by solute diffusion in the boundary layer. Therefore, \( k \) can also be calculated from the Levich–Cochran equation,[22] as shown in Eq. [10]:
where D, υ, and w are the diffusion coefficient of MgO in the melt, the kinematic viscosity of the melt, and the angular rotation rate of the MgO rod, respectively.
Equations [9] and [10] can be combined to obtain Eq. [11]:
For some specific melts, because the diffusion coefficient D, the kinematic viscosity υ, and the concentration difference \( \left( {C_{\text{MgO}}^{\text{i}} - C_{\text{MgO}}^{\text{b}} } \right) \) are all constants, the dissolution rate should be proportional to the square root of the angular rotation rate of the rod.
Considering the relationship between the mass fraction and the concentration, and changing the kinematic viscosity υ to the dynamic viscosity η, Eq. [11] can be expressed as follows:
The right side of Eq. [12] can be divided into two parts. One part, η −1/6 D 2/3 \( \left[ {\left( {\left( {{\text{mass}}\,{\text{pct}}\,{\text{MgO}}} \right)_{\text{i}} - \left( {{\text{mass}}\,{\text{pct}}\,{\text{MgO}}} \right)_{\text{b}} } \right)} \right] \), is dependent on the melt composition, and the other part, 0.0062\( \rho_{\text{flux}}^{7/6} \) w 1/2 (k 1), is independent of the melt composition. Hence, Eq. [12] can be expressed as shown in Eq. [13], which indicates that two main factors, the mass transfer coefficient k 1 and the concentration difference \( \left[ {\left( {{\text{mass}}\,{\text{pct}}\,{\text{MgO}}} \right)_{\text{i}} - \left( {{\text{mass}}\,{\text{pct}}\,{\text{MgO}}} \right)_{\text{b}} } \right] \), affect R d.
To analyze the relative importance of these two factors, the relationship between R d and the concentration difference was plotted as shown in Figure 8. A good linear relationship between the two can be observed in figure. However, if this linear relationship is extrapolated, it does not intersect the origin of the coordinate system, which means that the chemical composition of the melt may affect the mass transfer coefficient.
Figure 9 shows the influence of the MgO mass percentage on the viscosity and magnesium ion diffusivity. The radius of the ions has been reported by Haynes.[23] In this study, the viscosity was calculated using the viscosity module of the FactSage 6.2 thermodynamic software, and the diffusion coefficient was calculated from the Stokes–Einstein equation, shown as Eq. [7]. As Figure 9 shows, as the MgO content increases, the melt viscosity increases, and the magnesium ion diffusivity decreases. Figure 10 illustrates the effects of viscosity and diffusion on the relationship between the dissolution rate and the MgO concentration difference. This figure shows a better linear relationship between the dissolution rate and the MgO concentration difference. In addition, the linear relationship approaches the origin of the coordinate system when extrapolated. This indicates that the solute concentration difference in the solid and liquid phases greatly influences the dissolution rate and that the diffusion rate and the melt viscosity also influence the dissolution process.
In this study, the dissolution rate of MgO into calcium ferrite slag was found to be less than that of SiO2 but greater than that of Al2O3, which means that MgO promotes the formation of the liquid phase in sintering more than Al2O3 does but less than SiO2 does. Thus, a certain amount of MgO is helpful in forming an abundant liquid phase at the beginning of the sintering process. With increasing dissolution time, temperature, and rotation speed, the dissolution rate of MgO increases, whereas increasing the MgO content and the Fe2O3/CaO mass ratio in the initial slag decrease the dissolution rate.
Activation Energy of MgO Dissolution
Assuming that the dissolution is controlled by the mass transfer in the slag phase, the activation energy of the mass transfer in the slag phase can be obtained from the Arrhenius formula,[16] shown as Eq. [14]:
where k 0 , E, R, and T are the pre-exponential constant, activation energy, gas constant, and temperature, respectively. The value of k can be calculated using Eq. [9], and thus the relationship between ln k and 1/T can be obtained, and the activation energy and the value of the k 0 constant can be calculated by linear regression, as shown in Figure 11. The activation energies of MgO dissolution for A1, A2, and A3 were found to be 117.31,149.64, and 234.24 kJ mol−1, respectively, and the values of the k 0 constant of MgO dissolution for A1, A2, and A3 were found to be 63.94, 5.50 × 102, and 1.74 × 105 g cm−2 s−1, respectively. These results indicate that the initial MgO content has a great effect on the activation energy of MgO dissolution. The activation energy increases with the increasing MgO content in the initial slag.
Interface Observation and Reaction Mechanisms
To better understand the reaction at the solid–liquid interface, the interface microstructure between the MgO rod and the calcium ferrite melt was examined. The cross sections after the dissolution experiments were examined by scanning electron microscopy (SEM) and energy dispersion spectroscopy (EDS). Figure 12 shows the back-scattered electron imaging (BSE) images of the cross section of A2 at different times and that of C3. An apparent intermediate layer can be observed at the interface between the MgO rod and the melt. This layer can be considered the reaction layer.
Line scanning was performed to examine the elemental distributions of the solid–liquid interface of sample A2 after 30 minutes of immersion times of 30 minutes (A2-30 min), as shown in Figure 13. From the boundary to the center of the MgO rod, the elements of O, Al, and Ca are homogeneously distributed, Mg gradually increases, and Fe decreases. It can be inferred that strong erosion occurs between the calcium ferrite melt and the MgO rod. In addition, a comparison of the distributions of Fe and Ca shows that the locomotive ability of Ca is much better than that of Fe during the dissolution process. Figure 14 shows the elemental distributions of the solid–liquid interface of sample C3. The results are the same as those for sample A2-30 minutes. This indicates that the melt reacted with the surface of the MgO rod and eroded the surface of the rod.
To verify the results of the line scanning, spot scanning of the liquid–solid interface of the A2-30 minutes and C3 samples was carried out. Three points from outside to inside of the main line of the line scanning were chosen for spot scanning to analyze the elemental distribution and phase composition. Figures 15 and 16 show the chemical compositions of the main phases of the solid–liquid interphase of the A2-30 minutes sample and the C3 sample, respectively. The spot scanning results in Figure 15 indicate that the points 1 and 2 mainly exist in the forms of Ca2Fe1.52Al0.48O5 and MgFe2O4, respectively, and that point 3 exists in the form of MgO, because of the MgO rod. The elemental distribution results are consistent with the line scanning results, from outside to inside of the solid–liquid interface: the Fe content gradually decreases and the Mg content increases. The elemental distributions results for sample C3, shown in Figure 16, are consistent with those for the A2-30 minutes sample. Points 1 and 2 in this figure exist in the form of Ca2Fe1.052Al0.665Mg0.133Si0.133O5(SFCA) and MgFe2O4, respectively, and point 3 exists in the form of MgO.
These results demonstrate that the dissolution of the MgO rod into CaO-MgO-Fe2O3 melt is a dissolving diffusing process. The MgO dissolved and diffused into the melt, while Ca and Fe diffused into the MgO rod.
Conclusions
The rotating cylinder method was used to study the dissolution kinetics of MgO into CaO-MgO-Fe2O3 melts. The kinetic parameters were obtained by measuring the weight loss of solid samples. The diffusion coefficient and dissolution activation energy of MgO during the dissolution process were derived according to an existing diffusion model. The following conclusions can be drawn from the results of the experiments:
-
1.
The diffusion coefficient of MgO was found in this study to be within the range of 2.85 × 10−6 to 1.14 × 10−5 cm2 s−1. The dissolution activation energy of MgO was found to be greatly influenced by the initial MgO content during the MgO dissolution process. The activation energy from A1 to A3 ranged from approximately 117.31 to 234.24 kJ mol−1 and to increase with increasing MgO content.
-
2.
The MgO dissolution rate was found to be proportional to the dissolution time, temperature, and rotation speed. The MgO dissolution rate was negatively correlated to the Fe2O3/CaO mass ratio and the MgO content in the initial slag, and the dissolution rate was found to increase and then decrease with increasing SiO2 content. The MgO content was a main factor in the dissolution rate. Diffusion was the restrictive link in MgO dissolution into calcium ferrite systems.
-
3.
Cracking was observed at the solid–liquid interface for MgO dissolution into calcium ferrite systems, but an intermediate layer between melts and the MgO rod was not observed. The melt reacted with the rod samples by eroding the rod samples. The dissolution process was found to be primarily driven by the concentration difference between the solid and liquid phases. The dissolution rate was also found to be affected by the melt viscosity.
References
M. I. Pownceby and J. M. F. Clout: Trans. Inst. Min. Metall. C., 2003, vol. 112, pp. 44-51.
L.X. Yang and L. Davis: ISIJ international, 1999, vol. 39, pp. 239-245.
K. Sugiyama, A. Monkawa, and T. Sugiyama: ISIJ Int., 2005, vol. 45, pp. 560-568.
X. M. Guo, J. Zhang, C. Z. Li, and L. Zhu: Iron Stel., 2009, vol. pp. 16-19.
G. O. Oluwadare: Trends in Applied Sci. Res., 2007, vol. 2, pp. 483-491.
H. Kimura, T. Ogawa, M. Kakiki, A. Matsumoto, T. Hamano, and F. Tsukihashi: Tetsu. To. Hagane., 2006, vol. 92, pp. 748-754.
M. Umakoshi, K. Mori, and Y. Kawai: Trans. Iron Stel. Inst., 1984, vol. 24, pp. 532-539.
S. L. Xiang, X. W. Lv, B. Yu, J. Xu, and J. Q. Yin: Metall. Mater. Trans. B, 2014, vol. 45, pp. 2106-2117.
B. Yu, X. W. Lv, S. L. Xiang, and J. Xu: Metall. Mater. Trans. B, 2016, vol. 47, pp. 2063-2071.
M. I. Pownceby and T. R. C. Patrick: Eur. J. Mineral., 2000, vol. 12, pp. 455-468.
M. I. Clout and J. M. F. Pownceby: Trans. Inst. Min. Metall., 2000, vol. 109, pp. 36-48.
H. Matsuura, K. Yajima, and F. Tsukikashi: ISIJ Int., 2010, vol. 50, pp. 191-194.
F. Verhaeghe, J. H. Liu, M. X. Guo, B. Blanpain, and P. Wollants: J. Am. Ceram. Soc., 2007, vol. 90, pp. 3818-3824.
K. H. Sandhage and G. J. Yurek: J. Am. Ceram. Soc., 1990, vol. 73, pp. 3633-3642.
W. D. Kingery, and A. R. Cooper: J. Am. Ceram. Soc., 1964, vol. 47, pp. 37–43.
H. M. Ha, A. H. Bui, I. S. Chung, and H. G. Lee: ISIJ Int., 2005, vol. 45, pp. 1856-1863.
R. J. Pomfret, K. S. Coley, and X. Yu: Metall. Mater. Trans. B, 1997, vol. 28, pp. 275-279.
K. Nishioka, T. Maeda, K. Nakashima, and M. Shimizu: ISIJ Int., 2004, vol. 44, pp. 2046-2051.
K. R. Harris: J. Chem. Phys., 2009, vol. 131, pp. 54-58.
X. H. Huang: Metallurgical Principles, S. L. Cao, Beijing, 2008, pp. 211.
S. Taira, K. Nakashima, and K. Mori: ISIJ Int., 1993, vol. 33, pp. 116-123.
V. G. Levich: Physicochemical Hydrodynamics, Prentice-Hall, New York, 1962, pp. 60–72.
W. M. Haynes and D. R. Lide: Natl. Inst. Stand. Technol., 2010, vol. 45, pp. 12-15.
Acknowledgments
This study was performed with the financial support of the Natural Science Foundation of China (No. 51522403) and the Fundamental Research Funds for the Central Universities (No. CDJZR14135501).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted January 24, 2016.
Rights and permissions
About this article
Cite this article
Wei, R., Lv, X., Yue, Z. et al. The Dissolution Kinetics of MgO into CaO-MgO-Fe2O3 Slag. Metall Mater Trans B 48, 733–742 (2017). https://doi.org/10.1007/s11663-016-0842-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0842-3