Abstract
One of the most important casting defects in Al alloys is thought to be the double-oxide film defect (bifilm) which has been reported to have a deleterious effect on the reproducibility of the mechanical properties of Al castings. Previous research has suggested that the atmosphere inside such bifilms could be consumed by reaction with the surrounding melt, which might decrease the size of the defects and reduce their harmful effect on mechanical properties. In order to follow the change in the composition of the interior atmosphere of a bifilm, analog air bubbles were held inside Al alloy melts, for varying lengths of time, and subjected to stirring, followed by solidification. The bubble contents were then analyzed using a mass spectrometer to determine the changes in their compositions with time. The results suggested that initially oxygen and then nitrogen inside the bubble were consumed, and hydrogen dissolved in the melt diffused into the bubble. The consumption rates of O and N as well as the rate of H diffusion were dependent upon the type of oxide, which was dependent on the alloy composition. The reaction rates were the fastest with MgO (in an Al-5Mg alloy), slower with alumina (in commercial-purity Al alloy), and the slowest with MgAl2O4 spinel (in an Al-7Si-0.3Mg alloy). It was estimated that the times required for typical bifilm defects in the different alloys to lose their entire oxygen and nitrogen contents were about 345 seconds (~6 minutes), in the case of Al-5Mg; 538 seconds (~9 minutes), in the case of a commercial purity alloy; and 1509 seconds (~25 minutes), in the case of the Al-7Si-0.3Mg alloy (2L99) due to the different oxides that the different alloys would be expected to form.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
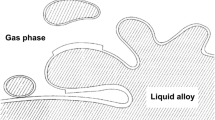
Double-oxide film defects, also known as bifilms, are significant defects in Al castings, which adversely affect the reproducibility of their mechanical properties.[1,2] They are created by surface turbulence during the transfer and/or pouring of the liquid metal, which causes the oxidized surface of the melt to be folded over onto itself and then submerged into the bulk liquid, with a portion of the mold atmosphere being entrapped within it as shown in Figure 1.
A bifilm would be expected to have no bonding between its inner faces, and therefore, would act as a crack in the solidified casting. In addition, hydrogen dissolved in the aluminum alloy melt may diffuse into the bifilm, causing its expansion into a pore. Finally, double-oxide films are also suggested to act as favorable substrates for the nucleation and growth of iron-rich intermetallic phases and initiators of shrinkage porosity. All these defects would not only reduce the tensile strength, elongation, and fatigue limit of aluminum castings, but would also increase the variability of their properties.[1,2]
In Nyahumwa et al.,[3] it was suggested that the transformation of the alumina constituting the bifilm from γ-alumina to α-alumina would occur in about 5 hours, and the associated volume change might initiate cracks in the film, allowing the atmosphere inside the defect (probably mainly oxygen and nitrogen) to react with the surrounding melt. Complete consumption of the internal atmosphere of double-oxide film may perhaps lead to the partial or complete deactivation of the defect. The studies by Raiszadeh and co-workers[4,5] have also suggested bonding between oxide surfaces, once the entire internal atmosphere of a bifilm has been consumed.
In order to study the history of a bifilm in aluminum castings, Raiszadeh and Griffiths[6] used real-time X-ray radiography to see the changes in volume of an air bubble with time, trapped within an Al melt. The results suggested that, in liquid pure Al, oxygen in the air bubble was consumed first, followed by nitrogen, forming Al2O3 and AlN, respectively. Estimates of the rates of consumption of the atmosphere inside the bubble were deduced from the reduction in bubble volumes observed in a real-time X-ray,[7] and extrapolated to estimate the duration of the atmosphere inside a double-oxide film defect of assumed dimensions. However, these experiments were carried out only with pure Al, where the entrained surface oxide film would be expected to be Al2O3. The alloying elements commonly occurring in Al alloys lead to the formation of different oxide films, and hence oxide film defects that may have different behaviors. For example, in Al-Si-Mg alloys, MgAl2O4 (spinel) would be expected to form,[8] while in Al alloys containing more than 2 pct Mg, the oxide film would be expected to be MgO.[8] Both Al2O3 and MgAl2O4 would be expected to form protective oxide films, while MgO forms a porous, nonprotective oxide film, and some differences in double-oxide film defect behavior might therefore be expected.
Studies on the effects of holding time before solidification on the mechanical properties of pure Al alloy, by Raisazadeh and Griffiths,[9] and by El-Sayed et al.,[10,11] suggested that two competing mechanisms might influence the behavior of double-oxide films. These would be (i) the consumption of the atmosphere inside the double-oxide films by reaction with the surrounding liquid Al (already discussed), which would reduce the size of double-oxide films and enhance casting properties; and (ii) the diffusion of hydrogen into the bifilms, causing them to expand, with a deleterious effect on mechanical properties.
In this paper, the experimental study by Raiszadeh and Griffiths[6] has been extended. The consumption rates of the gases inside air bubbles held in liquid Al alloys of varying compositions hve been studied using mass spectroscopy, as an analog for the behavior of double-oxide film defects. The consumption rates of oxygen and nitrogen in the bubble, and the rate of hydrogen diffusion from the melt into the bubbles, were measured, and differences in the behaviors of bifilms in the different alloys, having different oxide film compositions, inferred.
Experimental Procedure
A steel strip containing a blind hole of 6-mm diameter and 5-mm depth at each end was connected to a stirrer, and immersed into an Al alloy melt at a temperature of about 993 K (720 °C) ± 10 deg, thus trapping an air bubble in each of the blind holes. The stirrer was rotated at 540 rpm, corresponding to an angular velocity of 1.4 m s−1 at the radial distance of the holes, and the steel strip was held at a depth of about 5 mm below the surface of the melt (to avoid surface turbulence during rotation). Figure 2 shows a sketch of the experiment. The air bubbles trapped inside the holes reacted with the surrounding liquid Al during immersion in the melt, while hydrogen from the melt diffused into or out of the air bubble. After a period of rotation ranging from 2 to 40 minutes, the stirrer was halted, and the melt allowed to solidify, with the time taken for the solidification process being about 30 seconds. After solidification, a sample with dimensions of 15 × 15 × 15 mm was machined out of the casting, that contained a bubble trapped within the hole in the steel strip. This sample was then placed in a Pore Gas Analyser (PGA, Hiden Ltd., Warrington, UK)—and the bubble pierced under a vacuum of <10−4 Pa, and its contents passed through a mass spectrometer. Finally, the bubble interior was cut open and examined using an SEM to determine the presence of different reactants on the surface of the solidified Al alloy trapped within the hole in the steel rod.
At the beginning and at the end of the series of experiments with the PGA, a reference air bubble, containing ambient atmosphere, was created by soldering and sealing shut the ends of a Cu tube having the same dimensions as the holes in the steel strip. This was also tested in the PGA, at room temperature, to provide a calibration sample that should contain the normal composition of the atmosphere, and also to assess the accuracy and reproducibility of the procedure used to extract the gases from the bubble, and of the measurements provided by the mass spectrometer.
The following procedure was adopted to derive compositional information from the results obtained using the PGA. The results are presented as plots of the pressure variations of each gas in the bubble (in Torr) against time (in ms), shown, for example, in Figure 3. After subtraction of the background readings of the instrument, the relative amounts of the different gases in the bubble atmosphere were obtained by determining the total area under the curve for each gas. These relative amounts were then subjected to a library calibration factor to take into account the different sensitivities of the mass spectrometer with respect to the different gases. The initial dimensions of the bubble in the melt were assumed to be the same as the hole in the steel holder (6-mm diameter and 5-mm height), giving an assumed initial bubble volume of 141.4 mm3. Therefore, the initial volumes of oxygen and nitrogen in the air bubble were 29.7 and 110.2 mm3, respectively (with the balance being Ar), and the initial masses of oxygen and nitrogen inside the bubble were determined to be 4.1 × 10−7 and 1.52 × 10−6 mol, respectively. The corresponding values of the integrated areas under the pressure–time curve of the oxygen and nitrogen inside the room-temperature reference sample were 1.86 and 8.12 (Torr ms), respectively. The pressure–time curves from the PGA for the bubbles trapped in the different alloys for varying time periods were first integrated to obtain the relative amounts of the different gases inside the bubbles, and then the volume of each gas inside the bubble was estimated by comparing with the approximately known compositions of air obtained from the reference samples. The hydrogen content was estimated in the same way. Finally, the mass (in moles) was determined for each gas inside the bubbles of different ages and from the experiments with the different Al alloys.
Three different aluminum alloys of different compositions, expected to have different surface oxide films, were tested in this experiment. The oxides were expected to be alumina (Al2O3), in the case of commercial purity Al alloy (CP-Al); MgAl2O4 spinel, in the case of Al-7Si-0.3Mg (2L99) alloy; and magnesia, MgO, in the case of the Al-5Mg alloy.
Results
Pore Gas Analysis of the Bubbles
Figure 3 shows the results obtained by the PGA for the analysis of air. The results were plotted as the pressure of each gas (in Torr) against time (in ms). The largest peak was for nitrogen, a smaller peak was found for oxygen, and the smallest peak for argon, as would be expected for the normal composition of air. The integration of the total area under the curve (in torr ms) was determined for each gas to obtain a measure of the relative amounts of the different gases in the atmosphere.
The analysis of the reference (air) samples produced values of about 76.5 vol pct nitrogen, 20.6 vol pct oxygen, 0.8 vol pct argon, 1.5 vol pct hydrogen, and 0.6 vol pct water vapor, which were close to the nominal values of the gases in air. The amounts of hydrogen and water vapor detected were perhaps due to the slight contamination of the environment inside the PGA.
The overall changes in the bubble volume with time for the different alloys are shown in Figure 4, which shows that the reaction rates for 2L99 were much slower than those for CP-Al and Al-5Mg. Figures 5(a) to (c) show the variations in the compositions of the air bubbles when held for periods of up to 40 minutes, in the commercially pure Al (CP-Al), 2L99, and Al-5Mg alloy melts, respectively. The corresponding changes in the chemical composition of each bubble with time (expressed as a percentage) are shown in Figures 6(a) to (c), respectively. It was shown that the air bubbles lost most of their oxygen content within the first 8 minutes of holding, suggesting a rate of reaction of about 9 × 10−8 mol min−1 in each case. The similarity of the rates of reaction suggests a common mechanism. However, the rate of consumption of nitrogen inside the bubble and that of hydrogen diffusion into it were dependent on the alloy composition. CP-Al lost about 75 pct of its nitrogen content over a 40-minute holding period, while for the 2L99 alloy, the nitrogen content was reduced by about 25 pct over the same period. In Al-5Mg alloy, about 90 pct of the nitrogen content was lost. Over a 40-minute holding period, the estimated rates of reaction with nitrogen were 3.4 × 10−8, 1.1 × 10−8, and 5.7 × 10−8 mol min−1, respectively. Over the same time period, the estimated rates of H diffusion into the bubbles were 1.4 × 10−8, 1.5 × 10−9, and 2.8 × 10−8 mol min−1, respectively, i.e., the rate of hydrogen diffusion into the air bubble for the 2L99 alloy experiments was about an order of magnitude slower than those for the CP-Al and Al-5Mg alloys.
For all the alloys, shown in Figure 6, after about 8 minutes of holding, the composition of the bubble was mainly nitrogen and hydrogen, in varying amounts depending on the alloy type and the holding time of the bubble inside the melt. At the end of the 40-minute holding period the H–N ratio inside the air bubble was about 60 to 40, 30 to 70, and 80 to 20 pct for the CP-Al, 2L99, and Al-5Mg alloy melts, respectively.
Also, for all the alloys, the rate of nitrogen consumption and the rate of hydrogen diffusion into the bubbles were found to increase after consumption of most of the oxygen in the bubbles. For example, in the period from 0 to 4 minutes, (when oxygen was present in the bubbles), the rates of N consumption were 1x10−8, 5x10−9 and 3 × 10−8 mol min−1, for CP-Al, 2L99, and Al-5Mg alloys, respectively, and the rates of H diffusion into the bubbles were 1.4 × 10−8, 4.6 × 10−9, and 2.8 × 10−8 mol min−1, respectively. In comparison, during the period from 8 to 24 minutes (when the oxygen content of the bubble was negligible), N reaction rates were found to be 5 × 10−8, 1.4 × 10−8, and 9.1 × 10−8 mol min−1, respectively. These rates were about 3 to 5 times greater than those when oxygen was present. Similarly, H diffusion rates of about 2.6 × 10−8, 1.4 × 10−8, and 4.3 × 10−8 mol min−1, respectively, were found (about 2 to 3 times greater).
Finally, no changes in the amounts of either argon and water vapor were detected in the analysis of bubbles of different ages held in the different Al alloy melts. Constant values of about 0.02 and 0.01 mol, respectively, were obtained.
Microscopy of the Oxidized Surfaces
Figure 7(a) shows an SEM image of the surface of a sample from an experiment using CP-Al alloy, after 40 minutes holding time in the melt before solidification. This appears to show a tear in the film on the sample surface, revealing a layered structure. EDX spectra at locations X1, X2, and X3, presented in Figures 7(b) to (d), suggested the presence of AlN, Al2O3, and pure Al, respectively. The detection of an oxygen peak at location X1 (Figure 7(b)) suggests the presence of an Al2O3 layer underneath, upon which the AlN may have nucleated and grown. A higher magnification view of the AlN layer is shown in Figure 7(e).
The detection of pure Al on the sample at location X3 appears to show tearings of the Al2O3 and/or AlN layer at the interface between the bubble atmosphere and the melt, which would allow fresh melt to penetrate through the tear and be exposed to the bubble atmosphere. If this occurred at the end of the holding process, then there might be insufficient oxygen to form a detectable alumina layer. However, there would have been nitrogen (and hydrogen) present in the remaining atmosphere (see Figure 5(a)), and the absence of AlN at point X3, therefore, suggests that an incubation time may be required for the reaction of Al with N2 to form AlN.
Figures 8 and 9(a) show SEM images from the surfaces of samples taken from inside bubbles from experiments with 2L99 alloy (held for 32 minutes) and Al-5Mg alloy (held for 4 minutes), respectively, while higher magnification views of the AlN layers are shown in Figures 8 and 9(d). In both cases, EDX analyses at different locations on the surfaces suggested the presence of an oxide layer, which was expected to be MgAl2O4 spinel in the case of the 2L99 alloy, and MgO for the experiment with the Al-5Mg alloy. In both cases, a nitride was also formed (see Figures 8(b) and 9(b)), which may have been AlN, or Mg3N2, (which has been suggested to be a necessary precursor for the formation of AlN).[12] The oxygen peaks in the EDX spectra suggested that the nitride (whether AlN or Mg3N2), was again associated with an oxide substrate, either spinel or MgO.
It should be noted that the SEM images of the AlN layers formed in a previous experiment,[6] in which a static air bubble was held in an Al melt for durations of up to 8 hours, showed a fine and feather-like structure, while in the current study the AlN layers formed were coarser and with a more granular structure. These differences could be perhaps due to the different conditions under which the AlN was produced in the different experiments, e.g., different holding times, and stirring.
Discussion
The results of the compositional analysis of the gases within the bubbles held inside the different Al alloy melts for varying lengths of time, shown in Figure 5, showed that in these experiments, both oxygen and nitrogen were consumed with time (with oxygen being first consumed), while the hydrogen content of the bubbles was increased as the melt was kept longer inside the liquid metal, in agreement with previous results.[3,6] SEM micrographs of the surfaces of the samples obtained from the experiments with the different Al melts, shown in Figures 7, 8, and 9, showed that oxygen and nitrogen within the trapped air bubble were consumed by the surrounding melt producing different aluminum and/or magnesium oxides (depending on the alloy’s chemical composition), and aluminum nitride. The corresponding EDX spectra confirmed the presence of Al2O3, MgAl2O4, or MgO, on the surfaces of the samples from the experiments with pure Al, 2L99, and Al-5Mg alloy melts, respectively. The Gibbs free energies of formation (for the reaction of one mole of N2 at 1000 K (727 °C)) for AlN, Mg3N2, and Si3N4 were −423.6, −257.14, and −206.61 kJ mol−1 of N2, respectively,[13] indicating that AlN was the most favorable nitride to form for all the alloy compositions. However, Mg3N2 may also have been formed in the 2L99, and Al-5Mg alloys, as a precursor to the formation of AlN, as has been reported in another study.[12]
Sleppy[14] suggested that the presence of defects in the oxide layer such as cracks or pores can create leak paths for oxygen atoms to travel inward through the first formed oxide layer, allowing oxidation to continue. In these experiments, the rotational movement of the air bubble in the melt (intended to simulate the movement of a bifilm within flowing liquid metal during mold filling and after), would induce considerable stress in the oxide layer between the melt and the bubble, leading to its rupture. Such cracks would produce leak paths allowing the oxygen (and nitrogen) to react with the surrounding molten metal, at a greater rate than if diffusion through the oxide film was the controlling mechanism. Figure 7 showed examples of cracking in the interfacial layer that would allow the liquid alloy to come into contact with the bubble atmosphere.
In addition, the nitrogen content of the bubble started to decrease when the oxygen content declined to about 2 to 5 vol pct (depending upon the alloy). In other words, complete consumption of oxygen was not necessary for the commencement of nitrogen consumption, confirming the results of the study of Raiszadeh and Griffiths[6] who assumed that nitrogen within a static air bubble held inside an Al melt started to react with the surrounding liquid metal, when the concentration of oxygen reached about 5 vol pct.
To estimate the reaction rates of oxygen and nitrogen in the trapped air bubbles, a mean area over which the reaction occurred of 42.4 mm2 was assumed. The initial volume of the air bubble was considered to be 141.3 mm3, the same as that of the hole in the steel holder, and therefore, the initial amounts of oxygen and nitrogen within the bubble [at 973 K (700 °C)] were estimated be 4.1 × 10−7 and 1.5 × 10−6 mol, respectively.[13] Considering the reaction surface area between the bubble and the melt to be 4.24 × 10−5 m2, the consumption rates of oxygen, per unit area, were estimated to be around 2.5 × 10−6 mol m−2 s−1 for all the three alloys. In the case of nitrogen, varying reaction rates were estimated of about 1.32 × 10−6, 4.41 × 10−7, and 2.23 × 10−6 mol m−2 s−1 for the three alloys, CP-Al, 2L99, and Al-5Mg, respectively. The average rates of H diffusion into the bubble were determined to be about 5.2 × 10−7, 3.5 × 10−7, and 7.1 × 10−7 mol m−2 s−1, for the CP-Al, 2L99, and Al-5Mg alloy melts, respectively (The initial hydrogen content of the melt was not known). These values are also summarized in Table I.
An experimental study of the dimensions of bifilms using 3-D micro X-ray tomography suggested an initial bifilm size to be approximately of a rectangular shape with dimensions of 2.2 × 2.2 × 0.1 mm.[15] This would suggest a typical double-oxide film defect with a volume of 0.48 mm3, a surface area of 10.6 mm2, and hence a modulus of 0.045 mm. Using the consumption rates for oxygen and nitrogen estimated from these experiments, the times taken for a bifilm defect of the dimensions mentioned above to lose its entire oxygen and nitrogen by reaction with the melt, were estimated to be 538, 1509, and 345 seconds, for bifilms of alumina, spinel, and MgO, respectively (see Figure 10). However, the passage of H into the bifilm interior must also be taken into account. At these times, and assuming that H diffused into the bifilm at the rates mentioned above and that these were constant, the volumes of the bifilms (now almost completely hydrogen) would be reduced to about 40, 55, and 30 pct, respectively, of their initial volume (as shown in Figure 11). It should be noted that the rate of H diffusion would be strongly dependent on the difference in the concentration of H on either side of the oxide film, which could also affect the final size of the bifilm. H would stop diffusing into a bifilm once the H content of the bifilm atmosphere reached equilibrium with the surrounding melt.
The times taken for the oxygen to be consumed were estimated to be 61, 64, and 57 seconds, respectively, and this suggests that the reaction with N is the greatest factor in the duration of the interior atmosphere of a double-oxide film defect.
In these experiments, the main factor that was found to affect the reaction rates of the interior atmosphere, and the time required for the full consumption of the atmosphere inside a bifilm, was the alloy composition, specifically its Mg content, as this controlled the characteristics of the oxide layer forming the bifilm. The fastest rates of consumption were associated with MgO, perhaps due to its permeability. Alumina was the second fastest, perhaps because of the thinness of an alumina film, which could make it more sensitive to rupture in the experiment, and also because of movement of the bifilm within the melt. Finally, the spinel oxide film in the 2L99 alloy may, perhaps due to its thickness and impermeability, have led it to have the slowest estimated consumption rates, and its atmosphere was estimated to take the longest time to be consumed (about 25 minutes). These consumption rates were about 3 and 4 times slower than the estimates associated with bifilms formed of alumina and MgO, respectively. Of course the lifetime of the bifilm defect would be extended by allowing hydrogen to be diffused into the defect.
The reaction rates between the oxygen and nitrogen inside a static air bubble and the surrounding melt [of CP-Al at 973 K (700 °C)] were reported by Raiszadeh and Griffiths[7] to be 1.1 × 10−6 and 1.88 × 10−6 mol m−2 s−1, respectively, in order of magnitude agreement with the results reported here. However, in their study, the assumed dimensions of a bifilm were 10 × 5 × 0.04 mm (with a modulus of 0.02 mm), which resulted in an estimated lifetime of a bifilm (in CP-Al) of 144 seconds. In the current study, the consumption rates of oxygen and nitrogen (for CP-Al alloy) were determined to be 2.5 × 10−6 and 1.3 × 10−6 mol m−2 s−1, which were about double the rate of oxygen consumption, and about 30 pct lower than the rate in the case of the nitrogen consumption. The main difference in the consumption time was, therefore, due to the different dimensions of the bifilms assumed in each case. Raiszadeh and Griffiths used assumed dimensions for a bifilm as given by Campbell,[1] while in this study experimentally determined dimensions of real bifilms were used.[15]
Conclusions
-
1.
The atmosphere within a trapped air bubble in an Al melt was consumed by reaction with the surrounding liquid metal. First, oxygen was consumed to produce Al2O3, MgAl2O4, or MgO (for pure Al, 2L99, or Al-5Mg alloy melts, respectively). Then, nitrogen reacted to form AlN, or possibly Mg3N2. Also, hydrogen diffused into the bubble as the bubble was kept longer inside a melt.
-
2.
For pure Al, 2L99, and Al-5Mg alloy melts, the rates of oxygen consumption were estimated to be 2.5 × 10−6, 2.4 × 10−6, and 2.7 × 10−6 mol m−2 s−1, respectively, while the rates of nitrogen consumption were estimated to be 1.3 × 10−6, 4.4 × 10−7, and 2.2 × 10−6 mol m−2 s−1, respectively.
-
3.
The rates of H diffusion into the bubbles were estimated to be 3.4 × 10−7, 2.3 × 10−7, and 4.6 × 10−7 mol m−2 s−1, for pure Al, 2L99, and Al-5Mg alloy melts, respectively.
-
4.
It is suggested that the factor that would affect the rates of reaction of oxygen and nitrogen within the internal atmosphere of a bifilm defect, and the rate of hydrogen diffusion into it, was the type (and hence the characteristics) of the oxide layer forming the bifilm. Magnesia showed the fastest rates of consumption, while alumina showed the second fastest, and spinel (MgAl2O4) had the slowest rates.
-
5.
The times required for a double-oxide film defect, with estimated dimensions of 2.2 × 2.2 × 0.1 mm, to lose its entire oxygen and nitrogen by reaction with the surrounding melt, were estimated to be about 9, 25, and 6 min for CP-Al, 2L99, and Al-5Mg alloy melts, respectively.
-
6.
The obstacles to complete reaction of the oxygen and nitrogen in the interior atmosphere of a double-oxide film defect were found to be the rate of nitrogen consumption, and the diffusion of hydrogen into the defect interior.
References
J. Campbell: Castings. 2nd ed., Butterworth-Heinemann, Oxford, 2003.
J. Campbell: Mater. Sci. and Tech., 2006, vol. 22: pp. 127-145.
C. Nyahumwa, N.R. Green, and J. Campbell: AFS Trans., 1998. vol. 106: pp. 215-223.
M. Aryafar, R. Raiszadeh, and A. Shalbafzadeh: J. Mater. Sci., 2010. Vol. 45, no. 11, pp. 3041-3051.
F.N. Bakhtiarani and R. Raiszadeh: Metall. Mater. Trans. B, 2011, vol. 42, pp. 331-340.
R. Raiszadeh and W.D. Griffiths: Metall. Mater. Trans. B, 2006, vol. 37, pp. 865–871.
R. Raiszadeh and W.D. Griffiths: Metall. Mater. Trans. B, 2008, vol. 39, pp. 298-303.
S. Impey, D. Stephenson, and J.R. Nicholls: Int. Conf. Microsc. Oxid., University of Cambridge, Institute of Metals, 1990, pp. 238–44.
R. Raiszadeh and W.D. Griffiths: Metall. Mater. Trans. B, 2011, vol. 42, pp. 133-143.
M. El-Sayed, H. Salem, A. Kandeil, and W.D. Griffiths: 22nd Int. Conf. Comput. Aided Prod. Eng., Edinburgh, Scotland, 2011.
M. El-Sayed, H. Salem, A. Kandeil, and W.D. Griffiths: Metall. Mater. Trans. B, 2011, vol. 42B, pp. 1104–09.
H.Z. Ye, X.Y. Liu, and B. Luan: J. Mater. Process. Technol., 2005, vol. 166, pp. 79–85.
M.W. Chase: J. Phys. Chem. Ref. Data, 1985, vol. 14, p. 751.
W.C. Sleppy: J. Electrochem. Soc, 1961, vol. 108, no. 12, pp. 1097-1102.
J.M. Park: MRes Thesis, University of Birmingham, U.K., 2010.
Acknowledgments
The authors would like to thank Mr. Adrian Caden of the University of Birmingham; Eng. Ramy Wasfi of the American University in Cairo; and Mr. Salah Edrees of the Arab Academy for Science and Technology, Alexandria for their technical support during the laboratory work. Also, the authors wish to acknowledge the sponsorship of this study by the Arab Academy for Science and Technology, Alexandria, Egypt, and the American University in Cairo, Egypt. Finally, the authors would like to gratefully acknowledge the partial support of the EPSRC Centre for Innovative Manufacturing in Liquid Metal Engineering (grant no. EP/H026177/1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted October 6, 2013.
Rights and permissions
About this article
Cite this article
El-Sayed, M.A., Salem, H.A.G., Kandeil, A.Y. et al. Determination of the Lifetime of a Double-Oxide Film in Al Castings. Metall Mater Trans B 45, 1398–1406 (2014). https://doi.org/10.1007/s11663-014-0035-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0035-x