Abstract
To develop a simple and effective process for upgrading low-grade titanium ore (ilmenite, mainly FeTiO3), a new selective chlorination process based on the use of calcium chloride (CaCl2) as the chlorine source was investigated in this study. Titanium ore and a titanium ore/CaCl2 mixture were placed in two separate crucibles inside a gas-tight quartz tube that was then positioned in a horizontal furnace. In the experiments, the titanium ore in the two crucibles reacted with either HCl produced from CaCl2 or CaCl2 itself at 1100 K (827 °C), leading to the selective removal of the iron present in the titanium ore as iron chlorides [FeCl x (l,g) (x = 2, 3)]. Various kinds of titanium ores produced in different countries were used as feedstock, and the influence of the particle size and atmosphere on the selective chlorination was investigated. Under certain conditions, titanium dioxide (TiO2) with purity of about 97 pct was directly obtained in a single step from titanium ore containing 51 pct TiO2. Thus, selective chlorination is a feasible method for producing high purity titanium dioxide from low-grade titanium ore.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Titanium (Ti) is well known for its superior physical and chemical properties such as its light weight, high strength, and high corrosion resistance. These properties make it very useful in diverse fields such as the aerospace, chemical, and power generation industries. Therefore, the demand for titanium and its alloys continues to increase. In 2009, the worldwide production of titanium was 2.8 times higher than that in 1999.[1,2] Furthermore, titanium is the ninth most abundant element in the Earth’s crust and the concentration of titanium in the crust is about 1/20 that of aluminum (Al) and 1/10 that of iron (Fe).[3]
However, titanium is still not used nearly as much as aluminum or iron. The principal reason for this is that the production costs for titanium are high at all processing stages and the productivity of this processing is also quite low.[4,5] The cost of processing titanium ore is 15 times higher than that for processing iron ore and the cost for processing titanium metal is 20 times higher than that for processing iron metal.[6,7] Therefore, a reduction in these production costs from the ore to the metal is essential for increasing the use of titanium in various applications. In particular, an efficient process for upgrading titanium ore is required.
There are several kinds of titanium ores such as ilmenite (FeTiO3, 30 pct to 65 pct TiO2) and natural rutile (TiO2, 95 pct to 100 pct TiO2). Among the various kinds of titanium ores, ilmenite is the most significant feed mineral for titanium ore upgrading processes because about 94 pct of the world’s mine reserves of titanium ore are dominated by ilmenite.[8] In addition, the price of natural rutile (TiO2) is very high because of its scarcity. Therefore, all commercial titanium ore upgrading processes use ilmenite as the feedstock.[3,9–11]
Meanwhile, the purity of the product after the titanium ore upgrading process has to be above 90 pct TiO2 for it to be used as feedstock for the Kroll process,[12] the current titanium metal production process.[13,14] In particular, in some countries including Japan, a purity of over 95 pct TiO2 is required to reduce the amount of chloride wastes produced during the Kroll process and reduce the chlorine loss during processing; otherwise, the chloride wastes’ treatment cost and environmental burden increase significantly.
Representative processes for upgrading titanium ore include the Becher process,[15,16] the Benilite process,[17–19] and slag production.[20] However, there are several disadvantages to these processes, as shown in Table I. In the case of the Becher and slag processes, multiple steps are required to yield high purity TiO2. In the Benilite process, highly concentrated 18 pct to 20 pct HCl is required. In addition, the kinds of titanium ores that can be used as feedstock are limited.[11]
Many researchers have investigated many different processes with the goal of developing an efficient titanium ore upgrading process.[21–30] One representative group of processes is the chlorination processes. Chlorination processes can be classified into either direct chlorination or selective chlorination. A representative direct chlorination process is the chloride process, where both iron oxides and titanium oxides are chlorinated, the chlorinated metal chlorides are separated, and then TiCl4 gas is oxidized to obtain high purity TiO2.[36] On the other hand, in a selective chlorination process, only the iron oxides in the titanium ore are selectively chlorinated as iron chlorides [FeCl x (x = 2, 3)]. This selective chlorination process utilizes the difference of the physicochemical properties of the oxide/chloride stabilities that the titanium oxides are relatively more thermodynamically stable than the iron oxides under certain conditions.
Much research has been carried out on selective chlorination so far. However, most of the investigated processes were carried out under chlorine gas (Cl2) in the presence of carbon[24,29,30,33] or under a mixture of CO and Cl2.[22,23,34] This could be a disadvantage because the use of Cl2 gas has safety and environmental problems. Recently, Okabe and coworkers[25,26] developed a new process utilizing metal chlorides such as CaCl2 as the chlorine source for selective removal of iron from titanium ore under Ar or N2 atmospheres. However, even in the best result of their study, which was conducted under a mixed atmosphere of N2 and H2O at 1293 K (1020 °C) for 12 hours using CaCl2, 16.7 mass pct iron remained.
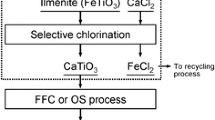
To improve these results, this study aims to develop a simple and effective process for upgrading low-grade titanium ore. In particular, a novel selective chlorination process that uses CaCl2 as the chlorinating agent is applied to selectively remove iron from the titanium ore. A flow diagram of this process is shown in Figure 1. The overall chlorination process investigated in this study consists of two selective chlorination processes. One process uses CaCl2 as a chlorinating agent to remove iron from the titanium ore, as suggested by Okabe and coworkers,[25,26] and the CaTiO3 produced by this process can be used as a feedstock for titanium metal production.[35] The other process suggested in this study uses the HCl gas produced from CaCl2 to remove iron from the titanium ore. This second process leads to better recovery of the chlorine source. The selective chlorination process investigated in this study has the following advantages: (1) It does not require the handling of highly concentrated HCl or Cl2 gas for the chlorination, (2) high purity titanium dioxide can be obtained in a single step through a simple and scalable process, and (3) various ilmenites can be used as the starting materials.
Experimental
Figure 2 shows a schematic and a photograph of the experimental apparatus. In this study, natural ilmenite, natural rutile, and titania slag were used as starting materials. In addition, CaCl2 (anhydrous; purity >95.0 pct; powder; Kanto Chemicals, Inc.) was used as the starting material for the chlorinating agent. The compositions of the titanium ores used in this study are shown in Table II.
Titanium ore was placed in the quartz crucible (ϕ = 31 mm, I.D.; d = 13 mm, depth) and a mixture of titanium ore and CaCl2 was placed in the molybdenum-lined quartz crucible (quartz crucible: ϕ = 26 mm, I.D.; d = 24 mm, depth; molybdenum lining: t = 0.1 mm, thickness; w = 10 mm, width; l = 10 mm, length; d = 25 mm, depth). These crucibles were then positioned in the gas-tight quartz tube (ϕ = 41.5 mm, I.D.; l = 545 mm, length), which was subsequently positioned in the horizontal furnace.
The CaCl2 was dried under vacuum for more than 3 days at 473 K (200 °C) in a vacuum dryer (EYELA, VOS-201SD) prior to use. The CaCl2 was premelted two times in the molybdenum-lined quartz crucible before the titanium ore was added to it under vacuum at 1100 K (827 °C) in order to prevent the overflow of the CaCl2 from the crucible. In each premelting step, half of the total weight of CaCl2 was premelted for 5 minutes. After the premelting of CaCl2 was finished, titanium ore was placed in both the quartz crucible and the molybdenum-lined quartz crucible.
Table III shows the experimental conditions used in this study. When the experiments were carried out under vacuum, the quartz tube was sealed with a silicone rubber plug and evacuated two times for 5 minutes each before the experiments. The quartz tube was then introduced into the horizontal furnace, which was already heated to 1100 K (827 °C). This temperature was then maintained for 5, 7, or 13 hours.
When the experiments were carried out under Ar gas (purity >99.9995 pct) atmosphere, the sealing and evacuation procedures were identical to those used when the experiments were conducted under vacuum. The Ar gas atmosphere was added using two methods. In the first method, the quartz tube was filled with Ar gas until the internal pressure was 0.31 atm or 0.41 atm. These values were chosen by considering the increase in the internal pressure caused by heat expansion when the quartz tube was introduced into the furnace. The internal pressure was maintained at 1 atm during the experiments carried out for 5, 7, and 11 hours at 1100 K (827 °C). In the second method, Ar gas flowed through the quartz tube at a rate of 50 sccm controlled using a MFC (mass flow controller). In this case, the internal pressure was also maintained at 1 atm during the experiment carried out for 5 hours at 1100 K (827 °C).
After the completion of the reactions, the quartz tube was immediately removed from the furnace and allowed to cool gradually to room temperature. Leaching was not conducted for the residues in the quartz crucible, while the residues in the molybdenum-lined quartz crucible were dissolved in deionized water for at least 12 hours and sonication was performed for at least 1 hour at room temperature. The chemical compositions of the residues obtained in the quartz crucible and in the molybdenum-lined quartz crucible were determined using X-ray fluorescence spectroscopy (XRF: JEOL, JSX-3100RII), their microstructures and compositions were analyzed using scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM/EDS: JEOL, JSM-6510LV), and their crystalline phases were identified using X-ray diffraction (XRD: RIGAKU, RINT 2500, RINT 2000, Cu-Kα radiation) analysis.
Mechanism of Selective Chlorination
From a thermodynamic viewpoint, titanium ore can be considered a mixture of FeO and TiO2 because the standard Gibbs energy of the reaction shown in Eq. [1][31] is small at 1100 K (827 °C). This makes it possible to carry out thermodynamic calculations for titanium ore by considering FeO and TiO2 separately and not FeTiO3. Therefore, the conditions for selective removal of iron from the titanium ore can be considered in terms of the chemical potential diagrams of the Fe-O-Cl system and the Ti-O-Cl system.
Figures 3 and 4 show the chemical potential diagrams of the Fe-O-Cl system and the Ti-O-Cl system, respectively, at 1100 K (827 °C), plotted with \( p_{{{\text{Cl}}_{ 2} }} \) as the abscissa and \( p_{{{\text{O}}_{ 2} }} \) as the ordinate. First, the case of both a high oxygen and a high chorine chemical potential should be considered. As shown in Figure 3, when the oxygen chemical potential is high (for example, at \( p_{{{\text{O}}_{ 2} }} \) = 0.1 atm), Fe2O3(s) is thermodynamically stable even when the chlorine chemical potential is very high (for example, at \( p_{{{\text{Cl}}_{ 2} }} \) = 0.1 atm). Therefore, it is impossible to chlorinate Fe2O3(s) with chlorine gas even at 1 atm under high \( p_{{{\text{O}}_{ 2} }} \). In addition, TiO2(s) cannot be chlorinated under these conditions either, as shown in Figure 4. Therefore, the selective chlorination process cannot progress even at very high \( p_{{{\text{Cl}}_{ 2} }} \) when \( p_{{{\text{O}}_{ 2} }} \) is high.
Chemical potential diagram of the Fe-O-Cl system at 1100 K (827 °C)[31]
Chemical potential diagram of the Ti-O-Cl system at 1100 K (827 °C)[31]
However, iron oxides will be chlorinated in the presence of carbon in this system under a high chlorine chemical potential (for example, at \( p_{{{\text{Cl}}_{ 2} }} \) = 0.1 atm) because the oxygen chemical potential is fixed by the C(s)/CO(g) equilibrium or CO(g)/CO2(g) equilibrium (both at \( p_{{{\text{Cl}}_{ 2} }} \) = 0.1 atm), as shown in Figure 3. In addition, titanium oxides can also be chlorinated under these low \( p_{{{\text{O}}_{ 2} }} \) conditions, as shown in Figure 4. For this reason, these conditions are employed in industrial processes (e.g., the Kroll process) to achieve direct chlorination and produce TiCl4 gas from upgraded titanium ore.
As shown in Figure 3, the ratio between the oxygen chemical potential and the chlorine chemical potential (\( p_{{{\text{O}}_{ 2} }} \)/\( p_{{{\text{Cl}}_{ 2} }}^{2} \)) of the Fe-O-Cl system can be determined by considering the relevant equilibrium reactions, such as the H2O(g)/HCl(g) eq. and CaO(s)/CaCl2(l) eq. in this system. The lines corresponding to the H2O(g)/HCl(g) eq.e and the CaO(s, CaTiO3)/CaCl2(l) eq.a in Figure 3 pass through the vicinity of the FeCl2(l) stability region. Therefore, FeO(s) can be chlorinated as FeCl2(l) by HCl(g), or by CaCl2(l) if the lines corresponding to the H2O(g)/HCl(g) eq.e and the CaO(s, CaTiO3)/CaCl2(l) eq.a pass through (or near) the FeCl2(l) stability region. Even when the H2O(g)/HCl(g) eq.e line does not pass through the FeCl2(l) stability region, the chlorination reaction could be achieved by a slight alteration of thermodynamic parameters or experimental conditions. In addition, the lines corresponding to the H2O(g)/HCl(g) eq.e and the CaO(s, CaTiO3)/CaCl2(l) eq.a do not pass through any region of TiCl2(s), TiCl3(s), or TiCl4(g) stability region, as shown in Figure 4. The line corresponding to the H2O(g)/HCl(g) eq.e is located a few magnitudes of \( p_{{{\text{O}}_{ 2} }} \) above the [TiCl x (x = 2, 3, 4)] stability region. Thus, this thermodynamic analysis indicates that the selective chlorination of FeO(s) from titanium ore can take place under conditions in which equilibrium reactions of the H2O(g)/HCl(g) eq.e and the CaO(s, CaTiO3)/CaCl2(l) eq.a are established.
The line corresponding to the H2O(g)/HCl(g) eq.e in Figure 3 can be calculated from Eq. [2] because it is expected that in the actual experiments, the dried CaCl2 will absorb H2O from the air while being prepared. The Gibbs energy of the reaction shown in Eq. [2][31] can be calculated using Eq. [3].
When H2O is present in the system during the premelting of the CaCl2, it reacts with the CaCl2(l) to produce CaO(s) and HCl(g) in the molybdenum-lined quartz crucible. Because of the low partial vapor pressure of HCl(g) in the system, this reaction proceeds even though the standard Gibbs energy of the reaction in Eq. [2] is positive. Then, when the titanium ore is added to the molybdenum-lined quartz crucible, the CaO(s) in the premelted CaCl2 reacts with the TiO2(s) in the titanium ore. As a result, the activity of CaO (a CaO) decreases as the reaction shown in Eq. [4] proceeds. Equation [3] shows that if the activity of CaO decreases or/and the partial vapor pressure of HCl(g) decreases, the reaction shown in Eq. [2] can progress. Therefore, the reaction shown in Eq. [2] will take place during the experiments, and the line corresponding to the H2O(g)/HCl(g) eq.e in Figure 3 can be determined according to Eq. [2]. In addition, if the activity of CaO is much lower than the activity calculated from Eq. [4][31], the line corresponding to the H2O(g)/HCl(g) eq.e in Figure 3 will pass through a FeCl2(l) stability region. The line corresponding to the CaO(s, CaTiO3)/CaCl2(l) eq.a in Figure 3 can be also determined from the activity of CaO, which is lowered by the production of CaTiO3(s).
Equation [5] shows the reaction between the titanium ore and CaCl2. According to the Gibbs phase rule, the system in this study is bi-variant at constant temperature and total pressure under the FeO(s)/FeCl2(l) eq. or H2O(g)/HCl(g) eq. or CaO(s)/CaCl2(l) eq. When the temperature and pressure were fixed under TiO2(s)/CaCl2(l)/CaTiO3(s) eq., FeCl2(l) could exist in this system. However, the vapor pressure of FeCl2(l) at 1100 K (827 °C) is 0.09 atm,[31] which is high enough that the FeCl2(l) will evaporate. As a result, FeCl2(l) is removed as FeCl2(l,g) at 1100 K (827 °C). Under the experimental conditions employed in this study, the activity of FeCl2(l) will decrease because the majority of the vaporized FeCl2(l) will solidify at the low temperature part of the quartz tube. As a result, the reaction shown in Eq. [5][31] progresses and the FeO(s) in the titanium ore is removed as FeCl2(l,g) in the molybdenum-lined quartz crucible, even though the standard Gibbs energy of the reaction for the formation of FeCl2(l) shown in Eq. [5] has a small positive value. Therefore, thermodynamic analysis suggests that the line corresponding to the CaO(s, CaTiO3)/CaCl2(l) eq.a in Figure 3 will pass through a FeCl2(l) stability region under these experimental conditions.
The HCl(g) produced by the reaction shown in Eq. [2] reacts with the FeO(s) present in the titanium ore placed in the quartz crucible, and this FeO(s) is also removed as FeCl2(l,g). The standard Gibbs energy of the reaction shown in Eq. [6][31,32] is close to zero and could be either a small positive or a small negative depending on the reference consulted. As a result, depending on the reference, the line corresponding to the H2O(g)/HCl(g) eq.e in Figure 3 may pass through the FeCl2(l) stability region. Therefore, it is expected that under certain conditions, the FeO(s) will be removed from the titanium ore and TiO2(s) will be produced in the quartz crucible.
Results and Discussion
Influence of the Particle Size of the Titanium Ore on Selective Chlorination
Figure 5 shows photographs of the low temperature part of the quartz tube, the residues in the quartz crucible, and the residues in the molybdenum-lined quartz crucible after the experiment. During the experiment, as shown in Figure 5(a), a white powder was deposited at the low temperature part of the quartz tube. In addition, the black color of the titanium ore was changed to the bright gray color of the residues after the experiment, as shown in Figure 5(b).
Figure 6 shows the XRD patterns of the white residues that condensed inside the low temperature part of the quartz tube. XRD analysis showed that the main crystalline phases of the white residues were FeCl2·(H2O)2 and FeCl2. It is expected that H2O adhered to the FeCl2 during the experiment or during the sample preparation for the XRD measurements. In addition, FeCl3·6H2O was also found; the detailed reason for this production of FeCl3·6H2O is still being investigated. Figure 7 shows SEM images of the microstructure of a titanium ore particle before the experiment and of the residues obtained in the quartz crucible after the experiment. As shown in Figure 7, microstructural pores were formed on the surfaces of the residues even though the leaching was not conducted after the experiment. These experimental results imply that the iron present in the titanium ore was removed mainly in the form of FeCl2(l,g), as expected from the thermodynamic analysis.
Table IV lists the analytical results for the residues obtained in the quartz crucible and in the molybdenum-lined quartz crucible after the experiments. Figures 8(a) and (b) show the XRD patterns of the residues obtained in the quartz crucible and in the molybdenum-lined quartz crucible, respectively, when the experiments were conducted under vacuum on Vietnamese titanium ore with various particle size ranges. The XRF and XRD results show that a purity of about 96 pct to 97 pct TiO2(s) was obtained in the quartz crucible when the particle size range was from 44 μm to 297 μm and a purity of about 94 pct TiO2(s) was obtained in the quartz crucible when the particle size range was from 297 μm to 510 μm.
(a) XRD patterns of the residues obtained in the quartz crucible when several ore particle size ranges were used: (1) 44 to 74 μm, (2) 74 to 149 μm, (3) 149 to 210 μm, (4) 210 to 297 μm, and (5) 297 to 510 μm. (b) XRD patterns of the residues obtained in the molybdenum-lined quartz crucible when several ore particle size ranges were used: (1) 44 to 74 μm, (2) 74 to 149 μm, (3) 149 to 210 μm, (4) 210 to 297 μm, and (5) 297 to 510 μm
However, 18.1 pct to 32.6 pct of the iron in the titanium ore remained in the residues in the molybdenum-lined quartz crucible, even though 17.1 pct to 31.6 pct of the iron present in the titanium ore was removed in the form of [FeCl x (l,g) (x = 2, 3)]. In addition, XRD analysis indicated that CaTiO3(s) was the main crystalline phase in the molybdenum-lined quartz crucible, as shown in Figure 8(b).
Figure 9 shows an SEM image and the EDS results found for a cross section of a titanium ore particle obtained from the molybdenum-lined quartz crucible. As shown in Figure 9, CaTiO3(s) was produced at the outer part of the titanium ore particle from the reaction between CaCl2 and the titanium ore particle. However, the iron present at the inner part of the titanium ore particle could not physically react with CaCl2 because of this formation of CaTiO3(s) at the outer part of the titanium ore particle. Therefore, CaTiO3(s) hindered the progress of the chlorination of the iron in the titanium ore, although the formation of CaTiO3(s) is preferable for the production HCl(g) from CaCl2(l) by lowering the activity of CaO(s).
It has been demonstrated that there is no influence of the particle size of the titanium ore on the ability of the selective chlorination process to produce high purity TiO2 when the titanium ore is upgraded by the HCl gas produced from CaCl2. Therefore, it is anticipated that the costs for sieving and crushing to select a particular particle size can be reduced.
Various Kinds of Titanium Ores Produced in Different Countries
Table V lists the analytical results for the residues obtained in the quartz crucible and in the molybdenum-lined quartz crucible after the experiments. Figures 10(a) and (b) show the XRD patterns of the residues obtained in the quartz crucible and in the molybdenum-lined quartz crucible, respectively, when the experiments were conducted using natural ilmenite produced in China and Australia and natural rutile produced in South Africa.
(a) XRD patterns of the residues obtained in the quartz crucible when various kinds of Ti ore were used as feedstock: (1) natural Chinese ilmenite, (2) natural Australian ilmenite, and (3) natural rutile produced in South Africa. (b) XRD patterns of the residues obtained in the molybdenum-lined quartz crucible when various kinds of Ti ore were used as feedstock: (1) natural Chinese ilmenite, (2) natural Australian ilmenite, and (3) natural rutile produced in South Africa
As shown in Table V and Figures 10(a) and (b), a purity of about 91 pct TiO2(s) was obtained in the quartz crucible, and CaTiO3(s) was the main crystalline phase in the molybdenum-lined quartz crucible when the Chinese titanium ore was used as feedstock. In addition, a purity of about 95 pct TiO2(s) was obtained in the quartz crucible when the Australian titanium ore and South African rutile were used as feedstock, and CaTiO3(s) and TiO2(s), respectively, were the main crystalline phases in the molybdenum-lined quartz crucible. The purity of the TiO2(s) obtained in the quartz crucible when the Chinese titanium ore was used was slightly lower than that obtained when the Vietnamese ore was used. This is because compared to the titanium ore produced in Vietnam, the Chinese titanium ore contains higher amounts of minor impurities such as Al and Si that cannot be chlorinated by HCl gas.
The minor impurities contained in the titanium ores produced in Vietnam, Australia, and China include Al, Si, Mn, and so on. Among these impurities, Al, Si, and Mn are the most abundant. It is difficult to chlorinate Al and Si with HCl gas, whereas Mn can be chlorinated by HCl gas through the reactions shown in Eqs. [7] through [9].[31] Therefore, it is expected that the purity of the TiO2(s) obtained in the quartz crucible when the Chinese titanium ore is used will be slightly lower because about 4.6 pct of minor impurities that cannot be chlorinated were already present in the Chinese titanium ore.
As shown in Figure 11, the main crystalline phase of the Canadian slag feedstock was FeTi2O5(s). This is a different phase from those in the other Vietnamese, Australian, and Chinese titanium ores, which have FeTiO3(s) as the main crystalline phase. Thermodynamically, FeTi2O5(s) can disassociate into FeTiO3(s) and TiO2(s) at 1100 K (827 °C), as shown in Eq. [10][32]. Therefore, the concentration of titanium increased from 76.7 pct to 89.1 pct in the quartz crucible when the experiment was conducted for 13 hours.
However, the purity of the TiO2(s) obtained in the quartz crucible was just below 90 pct, which is the criterion for the purity of the feedstock that can be used for the Kroll process. The reason for this low purity is probably that the total concentration of minor impurities in the Canadian slag that cannot be chlorinated by HCl gas is about 5.9 pct, which is much higher than that of the Chinese titanium ore.
When the experiment was conducted for 13 hours, the main product produced in the quartz crucible was TiO2(s), and TiO2(s) and CaTiO3(s) were the main products in the molybdenum-lined quartz crucible. In addition, Fe2TiO5(s) was found in both the quartz crucible and the molybdenum-lined quartz crucible, as shown in Figure 11. However, a detailed explanation for this is still being investigated.
Influence of Atmosphere on Selective Chlorination
Table VI lists the analytical results for the residues obtained in the quartz crucible and in the molybdenum-lined quartz crucible after the experiments. Figures 12(a) and (b) show the XRD patterns of the residues obtained in the quartz crucible and in the molybdenum-lined quartz crucible, respectively, when the experiments were conducted under Ar gas atmosphere. As shown in Table VI and Figures 12(a) and (b), when the experiment was conducted under static Ar gas atmosphere for 11 hours, a purity of about 97 pct TiO2(s) was obtained in the quartz crucible and mainly CaTiO3(s) was obtained in the molybdenum-lined quartz crucible. However, compared to the reactions conducted under vacuum, the reactions performed in static Ar gas atmosphere required 6 more hours to completely remove the iron from the titanium ore. In addition, the iron present in the titanium ore was not selectively chlorinated in the quartz crucible when the experiment was conducted under Ar gas flow.
(a) XRD patterns of the residues obtained in the quartz crucible when the experiments were conducted under a static Ar gas atmosphere and under an Ar gas flow: (1) 11 h under a static Ar gas atmosphere, (2) 7 h under a static Ar gas atmosphere, (3) 5 h under a static Ar gas atmosphere, and (4) 5 h under an Ar gas flow. (b) XRD patterns of the residues obtained in the molybdenum-lined quartz crucible when the experiments were conducted under a static Ar gas atmosphere and under an Ar gas flow: (1) 11 h under a static Ar gas atmosphere, (2) 7 h under a static Ar gas atmosphere, (3) 5 h under a static Ar gas atmosphere, and (4) 5 h under an Ar gas flow
Equation [11] describes the reaction establishing the line corresponding to the H2O(g)/HCl(g) eq. shown in Figure 3. If Ar gas is introduced into the quartz tube, the total pressure of the quartz tube is increased compared to that when the experiments were conducted under vacuum because of the increase in the total number of molecules. As a result, the partial vapor pressure of H2O(g) and HCl(g) decreases.
However, it is expected that the line corresponding to the H2O(g)/HCl(g) eq. in Figure 3 will still be located inside the area where iron oxide can be chlorinated in the scale of Figure 3 when the experiments are conducted under static Ar gas atmosphere. In addition, it is expected that it took 11 hours to remove the iron from the titanium ore because the amount of mass flux for the reaction was decreased or because the probability of collision between the produced HCl gas and the surface of the titanium ore was decreased. When the Ar gas flowed through the quartz tube, the partial vapor pressures of H2O(g) and HCl(g) were greatly decreased from those obtained when the quartz tube was simply filled with Ar gas because the Ar gas was forcibly introduced. Therefore, because the mass flux of the produced HCl gas was below the minimum mass flux required for the reactions to progress, the iron present in the titanium ore was not chlorinated.
Conclusions
With the purpose of establishing a novel, simple, and effective process for upgrading low-grade titanium ore based on selective chlorination, fundamental studies of the process were conducted and its feasibility was demonstrated. The iron present in the titanium ore was completely removed as [FeCl x (l,g) (x = 2, 3)] via a reaction between the titanium ore and HCl(g) produced from CaCl2(l) at 1100 K (827 °C). As a result, a high purity of TiO2(s) was obtained. In addition, the iron present in the titanium ore was also partially removed as [FeCl x (l,g) (x = 2, 3)] via the reaction between the titanium ore and CaCl2(l) at 1100 K (827 °C), and CaTiO3(s) was mainly obtained. However, the formation of CaTiO3(s) hinders the selective chlorination reaction in the titanium ore.
It was demonstrated that when the Vietnamese titanium ore with particle sizes in the range of 44 μm to 510 μm was used as feedstock under vacuum at 1100 K (827 °C) for 5 hours, a purity of about 94 pct to 97 pct TiO2(s) was obtained. In addition, when the titanium ores produced in China, Australia, and South Africa were used, a purity of about 91 pct to 97 pct TiO2(s) was obtained when the experiments were conducted under vacuum at 1100 K (827 °C) for 5 hours. However, when the Canadian slag was used, a purity of about 89 pct TiO2(s) was obtained. Moreover, a purity of about 97 pct TiO2(s) was obtained when the experiment was conducted under Ar gas atmosphere at 1100 K (827 °C) for 11 hours.
References
J. Gambogi: Mineral Commodity Summaries: Titanium and Titanium Dioxide, U.S. Geological Survey, Washington, DC, January 2011, pp. 172–73, http://minerals.usgs.gov/minerals/pubs/commodity/titanium/mcs-2011-titan.pdf.
J. Gambogi: Mineral Commodity Summaries: Titanium and Titanium Dioxide, U.S. Geological Survey, Washington, DC, January 2001, pp. 176–77, http://minerals.usgs.gov/minerals/pubs/commodity/titanium/670301.pdf.
F. Habashi, ed.: Handbook of Extractive Metallurgy, vol. 2, VCH Verlagsgesellschaft mbH, Weinheim, Germany, 1997, pp. 1129–80.
A. Moriya and A. Kanai: Shigen-to-Sozai, 1993, vol. 109, pp. 1164-69.
T. Fukuyama, M. Koizumi, M. Hanaki, and S. Kosemura: Shigen-to-Sozai, 1993, vol. 109, pp. 1157-63.
K. Faller and F.H. Froes: JOM, 2001, vol. 53, no. 4, pp. 27-28.
F.H. Froes, H. Friedrich, J. Kiese and D. Bergoint: JOM, 2004, vol. 56, no. 2, pp. 40-44.
J. Gambogi: Mineral Commodity Summaries: Titanium Mineral Concentrates, U.S. Geological Survey, Washington, DC, January 2011, pp. 174–75, http://minerals.usgs.gov/minerals/pubs/commodity/titanium/mcs-2011-timin.pdf.
J.E. Kogel, N.C. Trivedi, J.M. Barker, and S.T. Krukowski: Industrial Minerals & Rocks Commodities, Markets, and Uses 7th ed., Society for Mining, Metallurgy, and Exploration, Inc. (SME), Littleton, Colorado, USA, 2006, pp. 987–1013.
D. Filippou and G. Hudon: JOM, 2009, vol. 61, no. 10, pp. 36-42.
W. Zhang, Z. Zhu, and C.Y. Cheng: Hydrometallurgy, 2011, vol. 108, pp. 177-88.
W. Kroll: Trans. Electrochem. Soc., 1940, vol. 78, pp. 35-47.
T. Iida: Kinzoku, 2012, vol. 82, no. 3, pp. 218-21.
Y. Ito: Titan, 2012, vol. 60, no.3, pp. 212-18.
R.G. Becher, R.G. Canning, B.A. Goodheart and S.Uusna 1965, Proc. Aust. Inst. Min. Metall., vol. 21, pp. 21-44.
W. Hoecker: European Patent EP0612854, 1994.
J.H. Chen and L.W. Huntoon: United States Patent 4019898, 1977.
J.H. Chen: United States Patent 3967954, 1976.
J.H. Chen: United States Patent 3825419, 1974.
M. Gueguin and F. Cardarelli: Miner. Process. Extr. Metall. Rev., 2007, vol. 28, pp. 1-58.
T.S. Mackey: JOM, 1994, vol. 46, no. 4, pp. 59-64.
K.I. Rhee and H.Y. Sohn: Metall. Trans. B, 1990, vol. 21B, pp. 331-40.
K.I. Rhee and H.Y. Sohn: Metall. Trans. B, 1990, vol. 21B, pp. 321-30.
K.I. Rhee and H.Y. Sohn: Metall. Trans. B, 1990, vol. 21B, pp. 341-47.
H. Zheng and T.H. Okabe: in Proceedings of the 16th Iketani Conference: Masuko Symposium, 2006, Japan, pp. 1005–10.
R. Matsuoka and T.H. Okabe: Proceedings of the Symposium on Metallurgical Technology for Waste Minimization (134th TMS Annual Meeting), 2005, San Francisco, United States. http://www.okabe.iis.u-tokyo.ac.jp/japanese/for_students/parts/pdf/050218_TMS_proceedings_matsuoka.pdf. Accessed 29 November 2012.
J.S.J. van Deventer: Thermochim. Acta, 1988, vol. 124, pp. 205–15.
A.S. Athavale and V.A. Altekar: Ind. Eng. Chem. Process Des. Dev., 1971, vol. 10, no. 4, pp. 523-30.
S. Fukushima and E. Kimura: Titanium Zirconium, 1975, vol. 23, no. 2, pp. 67-74.
E. Kimura, A. Fuwa and S. Fukushima: Nippon Kogyo Kaishi, 1979, vol. 95, pp. 821-27.
I. Barin: Thermochemical Data of Pure Substances, 3rd ed., VCH Verlagsgesellschaft mbH, Weinheim, 1995.
A. Roine: HSC Chemistry ® 6.1 “Outokumpu HSC Chemistry for Windows”, version 6.1, Outokumpu Research Oy Information Center, Finland, 2006.
A. Fuwa, E. Kimura and S. Fukushima: Metall. Trans. B, 1978, vol. 9B, pp. 643-52.
L.K. Doraiswamy, H.C. Bijawat and M.V. Kunte: Chem. Eng. Progr., 1959, vol. 55, no. 10, pp. 80-88.
K. Jiang, X. Hu, M. Ma, D. Wang, G. Qiu, X. Jin and G. Z. Chen: Angew. Chem. Int. Ed., 2006, vol. 45, pp. 428-32.
M. K. Akhtar, S. Vemury and S. E. Pratsinis: AIChE J., 1994, vol. 40, no. 7, pp. 1183-92.
Acknowledgments
The authors are grateful to Professor Tetsuya Uda, Kyoto University; Professors Kazuki Morita and Takeshi Yoshikawa, the University of Tokyo; and Messrs. Susumu Kosemura, Masanori Yamaguchi, and Yuichi Ono, Toho Titanium Co., Ltd., for their valuable suggestions and the supply of samples used throughout this research. Furthermore, we thank Dr. Katsuhiro Nose for his valuable suggestions and technical assistance. We would like to specially thank Professor Haiyan Zheng of Northeastern University and Mr. Ryosuke Matsuoka of Global Advanced Metals Pty., Ltd., for their preliminary studies. This research was partly funded by a Grant-in-Aid for Next Generation World-Leading Researchers (NEXT Program). One of the authors (Jungshin Kang) is grateful for financial support provided by the MEM (Mechanical, Electrical and Materials Engineering) International Graduate Program from the Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 29, 2012.
Rights and permissions
About this article
Cite this article
Kang, J., Okabe, T.H. Upgrading Titanium Ore Through Selective Chlorination Using Calcium Chloride. Metall Mater Trans B 44, 516–527 (2013). https://doi.org/10.1007/s11663-013-9818-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-013-9818-8