Abstract
Recently, titanium metal production by molten salt electrolysis using CaCl2 as molten salt and TiO2 or rutile (94 to 96 pct TiO2) as feedstock has been drawing attention. However, when a low-grade Ti ore (mainly FeTiO3) is used as feedstock, removal of iron (Fe) from the ore is indispensable. In this study, the influence of reaction temperature, reaction time, particle size of the ore, and source country for the ore on the removal of iron by selective chlorination using CaCl2 was assessed. Experimental results showed that the mass percent of iron in the ore decreased from 49.7 to 1.79 pct under certain conditions by selective removal of iron as FeCl2. As a result, high-grade CaTiO3 was produced when the ore particles smaller than 74 µm reacted with CaCl2 at 1240 K (967 °C) for 8 to 10 hours. Therefore, this study demonstrates that the removal of iron from the ore is feasible through the selective chlorination process using CaCl2 by optimizing the variables.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Kroll process is the current commercial process for the production of Ti metal. However, the productivity of this process is low, owing to its slow production speed and batch-type processing. The FFC (Fray–Farthing–Chen),[1] the OS (Ono-Suzuki),[2] and the electronically mediated reaction/molten salt electrolysis (EMR/MSE)[3] processes have been proposed as alternatives to overcome the limitations of the Kroll process. The FFC and OS processes have been found to be promising among the new processes, because these are simple and semi-continuous processes. In the FFC or the OS process, Ti metal can be obtained directly from TiO2 in a molten CaCl2 by an electrochemical method or metallothermic method using calcium (Ca) as a reducing agent. During the electrochemical reductions in the FFC process, CaTiO3 is generated as an intermediate.[4,5] This CaTiO3 is eventually reduced to Ti metal, as reported by Jiang et al.[4] and Vishnu et al.[6]
Recently, Metalysis Ltd, U.K., reported an interesting result that rutile (94 to 96 pct TiO2) can be used as a feedstock in the FFC process.[7] This indicates that the removal of Fe from ilmenite, which is the most important Ti mineral resource, is indispensable when a low-grade Ti ore (mainly FeTiO3) is used in the FFC process. In the current Ti ore upgrading industry, synthetic rutile is prepared by the Becher,[8] Benilite,[9–11] or slag and UGS (Upgraded Slag)[12] process. The Becher, Benilite, and UGS processes use concentrated acid for the removal of Fe and other impurities from a low-grade Ti ore or Ti slag. As a result, acid waste solution is generated, and the treatment of this waste solution is costly in countries with stringent environmental regulations.
In 2007, Zheng[13] developed a novel method for the selective chlorination of Ti ore using CaCl2 as a chlorinating agent reacting with Fe and forming CaTiO3. However, the Fe in the ore was only partially removed: the mass percent of Fe decreased from 51 to 17 pct at 1293 K (1020 °C). The authors also reported that the mass percent of Fe decreased from 50 to 18 pct at 1100 K (827 °C) through the selective removal of Fe by CaCl2.[14] The authors analyzed that Fe was only partially removed because CaTiO3 formed at an outer layer on ore particles and impeded the reaction between Fe in the center parts of the particles and CaCl2.
Even though the mechanism of the selective chlorination using CaCl2 was investigated by Zheng,[13] the purity of the produced CaTiO3 is not enough to be used as a feedstock for the FFC process. Therefore, this study investigated the influence of reaction temperature, reaction time, and particle size of the ore on the amount of Fe removed as FeCl2 to produce high-grade CaTiO3 when CaCl2 was reacted with the ore through direct physical contact. High-grade CaTiO3 indicates that the amount of impurities except Ca in CaTiO3 are less than or equal to that of the impurities in rutile. In addition, the ores produced in several countries were used as feedstock for the verification of the feasibility of the developed selective chlorination process. However, the mines of the ores used were not specified although the compounds of the ores were analyzed.[15]
In this study, the iron removal ratio increased from 69 pct[14] or 82 pct[13] to 96-98 pct through the determination and the optimization of the effective experimental parameters for the selective removal of Fe from ilmenite to produce high-grade CaTiO3 in molten CaCl2. In addition, the amount of the feedstock was increased by two or twelve times compared to the previous study because the homogeneous chlorination reactions were difficult to achieve owing to the use of a small amount of Ti ore in the authors’ previous study.[14]
Figure 1 shows the flowchart of a novel Ti metal production process. First, Fe in a low-grade Ti ore is selectively removed as FeCl2 and high-grade CaTiO3 is obtained by the following reaction: FeTiO3 (s) + CaCl2 (l) = FeCl2 (l, g) + CaTiO3 (s). If high-grade CaTiO3 can be prepared from low-grade Ti ore by direct reaction with CaCl2, concentrated acid is not required to upgrade the ore. The recyclability of the generated FeCl2 has been reported in several studies.[16,17] The CaTiO3 produced is used as a feedstock for the FFC process, and Ti metal is obtained.
Experimental
Figure 2 shows the schematic and a photograph of the experimental apparatus, while Table I shows the experimental conditions and analytical results for feedstocks and residues. Prior to its use, CaCl2 (anhydrous; purity >95.0 pct; Kanto Chemicals, Inc.) was dried for more than 3 days at 473 K (200 °C) in a vacuum oven. To prepare the experiment, half of the total CaCl2 amount and the entire amount of the ore were uniformly mixed, and placed in a molybdenum-lined nickel crucible (nickel crucible: I.D. = 36 mm; depth = 36 mm; thickness of Mo-lining = 0.1 mm). Thereafter, the remaining CaCl2 was added to the mixture in the crucible. For Exp. no. 130509, the preparation was the same as described above, with the exception of the crucible, which had different dimensions (nickel crucible: I.D. = 60 mm; depth = 59 mm; thickness of Mo-lining = 0.05 mm). A top lid was loosely placed over the crucible, and the crucible was placed inside a vertical stainless steel reactor, which was then sealed with silicone rubber plugs.
Subsequently, the reactor was evacuated twice for 10 minutes each time, and Ar gas (purity > 99.9995 pct) was flowed through the reactor until the internal pressure reached 1 atm. The internal pressure was maintained at 1 atm during the experiments. The Ar gas flow was set to 200 ml/min and controlled with a flow meter. The temperature of the reactor was increased to the target reaction temperature at a rate of 4.9 K/min.
After the completion of the reactions, the reactor was cooled to room temperature, and the residues were obtained from the crucible. The residues were dissolved in deionized water with sonication for at least 2 hours at room temperature. Then, the residues were leached in stirred 6.47 M HCl aqueous solution at room temperature for 0.5 hours. The chemical compositions of the samples were determined using X-ray fluorescence (XRF: JEOL, JSX-3100RII) spectroscopy, and their compounds were identified by X-ray diffraction (XRD: RIGAKU, RINT 2500, RINT 2000, Cu-Kα radiation) analysis.
Results and Discussion
Figure 3(a) shows the presence of white deposits that condensed in the low-temperature regions of the reactor. These deposits were identified as FeCl2·(H2O)2 and FeCl2·4(H2O) by XRD analysis, as shown in Figure 4. When the XRD analysis of the deposits was carried out, double-sided adhesive tape and polymide tape were used for the adhesion of deposits and the prevention of adsorption of H2O present in air onto the deposits during analysis, respectively. The background pattern in Figure 4 was obtained when the blank XRD sample holder with this setup was analyzed. This setup was expected to prevent the adsorption of H2O; however, the results of XRD analysis showed that H2O was adhered to FeCl2. The adherence of H2O is expected to occur through either or both of the following routes: (1) H2O in the air adhered to FeCl2 when the top lid of the furnace was removed; (2) H2O adhered initially to the CaCl2 during the preparation of experiments and liberated when the reactor temperature was increased.
As shown in Table I, when chlorination reactions were carried out at 1240 K (967 °C) for 8 to 10 hours using ore particles smaller than 74 µm, the mass percent of Fe for feedstock decreased from 45.4-49.7 to 1.35-2.49 pct for the residues. In addition, Figures 6 and 7 show that TiO2 and CaTiO3 were obtained under the above conditions. These results show that the Fe in the ore was selectively removed as FeCl2 by reacting with CaCl2, according to Eq. [1] or [2][18]: The value of ∆G°r is slightly different depending on database.
The generated FeCl2 was removed as gas and condensed at the low-temperature regions of the furnace because the vapor pressure of FeCl2 is 0.22 atm at 1240 K (967 °C) which is sufficient to evaporate.[18] In addition, when some amount of FeCl2 dissolves in CaCl2, the activity of FeCl2 is decreased. Therefore, the Gibbs free energy of the reactions in Eqs. [1] and [2] would decrease, and chlorination reaction would proceed.
As shown in Eq. [3], when the activity of CaO (a CaO) is unity, it is difficult to remove Fe in the ore with CaCl2.[18] However, when a CaO decreases through the formation of CaTiO3 as in Eq. [4],[18] the reaction in Eq. [3] can proceed because of the decrease of ∆G r of Eq. [3]. In addition, the high solubility of CaO in CaCl2 at high temperatures also helps to decrease the activity of CaO:19.4 mol pct of CaO in CaCl2 at 1173 K (900 °C).[19] The activity of FeO (a FeO) by the formation of FeTiO3 is 0.663 at 1240 K when the activity of both TiO2 and FeTiO3 is unity.[18] However, the a FeO was assumed to be unity in this study owing to the high value of a FeO. As a result, the Fe in the ore can be selectively removed as FeCl2 by CaCl2.
The selective chlorination of Ti ore using CaCl2 can also be analyzed using the combined chemical potential diagram of Fe-O-Cl and Ti-O-Cl systems at 1240 K (967 °C), shown in Figure 5.[18] When the oxygen and chlorine chemical potentials are located in the hatched region (potential region for selective chlorination), Fe in the ore can be selectively chlorinated as FeCl x (l, g, x=2, 3). When the activity of CaO is unity, the CaO (s)/CaCl2 (l) eq.b line in Figure 5 does not pass through the potential region for selective chlorination. However, when the activity of CaO decreases by the formation of CaTiO3, the CaO (s)/CaCl2 (l) eq.a line in Figure 5 passes in the vicinity of the potential region for selective chlorination, where FeCl2 is stable. Therefore, the Fe in the Ti ore can be selectively removed as FeCl2, and CaTiO3 can be produced.
In this study, the degree of removal of Fe from Ti ore was evaluated by the Fe removal ratio R Fe introduced by Zheng, detailed in Eq. [5].[13] In addition, the obtained residue was evaluated on the basis of the total mass percent of all elements except Ti and Ca, C m (=100 − (C Ti + C Ca)), and the results of XRD analysis.
\( C_{\text{Fe}}^{\text{feed}} \) and \( C_{\text{Fe}}^{\text{residue}} \): the mass percent of Fe in the feedstock and residues, respectively,
\( C_{\text{Ti}}^{\text{feed}} \) and \( C_{\text{Ti}}^{\text{residue}} \): the mass percent of Ti in the feedstock and residues, respectively.
A report by Metalysis Ltd, U.K., indicated that rutile (94 to 96 pct TiO2) can be used as a feedstock in the FFC process.[7] Therefore, residues with C m smaller than 4 to 5 pct, approximately, are appropriate. In this study, a product with C m smaller than 4 pct is defined as high-grade. Furthermore, the compounds of the residues should be CaTiO3 or/and TiO2.
When the Vietnamese ore with particles smaller than 44 μm was reacted with CaCl2 at 1100 K, 1200 K, and 1240 K (827 °C, 927 °C, and 967 °C) for 10 hours, the mass percent of Fe in the ore decreased from 49.7 to 14.2, 4.16, and 2.49 pct, respectively. These results show that as the reaction temperature increased, more Fe in the Ti ore was selectively removed. The reaction rate was increased probably with increasing reaction temperature. In addition, R Fe increased to 96 pct and C m decreased to 3.43 pct at 1240 K (967 °C), because almost all Fe in the ore was selectively removed by CaCl2.
Table I also shows the influence of the reaction time on the amount of Fe removed from the ore by selective chlorination. When selective chlorination of the Vietnamese ore with particles smaller than 44 μm was carried out at 1240 K (967 °C) for 8, 6, 4, and 2 hours, the mass percent of Fe in the ore decreased from 49.7 to 1.87, 4.90, 4.13, and 8.25 pct, respectively. Experimental results showed the tendency of a greater amount of Fe being removed from the ore with increasing time until reaction times ranging from 8 to 10 hours. In addition, these results showed that reaction times longer than 8 hours are required to increase R Fe above 96 pct and to decrease C m to below 2.32 pct at most.
When selective chlorination of the Vietnamese ore with particle sizes in the ranges of under 44, 44 to 74, 74 to 149, 149 to 210, and 210 to 297 μm was conducted at 1240 K (967 °C) for 10 hours, the mass percent of Fe in the ore decreased from 49.7 to 2.49, 1.79, 3.07, 14.4, and 29.6 pct, respectively. As shown in the results, the mass percent of Fe was slightly increased from 1.79 to 2.49 pct when the ore with particle size below 44 μm was used. However, these results showed the tendency that as the particle size of the Ti ore increased, smaller amounts of Fe were selectively removed from the ore. In addition, the results showed that the R Fe increased to 97 pct and C m decreased to 2.41 pct at most, when the particle size of the ore is smaller than 74 μm.
The selective chlorination of Fe in the ore by CaCl2 proceeds from the outside to inside. During the chlorination reaction, CaTiO3 layer is generated to cause a diffusion-controlled process as in the shrinking core model.[20] When the CaTiO3 layer blocks the reaction between Fe in the ore and CaCl2, the removal of Fe significantly decreases. The effect of the diffusional barrier on the conversion is decreased with decreasing particle size. This implies that the larger the particle size, the more unreacted the core remains under identical conditions when the particle size is large enough to incomplete removal of Fe.
Figure 6 shows the XRD analysis results of residues from Ti ore with various particle sizes. As shown in Figures 6 and 7 (3), when the size of the ore particles was smaller than 74 μm, CaTiO3 and TiO2 were the main products. However, when the particle size was larger than 74 μm, FeTiO3 was also identified among the products. This can be understood based on the previous discussion[14]: with the ore particles in the 74 to 297 μm range, 3.07 to 29.6 pct of the Fe remained in the residues because chlorination of Fe by CaCl2 did not proceed in the center parts of the ore particles, owing to the formation of CaTiO3.
In addition, peaks corresponding to CaTi21O38 were also identified in Figure 6 depending on the samples, although the intensity of the peaks of the compound was weak. The main compounds of the Ti ores used are FeTiO3 and TiO2, as shown in Reference 15. However, small amounts of titanium sub-oxides (TiO n ) including magneli phases (Ti n O2n−1, 4 ≤ n ≤ 9)[21] may exist in the ores. As a result, CaTi21O38 may form by the reaction between the titanium sub-oxides with CaO. However, the exact reason for the formation of CaTi21O38 is still under investigation. The formation of CaTi21O38 also affects the activity of CaO. However, as shown in Figures 6 and 7, CaTiO3 is the main compound in the residues. Therefore, in this study, only the influence of the formation of CaTiO3 on the activity of CaO was discussed.
The investigation of the influence of reaction temperature, reaction time, and particle size of the ore on selective chlorination showed that the reaction temperature should be above 1240 K (967 °C), the reaction time should be longer than 8 hours, and the particle size of the ore should be less than 74 μm. Under these conditions, R Fe increased to 96 to 97 pct and C m decreased to 2.32 to 3.49 pct. On the basis of these results, when selective chlorination was conducted at 1240 K (967 °C) for 10 hours using the Australian and Chinese ores with particle sizes below 44 μm as feedstock, the mass percent of Fe in the ores decreased from 46.7 to 1.59 pct and from 45.4 to 1.35 pct, respectively. Figure 7 shows that CaTiO3 and TiO2 were mainly obtained after experiments. It can thus be concluded that selective chlorination using CaCl2 for the production of high-grade CaTiO3 can proceed using various types of Ti ores as feedstock.
Regarding the removal of impurities in the Ti ore, the experimental results showed that selective chlorination also reduced the mass percent of Mn in the ores. As shown in Table I, when the Vietnamese, Australian, and Chinese ores were used, the mass percent of Mn decreased from 3.47 to 0.13 pct, from 1.69 to 0.06 pct, and from 2.79 to 0.11 pct, respectively. It is expected that the Mn was removed through the reaction in Eq. [6].[18] The standard Gibbs energy of the reaction shown in Eq. [6] has a large positive value. Despite this, the reaction can proceed, because the activity of CaO is lowered to 1.74 × 10−4 at 1240 K (967 °C) when the formation of CaTiO3 is taken into consideration.[18]
In order to check the feasibility of scale-up of the selective chlorination using CaCl2 for the production of CaTiO3, the mass change of feedstock was measured. The weight of the Ti ore used was increased from 0.5 to 3.0 g, where ore with particle size below 44 μm was used for the reaction carried out at 1240 K (967 °C) for 20 hours. Consequently, the mass percent of Fe decreased from 49.7 to 2.22 pct owing to the selective removal of Fe, as shown in Table I. In addition, the results of XRF analysis showed that Mn was also removed by the selective chlorination: the mass percent of Mn decreased from 3.47 to 0.25 pct. These results show that selective chlorination using CaCl2 for the production of CaTiO3 can be scaled up. However, because the upgrading of 3.0 g of Ti ore required 20 hours, further research on the scaling up would be required to improve the efficiency of the process.
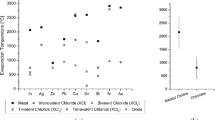
The maximum R Fe calculated from the results reported in the previous study[14] was 56 to 69 pct when the Vietnamese, Australian, or Chinese ore with particle sizes in the range of 74 to 149 μm was reacted with CaCl2 at 1100 K (827 °C) for 5 hours, as shown using solid black circles in Figure 8. A possible explanation for R Fe and C m not reaching 96 to 98 and 4 to 5 pct, respectively, is that the particle size was not smaller than 74 μm, the reaction temperature was not higher than 1240 K (967 °C), and the reaction time was not longer than 8 hours. In addition, the maximum R Fe reported by Zheng was 82 pct when the Vietnamese ore (4.0 g) was reacted with CaCl2 (2.0 g) at 1293 K (1020 °C) for 12 hours. The present results show that low R Fe was achieved because pulverization of the ore was not enough to make the particles smaller than 74 μm and/or the reaction time for chlorination of 4.0 g of the ore was not enough. It is evident from these results that sufficient Fe removal is difficult to achieve when the reaction temperature, particle size, and reaction time are not optimized.
Table II compares the results of the selective removal of Fe from Ti ore obtained in this paper with those obtained by Zheng[13] and Kang et al.[14,22] As shown in Table II, one of the important findings of this study is that it demonstrated the feasibility of the scale-up of selective chlorination using CaCl2 for the production of high-grade CaTiO3. In addition, a Fe removal ratio of 96 to 98 pct was achieved in this study, significantly higher than the 82 pct ratio obtained by Zheng.[13]
Conclusions
An effective selective chlorination process using CaCl2 as a chlorinating agent for upgrading Ti ore was investigated for the production of high-grade CaTiO3. After assessing the effects of various experimental parameters on the selective chlorination, an experiment was conducted to evaluate the scaling-up feasibility of the selective chlorination process. Experimental results showed that Fe was selectively and directly removed as FeCl2 (l, g) from the various types of Ti ores studied, and that CaTiO3 was produced in a single step. The experimental results also showed that the amount of Fe removed from the Ti ore increased with increasing reaction temperature and time, as well as with decreasing particle size of the Ti ore.
When selective chlorination was conducted using Vietnamese ore particles of size below 74 μm at 1240 K (967 °C) for more than 8 hours, the mass percent of Fe in the Ti ore decreased from about 50 to 1.8 pct by XRF analysis. In addition, the mass percent of Fe in the Australian and Chinese Ti ores decreased from 47 to 1.6 pct and from 45 to 1.4 pct by XRF analysis, respectively, when the experiments were conducted at 1240 K (967 °C) for 10 hours and with particles smaller than 44 μm. Furthermore, the experiments demonstrated the scaling-up feasibility of the selective chlorination process using CaCl2, a process that can therefore be applied for the production of high-grade CaTiO3.
References
G.Z. Chen, D.J. Fray, and T.W. Farthing: Nature, 407 (2000), 361–64.
K. Ono and R.O. Suzuki: JOM, 54 (2002), 59-61.
T.H. Okabe and Y. Waseda: JOM, 49 (1997), 28-32.
K. Jiang, X. Hu, M. Ma, D. Wang, G. Qiu, X. Jin, and G.Z. Chen: Angew. Chem. Int. Ed., 45 (2006), 428-32.
K. Dring, R. Dashwood, and D. Inman: J. Electrochem. Soc., 152 (2005), D184–90.
D.S.M. Vishnu, N. Sanil, L. Shakila, R. Sudha, K.S. Mohandas, and K. Nagarajan: Electrochim. Acta, 159 (2015), 124–30.
K. Rao, J. Deane, L. Grainger, J. Clifford, M. Conti, and J. Collins: United States Patent US 2014/0231262 A1, (2014).
R.G. Becher, R.G. Canning, B. A. Goodheart and S. Uusna: Proc. Aust. Inst. Min. Metall., 21 (1965) 21–44.
J.H. Chen and L.W. Huntoon: United States Patent 4019898, 1977.
J.H. Chen: United States Patent 3967954, 1976.
J.H. Chen: United States Patent 3825419, 1974.
M. Guéguin and F. Cardarelli: Miner. Process. Extr. Metall. Rev., 28 (2007) 1–58.
H. Zheng: Doctoral Thesis, The University of Tokyo, 2007.
J. Kang and T.H. Okabe: Metall. Mater. Trans. B., 44B (2013), 516–27.
J. Kang and T.H. Okabe: Mater. Trans., 55 (2014), 591–8.
H. Zheng and T.H. Okabe: J. Alloy. Compd., 461 (2008), 459–66.
Y. Taninouchi and Y. Hamanaka, and T.H. Okabe: Mater. Trans., 56 (2015), 1–9.
I. Barin: Thermochemical Data of Pure Substances, 3rd ed., VCH Verlagsgesellschaft mbH, Weinheim, 1995.
R.O. Suzuki and S. Inoue: Metall. Mater. Trans. B., 34B (2003), 277–85.
O. Levenspiel: Chemical Reaction Engineering 3rd ed., Wiley, United States of America (1999), 566–88.
D.C. Lynch and D.E. Bullard: Metall. Mater. Trans. B., 28B (1997), 447-53.
J. Kang and T.H. Okabe: Mater. Trans., 54 (2013), 1444–53.
Acknowledgments
The authors would like to thank Professor Haiyan Zheng of Northeastern University and Mr. Ryosuke Matsuoka of Global Advanced Metals Pty. Ltd. for their preliminary studies. This research was partly funded by a Grant-in-Aid for the NEXT Program for the Research Project for Development of Environmentally Sound Recycling Technology of Rare Metals (#GR019), and Scientific Research (S) (KAKENHI Grant #26220910) by JSPS. Jungshin Kang is grateful for the financial support that was partly provided by the National Research Council of Science & Technology (NS grant by the Korea government (MSIP) (No. CRC-15-06-KIGAM)), the MEM International Graduate Program from MEXT, Japan, and the Grants for Excellent Graduate Schools from MEXT, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 3, 2015.
Rights and permissions
About this article
Cite this article
Kang, J., Okabe, T.H. Selective Removal of Iron from Low-Grade Ti Ore by Reacting with Calcium Chloride. Metall Mater Trans B 48, 294–301 (2017). https://doi.org/10.1007/s11663-016-0820-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0820-9