Abstract
With a goal to estimate the sulfide capacities of slags used in the pretreatment of hot metal, the sulfide capacities of CaO-Al2O3-SiO2 slags were measured at 1673 K to 1773 K (1400 °C to 1500 °C). The gas–slag equilibrium technique has been used for this measurement. From the results obtained, it was found that the temperature dependence of the sulfide capacity of this slag is independent of the slag compositions. Therefore, a new empirical model based on optical basicity for sulfide capacity estimation of this slag was developed using the measured values of the current work and literature. With the use of the new model, the isosulfide capacity curves at 1673 K (1400 °C) were mapped.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the years, CaF2-containing fluxes had an important role in desulfurization in steelmaking processes including hot metal pretreatment. However, it has become increasingly difficult to use CaF2 because of the environmental problems caused by fluoride emissions. In the case of hot metal treatment, it is common practice to employ CaO-based flux. In view of the fact that the solubility of CaS in CaO is low,[1] it is considered that a molten slag that coexists with solid CaO has an important role for the desulfurization reaction in the hot metal pretreatment process, and the sulfide capacity of the molten slag is one of the important properties that must be considered. In view of the carryover of a certain quantity of blast furnace slag in the hot metal ladle, CaO-Al2O3-SiO2 slag is likely to be formed during the hot metal pretreatment. Whereas the experimental studies of the sulfide capacity values for this slag are abundant in the literature,[2–11] many previous works focused on the refining process. To the knowledge of the authors, no measurement of the sulfide capacity of this slag at temperatures less than 1773 K (1500 °C) has been reported. Therefore, in the current work, the sulfide capacities of the CaO-Al2O3-SiO2 slags were measured in the temperature range 1673 K to 1773 K (1400 °C to 1500 °C).
Experimental
The classic gas–slag equilibrium technique was employed in the current study to measure sulfide capacities. The schematic diagram of the experimental setup is shown in Figure 1. A horizontal resistance furnace with MoSi2 heating elements was used for all the measurements. The furnace was controlled by a PID controller within ±1 K using a Pt-30 pct Rh/Pt-6 pct Rh thermocouple, fixed close to the heating element, just outside the furnace tube. An alumina reaction tube (outer diameter: 0.06 m, inner diameter: 0.05 m, length: 1.2 m) was positioned inside the furnace. The even temperature zone of the furnace (with a temperature fluctuation of ±2 K) extended over a length of 0.1 m in a reaction tube. A separate thermocouple was positioned just above the sample to measure the sample temperature. The overall errors in temperature control are considered to be less than ±3 K.
A gas mixture consisting of Ar, CO, CO2, and SO2 was used to impose predetermined sulfur and oxygen partial pressures in the gas phase above the slag samples equilibrated in the furnace. The flow rates of the gases were controlled by mass flow meters F201C supplied by Bronkhorst High-Tech. Suitable purification steps were adopted for these gases before mixing. The schematic diagram of the gas cleaning system is shown in Figure 2. Silica gel and Mg(ClO4)2 were used to remove the traces of moisture (except in the case of SO2 for which Mg(ClO4)2 was not used). Ascarite (sodium-hydroxide-coated silica) was used to remove the CO2 impurity, and in the case of Ar gas, Cu and Mg chips that were kept at 773 K (500 °C) were used to remove the traces of oxygen.
The slag samples were prepared by mixing appropriate proportions of CaO, Al2O3, and SiO2 reagent powders. The oxide powders were calcined at 1273 K (1000 °C) for 12 hours in a muffle furnace to remove any trace of moisture before mixing. The samples were pressed into small pellets of 1 g and placed in Pt crucibles positioned on an alumina boat. The Pt crucibles were made of 1.27 × 10−4 m thickness Pt (99.9 mass pct [metal basis]) foil.
In a general run, two to four Pt crucibles with the slag samples were pushed inside the furnace at 1673 K (1400 °C) and kept for 12 hours in Ar (99.999 pct, AGA) gas stream for premelting. The Ar-CO-CO2-SO2 gas mixture was then introduced into the reaction tube, and the temperature was changed to the target value. A constant flow rate of 400 mL/min was maintained during the equilibration of the slag with the gas mixture at the experimental temperature for at least 6 hours. This soaking time was found to be sufficient for the slag to reach the equilibrium with the gas phase.[12,13] After the equilibration, the samples were pulled out toward the cold end of the furnace and quenched with the gas mixture flowing. The gas atmosphere was changed to Ar, and the samples were taken out and subjected to a chemical analysis. The sulfur content was analyzed using a LECO combustion-infrared spectrometer (LECO Corporation, St Joseph, MI).
Results and Discussion
The concept of sulfide capacity as expressed by Richardson and Fincham[2] is as follows:
where (mass pct S) is the mass pct of the sulfur content in slag, and \( P_{{\text{O}_{2} }} \) and \( P_{{\text{S}_{2} }} \) are the partial pressures of oxygen and sulfur gas, respectively, in the gas phase.
In the current experiments, the partial pressures of sulfur and oxygen were calculated using Thermo-Calc version R with the SSUB3 database (Thermo-Calc Software, Inc., McMurray, PA). To check the reliability and accuracy of the SSUB3 database, a comparison of the sulfide capacities has been made between the reported values by Hino et al.[8] and those calculated by the database. The calculated values agree well with their results. The partial pressure of oxygen in the gas mixture just above the sample was maintained between 3.5 × 10−4 and 2.6 × 10−3 Pa, and the partial pressures of sulfur were found to vary between 1.2 × 103 and 1.5 × 103 Pa.
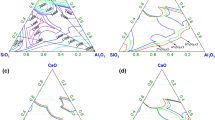
The experimental compositions and results are summarized in Tables I and II. The values of optical basicity were calculated using the following data reported by Sosinsky and Sommerville[14]: CaO = 1.0, Al2O3 = 0.61, and SiO2 = 0.48. The error ranges of log C S are mainly the result of standard deviations in the chemical analysis of sulfur. The experimental compositions of this work are shown in Figure 3. The dashed lines in this figure indicate the liquidus line of this slag at 1673 K (1400 °C). It is observed that this slag system has two liquidus phase areas at 1673 K (1400 °C): one in the high SiO2 concentration corresponding to the upper liquid region in Figure 3 and the other in the low SiO2 concentration corresponding to the lower liquid region in the same figure.
Sulfide Capacity in High SiO2 Concentration
The sulfide capacities of CaO-Al2O3-SiO2 slags in the high SiO2 concentration region were determined first at 1773 K and 1923 K (1500 °C and 1650 °C) by Richardson and Fincham[2] in the 1950s. Their results were later corrected by Abraham and Richardson.[5] Kalyanram et al.[4] measured the activity of CaO in this slag at 1773 K (1500 °C). The sulfide capacity was not calculated directly in their work, but can be calculated from their results. Kärsrud[7] (1773 K [1500 °C]) as well as Görnerup and Wijk[9] (1823 K, 1873 K, and 1923 K [1550 °C, 1600 °C, and 1650 °C]) also reported the values. However, Drakaliysky et al.[10] published larger values than the former researchers in 1997. To obtain a reasonable description for this slag system, it is important to check the accuracy of the reported values. Thus, the measurement at 1773 K (1500 °C) was rechecked in the current work. The values of log C S at 1773 K (1500 °C) in high SiO2 concentration are plotted against (pct CaO) in Figure 4. The literature data at 1773 K (1500 °C) mentioned previously are also plotted for comparison. As shown in the figure, the current results increased with an increase in (pct CaO) and are in good agreement with the data obtained by Kalyanram et al.,[4] Abraham and Richardson,[5] and Kärsrud.[7] It is also evident that the effect of (pct Al2O3) on sulfide capacity is smaller than the effect of (pct SiO2).
The values of log C S at 1673 K (1400 °C) in high SiO2 concentration are plotted against (pct CaO) in Figure 5. The current results at 1673 K (1400 °C) also increased with an increase in (pct CaO). The effect of (pct Al2O3) and (pct SiO2) on the sulfide capacity is similar to the case at 1773 K (1500 °C).
Figure 6 shows the relationship between log C S and (pct CaO/pct SiO2). The calculated sulfide capacities from the results of Kalyanram et al.[4] are also plotted in the figure. As shown in Figure 6, it is evident that at fixed (pct CaO/pct SiO2), the values of log C S are independent of (pct Al2O3). This indicates that the isosulfide capacity lines in high-SiO2 concentration are almost parallel to the lines that are drawn from the Al2O3 corner to the binary CaO-SiO2 side.
Sulfide Capacity in low SiO2 Concentration
The sulfide capacities of CaO-Al2O3-SiO2 slags in low-SiO2 concentration have been studied by many researchers[3,6,8–10] using the gas–slag equilibrium technique, and their results agree well with each other. Thus, only the measurement at 1673 K (1400 °C) had been carried out in this work. The values of log C S at 1673 K (1400 °C) in the low-SiO2 concentration region are shown in Figure 7. The values of log CS in high-SiO2 concentration are also plotted for a comparison. As shown in Figure 7, the sulfide capacity values in low-SiO2 concentration are higher than the values in high-SiO2 concentration. Furthermore, in the case of low-SiO2 concentration, it was found that the isosulfide capacity line is almost parallel to the liquidus of 3CaO·Al2O3. A similar tendency was reported for this slag by Hino et al.[8] at 1873 K (1600 °C). In a real process, a large amount of solid CaO exists in the slag phase. Thus, the composition of the molten slag is likely to become saturated with 3CaO·Al2O3 during the hot metal pretreatment. This finding indicates that the effect of SiO2 on sulfide capacity in low-SiO2 concentration is almost negligible. Although SiO2 is known to decrease the sulfide capacity,[8,11] considering that the CaO-Al2O3 slag system has a small liquid phase area at 1673 K (1400 °C) and has no liquid phase below 1668 K (1395 °C),[1] a small amount of SiO2 is expected to be useful for the stability of desulfurization.
Temperature Dependence
In the current work, the sulfide capacities of slags of constant composition at different temperatures were measured to investigate the effect of temperature. The measured values were found to increase with increasing temperature. Figure 8 shows the relationship between log C S and temperature. The results by Richardson and Fincham,[2] and by Görnerup and Wijk[9] are also plotted in Figure 8 for a comparison. As shown in this figure the slope of log C S vs 104/T measured in the present work is agreed well with their results and it was also found that the slope is virtually constant with the slag compositions.
In this work, as mentioned, there were values only at 1673 K (1400 °C) in low-SiO2 concentration. The values of –2.538 (52-CaO, 40-Al2O3, and 8-SiO2 in mass pct) and –2.567 (51-CaO, 45-Al2O3, and 4-SiO2 in mass pct) at 1873 K (1600 °C) were predicted by Thermoslag Software (Royal Institute of Technology, Stockholm, Sweden), based on the KTH model.[12,15,16] Note that the KTH model was optimized using the reported values by Hino et al.,[8] which are in general considered reliable, and the difference between the predicted and measured values especially near the compositions of the current work are almost negligible as shown in Figure 9. Thus, the model predictions in low-SiO2 concentration of the system CaO-Al2O3-SiO2 at 1873 K (1600 °C) is most likely to be reliable.
Modeling of Sulfide Capacity
Comparison with the sulfide capacity models
A comparison of the current values of sulfide capacity at 1673 K (1400 °C) along with the predicted values of sulfide capacity by Sommerville’s model[14], Young’s model,[17] and the KTH model[12,15,16] is shown in Figure 10. Figure 11 shows the relationship between the sulfide capacity of the slag that has a composition of 52-CaO, 40-Al2O3, and 8-SiO2 in mass pct predicted by these models and temperature. The slope of the current work is shown also in this figure. As shown in Figure 10, the predicted values of Sommerville’s model become larger than the current values with decreasing the sulfide capacity obviously because the temperature coefficient of this model has an optical basicity term as shown in Eq. [2].
Sommerville’s model[14]
The optical basicity of the current work varied from 0.592 to 0.752. The corresponding temperature coefficients of Sommerville’s model are –9,655 at Λ = 0.592 and –18,423 at Λ = 0.752. However, as mentioned in the current work, the temperature dependence of the sulfide capacity of the CaO-Al2O3-SiO2 slags is independent of the slag composition as well as the optical basicity. Unlike in the case of the Sommerville’s model, the predicted values of Young’s model become smaller than the current values with decreasing the sulfide capacity.
Young’s model[17]
The slope of log C S vs 104/T is almost the same with the current work as shown in Figure 11; this may be because the (pct SiO2) and (pct Al2O3) terms of this model (Shown in Eq. [3]) have a stronger effect on the predicted value when the both concentrations are high. The predicted values of the KTH model are larger than the current values over the complete range of the measured compositions. Because this model has complex expressions, it is impossible to derive the slope of log C S vs 104/T mathematically. However, it can be observed in Figure 11 that the slope is larger than in the current work. This could explain the difference between the predicted and measured values at 1673 K (1400 °C). As shown in the literature,[12,15,16] the prediction of the KTH model has achieved a good agreement with the experimental values at 1873 K (1600 °C). In view of the accuracy of this model at 1873 K (1600 °C), the deviation is probably caused by the experimental error of the values used to the optimization for lower than 1873 K (1600 °C). Thus, a new model for sulfide capacity prediction was developed in this work.
New Sulfide Capacity Model
The concept of optical basicity was developed by Duffy and Ingram[18] and was expressed as follows:
The values of log C S are plotted against optical basicity in Figure 12. From this figure, it can be observed that sulfide capacity and optical basicity have a good correlation in the CaO-Al2O3-SiO2 system. Thus, it would be convenient to express this new model in terms of optical basicity.
To obtain a complete description of the CaO-Al2O3-SiO2 system, the corresponding binary systems must be taken into account. The sulfide capacities of the CaO-SiO2 slags were determined by Richardson and Fincham[2] as well as Carter and Macfarlane.[3] Their values were later recalculated by Abraham and Richardson.[5] Görnerup and Wijk[9] also reported the values. Figure 13 shows the relationship between log C S and temperature in the CaO-SiO2 slag. As shown in Figure 13, the slope of log C S vs 104/T is also virtually constant with the slag compositions. The sulfide capacity of the CaO-Al2O3 slags has been studied by several researchers.[2,3,5,6,8,11,19–22] The relationship between log C S and temperature in the CaO-Al2O3 slag is shown in Figure 14. In this figure, the slopes were derived from the data that were read off from Hino et al.’s paper[8] and agree well with the data measured at 1773 K (1500 °C) by Sharma and Richardson[19] as well as Kor and Richardson.[20] It is evident from Figure 14 that the slope of log C S vs 104/T is also virtually constant with the slag compositions. The temperature dependence of the sulfide capacity in the CaO-Al2O3-SiO2, CaO-SiO2, and CaO-Al2O3 slags are summarized in Figure 15. The data shown in the plot of the Figure 15 correspond to the same data represented in Figures 8, 13, and 14. As shown in Figure 15, it was found that the difference between the slopes of log C S vs 104/T in these slags is almost negligible. Based on the preceding facts, a new model for sulfide capacity prediction based on optical basicity has been developed using the measured values of the current work and literature,[3–5,7–9,19,20] and it is shown in Eq. [5]. The prediction of sulfide capacity by this model is shown in Figure 16. It is evident from Figure 16 that this model predicts sulfide capacity well over the whole range of measured sulfide capacity for the current slag system as compared with other models.
This equation has been developed for slags having CaO of 20 to 63 mass pct, Al2O3 of 0 to 65 mass pct, SiO2 of 0 to 68 mass pct, and temperature of 1673 K to 1928 K (1400 °C to 1655 °C). A total of 187 data points was used for regression analysis, and the multiple correlation coefficient was 0.99. Furthermore, this model has been developed for slags with an optical basicity between 0.56 and 0.80, and a sulfide capacity between –5.3 and –1.9 in logarithm. The range of optical basicity, sulfide capacity, and temperature is applicable to iron making, hot metal pretreatment, and as secondary refining slags. The calculated isosulfide capacity curves at 1673 K (1400 °C) using the current model were mapped in Figure 17. The curves show a slightly different curvature than the contours reported by Abraham and Richardson[5] at 1773 K (1500 °C) as well as Görnerup and Wijk[9] at 1823 K, 1873 K, and 1923 K (1550 °C, 1600 °C, and 1650 °C).
Conclusions
In the current work, the sulfide capacities of CaO-Al2O3-SiO2 were measured experimentally at 1673 K to 1773 K (1400 °C to 1500 °C) using the gas–slag equilibrium method. The measured values at 1773 K (1500 °C) were in good agreement with the reported values by Kalyanram et al.,[4] Abraham and Richardson,[5] and Kärsrud.[7] In high-SiO2 concentration, it was found that at fixed (pct CaO/pct SiO2), the measured values are fairly independent of (pct Al2O3). In low-SiO2 concentration, the isosulfide capacity line is almost parallel to the liquidus of 3CaO·Al2O3. The temperature dependence of sulfide capacity of this slag is independent of the slag compositions. Based on the facts mentioned, a new empirical model based on optical basicity for sulfide capacity estimation of this slag was developed using the measured value of the current work and the literature. This model is applicable to iron making, hot metal pretreatment, and as secondary refining slags.
References
Slag Atlas, 2nd ed., VDEh, Verlag Stahleisen GmbH, Düsseldorf, Germany, 1995.
F.D. Richardson and C.J.B. Fincham: J. Iron Steel Inst., 1954, vol. 178, pp. 4–15.
P.T. Carter and T.G. Macfarlane: J. Iron Steel Inst., 1957, vol. 185, pp. 54–62.
M.R. Kalyanram, T.G. Macfarlane, and H.B. Bell: J. Iron Steel Inst., 1960, vol. 195, pp. 58–64.
K.P. Abraham and F.D. Richardson: J. Iron Steel Inst., 1960, vol. 196, pp. 313–17.
J. Cameron, T.B. Gibbons, and J. Taylor: J. Iron Steel Inst., 1966, vol. 204, pp. 1223–28.
K. Kärsrud: Scand. J. Metall., 1984, vol. 13, pp. 144–50.
M. Hino, S. Kitagawa, and S. Ban-Ya: ISIJ Int., 1993, vol. 33, pp. 36–42.
M. Görnerup and O. Wijk: Scand. J. Metall., 1996, vol. 25, pp. 103–07.
E. Drakaliysky, D. Sichen, and S. Seetharaman: Can. Metall. Q., 1997, vol. 36, pp. 115–20.
M. Ohta, T. Kubo, and K. Morita: Tetsu-to-Hagane, 2003, vol. 89, pp. 742–49.
M.M. Nzotta, D. Sichen, and S. Seetharaman: ISIJ Int., 1998, vol. 38, pp. 1170–79.
A. Shankar, M. Görnerup, A.K. Lahiri, and S. Seetharaman: Metall. Mater. Trans. B, 2006, vol. 37B, pp. 941–47.
D.J. Sosinsky and I.D. Sommerville: Metall. Trans. B, 1986, vol. 17B, pp. 331–37.
R. Nilsson and S. Seetharaman: Scand. J. Metall., 1994, vol. 23, pp. 81–86.
M.M. Nzotta, D. Sichen, and S. Seetharaman: Metall. Mater. Trans. B, 1999, vol. 30B, pp. 909–20.
R.W. Young, J.A. Duffy, G.J. Hassall, and Z. Xu: Ironmaking Steelmaking, 1992, vol. 19, pp. 201–19.
J.A. Duffy and M.D. Ingram: J. Am. Ceram. Soc., 1971, vol. 93, pp. 6448–54.
R.A. Sharma and F.D. Richardson: J. Iron Steel Inst., 1961, vol. 198, pp. 386–90.
G.J.W. Kor and F.D. Richardson: J. Iron Steel Inst., 1968, vol. 206, pp. 700–04.
B. Ozturk and E.T. Turkdogan: Met. Sci., 1984, vol. 18, pp. 299–305.
E. Drakaliysky, R. Nilsson, D. Sichen, and S. Seetharaman: High. Temp. Mater. Proc., 1996, vol. 15, pp. 263–72.
Acknowledgment
The authors are thankful to Nippon Steel Corporation, Japan for sponsoring the current work and the financial support for the same.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 11, 2008.
Rights and permissions
About this article
Cite this article
Taniguchi, Y., Wang, L., Sano, N. et al. Sulfide Capacities of CaO-Al2O3-SiO2 Slags in the Temperature Range 1673 K to 1773 K (1400 °C to 1500 °C). Metall Mater Trans B 43, 477–484 (2012). https://doi.org/10.1007/s11663-011-9621-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-011-9621-3