Abstract
The effect of CaF2 on the viscosity of high-basicity Al2O3-CaO-MgO-SiO2 (-CaF2) slags for secondary steelmaking was studied using a Brookfield digital viscometer. The addition of approximately 3 mass pct CaF2 could decrease the liquidus temperature substantially in the case of high CaO containing slags, leading to good flowability of the slag at the temperature of the ladle treatment. The addition of CaF2 had the strongest effect on the viscosity of liquid slag with high SiO2 content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In most steelmaking processes, it is of considerable importance to keep the slag viscosity low to ensure fast heat and mass transfer. Low viscosity is also often one of the foremost factors when choosing slag for secondary steelmaking. One of the most common measures to lower the slag viscosity and its melting temperature is to add a certain amount of calcium fluoride to the slag. In line with this direction, the effect CaF2 on slag viscosity has been studied over the years. One example is the investigation of the ternary system FeO-SiO2-CaF2 and quaternary system CaO-FeO-SiO2-CaF2 by Shahbazian,[1,2] who reported that the effect of CaF2 addition on the viscosities of “FeO”-SiO2 slags is not as significant as in the case of CaO-SiO2 slags. Another example is the work by Behera and Mohanty,[3] who studied the viscosity of Al2O3-Cr2O3-CaO-CaF2 slags. Some researchers have also investigated the mechanisms of the effects of calcium fluoride on slag viscosity.[4–9] In addition, several models have been proposed to estimate viscosity of slags containing calcium fluoride.[10–13] However, the viscosity data for multicomponent slags containing calcium fluoride are still scarce, especially for high-basicity slags used in secondary steelmaking. In this context, the current work aims to investigate the effects of CaF2 on the viscosity of high-basicity Al2O3-CaO-MgO-SiO2 (-CaF2) slags at steelmaking temperatures.
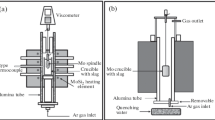
The experimental setup is shown schematically in Figure 1. A Brookfield digital viscometer (model: DV-II+ Pro; Brookfield Engineering Laboratories, Inc., Middleboro, MA) was used. The viscometer was placed in a vacuum tight poly(methyl methacrylate) (PMMA) box on the top of the furnace. The viscosity could be followed by a personal computer (PC) as a function of time using the software supplied by Brookfield.
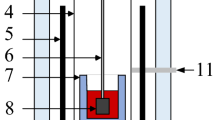
A graphite resistance furnace was used for this investigation. The variation of the temperature in the even temperature zone of the furnace was less than ± 3 K. The furnace could be moved along the vertical direction by a hydraulic moving system. The temperature of sample was measured by Pt-6 pct Rh/Pt-30 pct Rh thermocouple in a molybdenum rod connected to the molybdenum platform. The thermocouple was kept in an alumina tube with closed upper end. Molybdenum crucible (inner diameter: 42 mm, and inner height: 153 mm) was used for all the measurements. The crucible was placed on the molybdenum platform. The spindle was also made of Mo. The setup allowed fast quenching of the sample along with crucible by moving them rapidly into the quenching chamber. The spindle consisted of a bob and a shaft. The dimensions of the bob were 19 mm in diameter and 18 mm in height. The diameter of the shaft was 4 mm, and its length was 50 mm.
Four different master slags were studied. Reagent grade MgO, SiO2, Al2O3, and CaO were calcinated at 1273 K (1000 °C), whereas the CaF2 (>99.99 pct) powder was dried at 773 K (500 °C) before use. The slags were prepared in two steps. First, the master slag containing Al2O3-CaO-MgO-SiO2 was premelted in Mo crucible in the same furnace. The premelted master slag was quenched rapidly to avoid the expansion of the sample as a result of phase transformation of 2CaO·SiO2, which would damage the Mo crucible. CaF2 powder was added to the master slag to achieve the desired CaF2 concentration. The master slag-CaF2 mixture was then heated and melted again in the same furnace. In the process, a Mo cover was put on the top of the crucible to avoid the extensive volatilization of CaF2 before it dissolved into the slag. Again, the sample was quenched to keep the Mo crucible from cracking. The Mo cover was removed before the furnace was heated up again for viscosity measurements. Purified argon gas atmosphere was used throughout the premelting procedures. Some of the master slags had a high melting temperature. In such case, only step two was used. Each premelted slag was approximately 160 g.
In a general run, the reaction chamber along with the PMMA box was evacuated after placing the Mo crucible on the platform and the spindle just above the sample. Purified argon gas was introduced from the gas inlet situated at the bottom of the reaction chamber and led out from the top of the PMMA box. An argon flow (about 0.05 l/min) was maintained throughout the whole experiment. The sample was heated up to a temperature of 1913 K (1640 °C) and kept there for 4 hours to ensure complete melting. The furnace was moved up slowly to immerse the spindle into the liquid. A low rotational rate was employed to ensure homogenization of the sample. Thereafter, the sample was cooled down slowly or heated up to the measurement temperature, and the viscosity–time curve was recorded.
After measurement, the sample along with the crucible was quenched by moving the sample to the quenching chamber. At the same time, argon gas with a high flow rate was impinged on the sample to increase the cooling. The sample temperature was followed by the thermocouple attached to the bottom of the crucible. Usually, the sample could be cooled to 1573 K (1300 °C) in 30 seconds.
Several pieces of the quenched sample were collected for the analysis of CaF2. The CaF2 content was analyzed by an ion selective electrode.
The composition of the master slags and the analyzed CaF2 content are presented in Table I. The experimental results are presented in Figures 2–5. The different figures show the results involving the four different master slags, respectively. The data for master slag 2 without CaF2 addition are from a previous work.[14] To provide the readers raw data, the experimentally determined viscosities are listed in Table II.
Master slags 1 and 2 have relatively low liquidus temperatures. As shown in Figures 2 and 3, the addition of CaF2 leads to a substantial decrease of the viscosity in the case of both master slags. An addition of 3.1 mass pct CaF2 to master slag resulted in an approximately 40 pct viscosity decrease over the temperature range of 1700 K to 1900 K (1427 °C to 1627 °C). Note that the main purpose of the study of master slag 2 was to examine the effect of CaF2 on the slags with low liquidus temperature. Because the slag composition is somewhat different from the typical basic ladle slags used for desulfurization, only one CaF2 addition was studied for this master slag. The phase diagram of the Al2O3(25mass pct)-CaO-MgO-SiO2 system[15] indicates that master slag 1 is likely to be in a two phase region below 1850 K (1577 °C). The presence of the solid phase would explain the dramatic increase in viscosity observed at this temperature. In contrast, the viscosity data for slags 1A and 1B reveal good continuity, increasing smoothly with decreasing temperature. These two curves would suggest that there was no solid precipitation in either of these samples. This implies that the addition of low amount of CaF2 (2.9 mass pct or greater) can decrease the liquidus temperature substantially. Even the viscosity is considerably lowered by the addition. The bigger addition leads to a larger viscosity decrease.
Variation of viscosity with temperature at different CaF2 addition for master slag 2. The data points for sample 2 are from Song et al.[14]
In the case of master slags 3 and 4, the liquidus temperatures were too high to make viscosity measurements. Hence, only the slags with CaF2 additions were studied in these instances. It is evident in Figures 4 and 5, that there are almost no differences in viscosity between the A and B slags at higher temperatures (above ~1800 K [1527 °C]). The moderate viscosity increase and the absence of discontinuity of the viscosity–temperature curves suggest that these slags are fully liquid above 1800 K (1527 °C). Note that both master slags 3 and 4 have a high liquidus temperature (>1900 K [1627 °C]). It is apparent from this behavior that the addition of about 3 mass pct CaF2 can lower the liquidus temperature substantially. Although the real liquidus temperature of slags 3A, 3B, 4A, and 4B still need additional work to quantify exactly, the viscosity curves in Figures 4 and 5 suggest strongly that all the four slags have very good flowability at the temperature of ladle treatment. Above 1800 K (1527 °C), all four slags have viscosity values lower than 0.1 Pa.s. Below 1800 K (1527 °C), the viscosity increases dramatically with decreasing temperature, in the case of slags 3A, 3B, and 4A. This dramatic increase would suggest the precipitation of solid particles below 1800 K (1527 °C). Additional study is needed to confirm this aspect. Because most of the steel refining processes are conducted above 1800 K (1527 °C), the addition of CaF2 of 2–3 mass pct might help the steelmakers to operate the slag in a much higher basicity region.
Figures 4 and 5 also indicate that there is a distinct difference between the slags with different amounts of CaF2 addition. Increasing the CaF2 addition seems to push further down the liquidus temperature of the slag, thereby decreasing the amount of solid particles. It is well known that the presence of bigger fraction of solid particles would result in higher viscosity.[16]
A comparison of Figures 2–5 indicates that the addition of CaF2 has the strongest effect on the viscosity of master slag 2. This effect is believed to be caused by the higher silica content of slag 2. The presence of CaF2 would tend to depolymerize of the silicate chains/molecules, leading to significant viscosity decrease. In contrast, as shown in Table I, the other master slags have low SiO2 content, and therefore the disruption of silicate chains/molecules structure is likely to be less apparent.
It can be concluded that the effect of CaF2 depends strongly on the composition of the master slag. For high-basicity slags (master slags 3 and 4), CaF2 suppresses the precipitation of solid phases at lower temperatures, leading to a lower viscosity compared with CaF2-free slags. When these slags are completely liquid, the effect of CaF2 on viscosity is negligible. For slags with higher SiO2 contents (master slags 1 and 2), CaF2 lowers both the viscosity of the liquid phase and suppresses the precipitation of solid phases.
References
F. Shahbazian: ISIJ Int., 1999, vol. 39, pp. 687–96.
F. Shahbazian: Physico. Scand. J. Metall., 2001, vol. 30, pp. 302-08.
R.C. Behera and U.K. Mohanty: ISIJ Int., 2001, vol. 41, pp. 834-43.
P.M. Bills: JISI, 1963, vol. 201, pp. 133-40.
T. Iida, M. Ueda, and Z. Morita: Tetsu to Hagane, 1976, vol. 62, pp. 1169-78.
S. Seetharaman, S.C. Du: Metall. Mater. Trans. B, 1994, vol. 25B, pp. 589-95.
S. Seetharaman, S.C. Du, F.-Z. Ji: Metall. Mater. Trans. B, 2000, vol. 31B, pp. 105-09.
L.S. Darken: TMS-AIME, 1967, vol. 239, pp. 80-89.
S. Seetharaman, S. Sridhar, S.C. Du, K.C. Mills: Metall. Mater. Trans. B, 2000, vol. 31B, pp. 111-19.
Q. Shu and J. Zhang: J. Univ. Sci. Technol. Beijing, 2005, vol. 12, pp. 1-5.
U. Georges: Steel Res., 1987, vol. 58, pp. 111-16.
K.C. Mills and S. Sridhar: Ironmaking Steelmaking, 1999, vol. 26, pp. 262-68.
T. Iida, H. Sakai, and Y. Kita: High Temp. Mater. Proc., 2000, vol. 19, pp. 153-64.
M. Song, Q. Shu, and Du Sichen, Steel Res. Int., 2011, vol. 82, pp. 260–68.
M. Allibert and H. Gaye et al.: Slag Atlas, 2nd ed., V.D. Eisenhuttenleute, ed., Verlag Sthaleisen GmbH, Dusseldorf, Germany, 1995, pp. 158.
L. Wu, M. Ek, M. Song, and S. Du: Steel Res. Int., 2011, vol. 82, pp. 388-97.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted May 6, 2011.
Rights and permissions
About this article
Cite this article
Wu, L., Gran, J. & Sichen, D. The Effect of Calcium Fluoride on Slag Viscosity. Metall Mater Trans B 42, 928–931 (2011). https://doi.org/10.1007/s11663-011-9546-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-011-9546-x