Abstract
The present study reports an experimental investigation of the effects of CaO/SiO2 mass ratio (0.4 to 1.2) and Fe/SiO2 ratio (1.26 and 1.7) on viscosity of the heterogeneous system of the CaO–SiO2–FeO–Fe2O3–PbO–MgO–ZnO–S slag at 1300°C. The viscosity was measured using rotating cylindrical viscometer, and structure of quenched slags was studied by X-ray diffraction and SEM-EDS analysis. It was found that the viscosity of the slurry decreased with an increase in CaO/SiO2 ratio and increased with an increase in Fe/SiO2 ratio. The sulphur content in the slag decreases the FeO/Fe2O3 ratio in the liquid slag and consequently increases the viscosity. The FactSage thermodynamic software is utilized for the phase equilibrium and viscosity predictions. The effect of solid fractions in slag phase on the viscosity is calculated using the Einstein–Roscoe equation. Calculations show that an increase in solid fractions increases the viscosity of the slag phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The control of slag properties such as viscosity is essential in maintaining the pyrometallurgical processes efficiency. The slag viscosity is very sensitive to the changes of temperature and composition. The suitable viscous flow of molten slag can facilitate the chemical reaction to eliminate impurities and control the heat transfer, mass transfer and smelting stability related to the active multiphase reaction among molten slag, liquid metal, and gas.
Viscosities of liquid slag systems have been extensively studied and many theoretical models have been developed to predict the viscosity of a fully liquid silicate [1–4]. However, not all slag melts are fully liquid at the operating temperatures. Silicate slags may become quite viscous due to the precipitation of solid phases, leading to problems with slag tapping or appropriate separation of metallic phase. Sycheva et al. [5–7] have studied the nucleation of crystals in glass obtained by melting furnace slags with the additive of SiO2.
Several viscosity models have been established to correlate the viscosity and solid fraction. The most widely used viscosity model for heterogeneous system is the Einstein–Roscoe (E–R) equation [8, 9]. Kondratiev and Jak [10] validated the applicability of the E–R equation with a large experimental data set in the Al2O3–CaO–FeO–SiO2 slag.
Liu et al. [11] discussed a review of the viscosity of solid-bearing silicate melts where it is mentioned that several characteristics of the high-temperature melts, such as temperature, dwelling time, and shear rate, which leads to change in crystal fraction should be noticed. Wang et al. [12] showed that an increase in the CaO/SiO2 ratio led to the decrease in viscosity in silicate mould fluxes with B2O3. Wright et al. [13] reported an experimental investigation of the effect of solid suspension on the viscosity of molten slags. They used up to about 20 vol % of spinel (MgAl2O4) particles of the size range from 0.10 to 0.99 mm.
FactSage thermodynamic software has been used for a better understanding of slag-metal interaction in both ferrous and non-ferrous processes. It is well known for predicting metallurgical processes, multi-phase equilibria, liquidus temperature and chemical and metallurgical process modelling [14]. This software was also used by Mishra et al. [15] to study the deposition characteristics of ash particles based on the viscosity and phase transformation properties in boiler operation. They also studied the effect of solid or crystalline fractions in slag phase on the viscosity.
The lead ore produced in Mexico has historically been different from that around the rest of the world as the Mexican lead ores contains high concentrations of silver and bismuth. The recovery of precious metals during lead production in a blast furnace makes this process more profitable. Losses of silver and lead are common in this process and can be due to both physical and chemical phenomena. Lead can be trapped as metallic particles in the slag [16], due to its composition and viscosity.
The present study has been conducted to provide experimental data of the viscosity of the heterogeneous system of the CaO–SiO2–FeO–Fe2O3–PbO–MgO–ZnO–S slag at 1300°C (1573 K) with the following mass ratios: CaO/SiO2 = 0.4 to 1.2 and Fe/SiO2 = 1.26 and 1.7. These composition parameters have been selected because they represent the slags typically encountered in the Mexican lead blast furnaces. The equilibrium-quenching method was also used to estimate the solid fraction and the species that are stable at high temperature experimentally.
Factsage thermodynamic software [14] has incorporated an extensive amount of results on the PbO–ZnO–CaO–SiO2–Fe2O3–FeO–Al2O3 slag systems in order to obtain one set of model equations for the Gibbs energy of the liquid slag. This software is used in this work as a tool to calculate the solid fraction of the system. This computer program can also calculate the viscosities of fully liquid silicate slags. The model links the viscosities of silicate melts to their structure and thermodynamic properties.
EXPERIMENTAL
Materials
A typical slag sample obtained from the smelting of lead concentrate in a blast furnace was used as master slag. The as-received sample was crushed to –100 mesh size for the X-ray fluorescence analysis. Table 1 shows the chemical composition of the slag. The master slag contains the following mass ratios Fe/SiO2 = 1.26 and CaO/SiO2 = 1.027. It is worth to note that the chemical analysis reported 3.7 mas. % of sulphur, which is considered as ZnS since this sulphide is thermodynamically more stable than FeS and PbS. To estimate the effect of the CaO/SiO2 and Fe/SiO2 ratios on the viscosity and the solid fraction at high temperature, reagent-grade chemicals (above 99.5 mas. % purity) of SiO2, CaO and Fe2O3 powders were added to the master slag. The slag compositions used in the viscosity and the equilibrium-quenching trials are reported in Table 2.
Procedure to Estimate Slag Viscosity
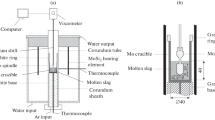
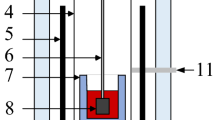
The viscosity was measured by the rotating cylinder method. The scheme of the apparatus used is shown in Fig. 1a, where the slag sample was melted in a chrome-magnesite crucible 4.4 mm in diameter and 80 mm in height and placed in a vertical tubular resistance furnace, using a molybdenum spindle. The industrial slag contains some sulphur, so argon atmosphere were used as a protective gas to prevent the corrosion at high temperature. The temperature was measured by a Pt/Pt–Rh 10 thermocouple. The system was calibrated using a standard oil with known viscosity for the range from 0.29 to 1.8 Pa⋅s at 298 K. The reproducibility of the measurements was approximately ±10%.
Equilibrium-Quenching Trials
Figure 1b shows a schematic illustration of the experimental setup to estimate the mineralogical species and the fraction of solid in equilibrium with liquid slag at high temperature. It consists of a tubular resistance furnace where the tube was of alumina with an internal diameter of 65 mm. 20 g of each slag sample with the composition given in Table 2 were homogenized and equilibrated in molybdenum crucible (15 mm inner diameter and 40 height) in argon, at 1300°C. After holding for 2 h at the set temperature (the time required to achieve equilibrium between the condensed phases of slag) the samples were quenched in iced water. On rapid cooling, the silica-containing liquid phase converts to glass so that the phase assemblage that exists at high temperature is frozen.
The phases present were determined by first mounting and polishing the samples, and then by examination using optical microscopy and scanning electron microscopy coupled with an energy-dispersive spectra analyzer (FEI Quanta 600, EDAX EDS). Samples of each slag were also characterized by X-ray diffraction (XRD Bruker D8 Focus) using CuKα (λ = 1.5406 Å) radiation over a 2θ of 20° to 100° at a speed of 2 min–1 to determine the mineralogical species.
Viscosity Calculations Using FactSage and the Einstein–Roscoe Equation
The simple and most well-known model based on Einstein’s approach was due to Roscoe. The Einstein–Roscoe type equation is given as follows [8, 9, 17–21]:
where η and ηL are the viscosity of the solid-containing and the solid-free melt, respectively, ϕs is the volume fraction of solid particles in the melt, a and n are empirical parameters that depend on the morphology of the solids. a and n are considered equal to 1 and 2.5, respectively, for rigid spheres of diverse sizes [9]. The volume fraction of solids is expressed as:
where Vs and VL are the volumes of the crystallized solids and the residual liquid, ν is the partial molar volume, m is mass and M is molar mass of the component in solid or liquid phases.
The values of the molar partial volume are not very well-known for liquid slags, so in the present work the partial molar volumes are assumed to be equivalent to the molar volumes of pure components which are shown in Table 3.
FactSage consists of several thermodynamic calculation modules such as (1) Reaction, (2) Equilib, (3) Predom, (3) Viscosity, etc. Multiphase equilibrium in the present study is calculated by using the “Equilib” module [14]. Gibbs free energy minimization and the modified non-ideal associate species models are used for the phase equilibrium calculations. The “Viscosity” module calculates the viscosity of a liquid slag solution at a given temperature and composition. The flow sheet to estimate the viscosity of a solid + liquid mixture using the Factsage software and the Einstein–Roscoe equation is shown in Fig. 2.
RESULTS AND DISCUSSION
Thermodynamic and Experimental Viscosity Results
The FactSage computational thermodynamic package [14] can be used to determine the equilibrium species in the slag since it contains an extensive optimized solution database. This system uses the quasi-chemical approximation [22] for the liquid oxide-based slags. The general free energy minimization method is used to estimate the equilibrium concentration once the models and the thermodynamic properties of the species in the system have been selected. For a given set of constraints (such as fixed temperature, pressure and overall concentration), the free energy minimization algorithm finds the set of mole numbers of each species as well as the compositions of all solution phases which globally minimize the free energy function. This software was used to estimate the liquid and solid phase distribution for the slags in terms of slag basicity (CaO/SiO2) and Fe/SiO2 mass ratios and 1300°C (1573 K).
Figures 3, 4 show the amount of liquid and solid phases at equilibrium at 1300°C in terms of CaO/SiO2 mass ratio for slags with Fe/SiO2 ratio of 1.26 and 1.7, respectively. These figures show that increasing the slag basicity the amount of liquid phase is increased at both Fe/SiO2 ratios. It is also observed that a given basicity the liquid is slightly higher for slags with low Fe/SiO2 ratio (1.26). The main solid phases calculated by the FactSage software are wurtzite (ZnS), pyrite (FeS2) and spinel, which is a solid solution mainly formed by zinc ferrite (ZnFe2O4). It is observed that most of the sulphur content is associated with zinc.
FactSage only calculate the amount of equilibrium phases in mass (g/mol). To estimate the molar volume of the liquid phases the results in mass are combined with the molar volume given in Table 3 and equation 3. The volume of each solid is calculated considering the reported density, 5 g cm–3 for pyrite and 3.98 g cm–3 for wurzite. Spinel is considered mainly formed by ZnFe2O4 with density 5.322 g cm–3. The volume fraction of solid is straightforward calculated with equation 2.
The fraction of solid phases and composition of the liquid slag phase at different CaO/SiO2 and Fe/SiO2 ratios were calculated by using the module “Equilib” of the FactSage software. The viscosity of the solid-free liquid (ηL) was calculated through the module “Viscosity”. The mass fraction of solids was transformed to volume fraction and then the Einstein–Roscoe equation was used to estimate the viscosity of the slurry system.
Figures 5, 6 show the calculated and the experimental viscosities results of the slags with Fe/SiO2 ratio of 1.26 and 1.7, respectively. From these calculations, it is evident that viscosity value increases by increasing the Fe/SiO2 due to the increase of the volume fraction of solids. These figures also show that there is a qualitative agreement between the experimental and calculated results, where it is observed that the viscosity decreases as the slag basicity is increased. However, there are discrepancies between experimental and calculated viscosities which may be mainly due to the fact that the lead blast furnace slag contains many components and there is no thermodynamic database to perform the phase equilibrium calculation considering all compositions and temperatures of this silicate-based complex system. The FactSage software performs an extrapolation of thermodynamic properties to determine the phase equilibrium at compositions and temperatures that have not been obtained experimentally. Another cause of the differences between experimental and calculated results is the fact that the Viscosity module of the FactSage program does not consider the presence of metal sulphides. Finally, the Einstein–Roscoe equation employs the empirical shape parameters “a” and “n” which might vary according to the size and shape of the solid particles.
Microstructure
There is no experimental evidence that at the temperature tested (1300°C) there exists in equilibrium a mixture of liquid slag and solid phases in the lead blast furnace process, due to the many components present in this industrial system, although thermodynamically the presence of these phases in equilibrium was determined using the FactSage software. For this reason, equilibrium and quenching tests were carried out in this work, considering the same compositions of the slags used in the viscosity tests, reported in Table 2.
The microstructures of the quenched slags with Fe/SiO2 = 1.26 and CaO/SiO2 = 0.4 and 1.2 are shown in Fig. 7. It is observed that at 1300°C the slag is partly melted and the rapid cooling to room temperature produces a dendritic structure. The SEM-EDS results show that in Fig. 7a the solid phases in equilibrium with liquid are wustite (FeO) and zinc ferrite (ZnFe2O4) for the system with low slag basicity (CaO/SiO2 = 0.4). There were also observed in this sample rounded particles with combined metallic sulphide phases, pyrite (FeS2) and wurtzite (ZnS). The slag with high slag basicity (CaO/SiO2 = 1.2) also shows solid particles of wustite and zinc ferrite together with pyroxene hedenbergite CaFeSi2O6.
The micrographs of the quenched slags were used to estimate the volume fraction of solids through an image analyzer, such as is shown in Fig. 8. These images correspond to slags with CaO/SiO2 = 1.027. The experimental volume fractions of solids are 26.1 and 31.9% for slags with Fe/SiO2 with 1.26 and 1.7, respectively. These results are close to the calculated volume fractions of solids calculated by the software FactSage, which are 25.7 and 28.1%, respectively. The average difference between the experimental and the calculated volume fraction of solids were less than 10%.
The X-ray diffraction (XRD) analysis was used to confirm phase identification carried out by SEM-EDS. Figures 9, 10 show the XRD patterns for slags with different Fe/SiO2 ratio and CaO/SiO2 being 0.4 and 1.2, respectively, equilibrated at 1300°C and quenched to room temperature. Zinc ferrite ZnFe2O4 (JCPD file 22-1012), wustite Fe0.932O (JCPD file 06-0615) and CaFeSi2O6 (JCPD file 70-1876) were observed at most of the experiment conditions as the main solid phases obtained in these slags. Small peaks of wurtzite ZnS (JCPD file 39-1363) and CaO (JCPD file 37-1497) were also observed. It is worth noting that the presence of crystals of CaO and CaFeSi2O6 were not clearly observed in the SEM-EDS microanalysis. These figures also show that increasing the Fe/SiO2 basicity, the amount of wustite and zinc ferrite was increased.
The thermodynamically determined solid species were spinel, which consists mainly of zinc ferrite, and two sulphides (FeS2 and ZnS). It should be mentioned that wustite (Fe0.932O) was not determined as a solid phase but it was reported as a component of the liquid slag in the equilibrium calculations; however, this phase was detected in the experimental tests by XRD and SEM-EDS.
Park et al. [23] studied the viscous behavior of FeOt–Al2O3–SiO2 copper smelting slags and found that the slag viscosity as a function of Fe/SiO2 ratio showed a common pattern of decreasing viscosity with increasing Fe/SiO2. Osugi et al. [24] also found that the viscosity of silicate melts decreased with increasing the amount of FeO, since it behaves as network modifier and depolymerizes the silicate melts. Unlike Park’s and Osugi’s works, we observed that the slag viscosity is increased by increasing the Fe/SiO2 ratio. The reason is that in the slags from the lead blast furnace at 1300°C the fraction of solid phases, as well as the viscosity of the slag, increase as Fe/SiO2 increases.
Despite the simplifications made in this work to calculate the viscosity of a heterogeneous slag using the FactSage thermodynamic software and the Einstein–Roscoe, this method provides an insight of the rheological behavior of complex silicate-based slags.
CONCLUSIONS
An experimental study of the viscosity and the equilibrium and quenching of slags from the lead blast furnace with the mass ratios: CaO/SiO2 = 0.4 to 1.2 and Fe/SiO2 = 1.26 and 1.7 at 1300°C was carried out. The major results are summarized as follows:
Change in the CaO/SiO2 ratio from 0.4 to 1.2 lowers the solid fractions as well as the slag viscosity. Whereas a change in the Fe/SiO2 ratio from 1.26 to 1.7 increases the fraction of solid phases and the slag viscosity.
The calculated slag viscosity combining the Factsage thermodynamic software and the Einstein–Roscoe equation provides a method to have an insight of the rheological behavior of heterogeneous slags systems at high temperature. The experimental viscosity behavior compares qualitatively with the calculated results.
The equilibrium-quenching experiments and thermodynamic modelling show similar solid fractions in slags at 1300°C. These results also agree in the formation of spinel, mainly formed by ZnFe2O4, wurzite and pyrite as solid phases. Wustite was also obtained experimentally, even though it was not obtained in the thermodynamic calculations.
REFERENCES
Zhang, G.-H., Chou, K.-C., Xue, Q.-G., and Mills, K., Modeling viscosities of CaO–MgO–FeO–MnO–SiO2 molten slags, Metall. Mater. Trans. B, 2012, vol. 43, pp. 64–72.
Lopez Rodriguez, J., Romero-Serrano, A., Hernández, A., Pérez Labra, M., Cruz, A., and Rivera, E., Use of a structural model to calculate the viscosity of liquid silicate systems, ISIJ Int., 2018, vol. 58, pp. 220–226.
Han, C., Chen, M., Zhang, W., Zhao, Z., Evans, T., and Zhao, B., Evaluation of existing viscosity data and models and developments of new viscosity model for fully liquid slag in the SiO2–Al2O3–CaO–MgO system, Metall. Mater. Trans. B, 2016, vol. 47, pp. 2861–2874.
Kim, W., Pelton, A.D., and Decterov, S.A., Modeling the viscosity of silicate melts containing lead oxide, Metall. Mater. Trans. B, 2012, vol. 43, pp. 325–336.
Sycheva, G.A. and Polyakova, I.G., Volume nucleation of crystals in glass based on blast-furnace slag, Glass Phys. Chem., 2013, vol. 39, pp. 248–260.
Sycheva, G.A., Polyakova, I.G., and Kostyreva, T.G., Volumetric nucleation of crystals catalyzed by Cr2O3 in glass based on furnace slags, Glass Phys. Chem., 2016, vol. 42, pp. 238–245.
Sycheva, G.A., Nucleation of crystals in glass based on blast-furnace slag: Influence of chemical differentiation on the process of nucleation, Glass Phys. Chem., 2019, vol. 45, pp. 19–28.
Einstein, A., A new determination of molecular dimensions, Ann. Phys., 1906, vol. 324, pp. 289–306.
Roscoe, R., The viscosity of suspensions of rigid spheres, J. Appl. Phys., 1952, vol. 3, pp. 267–69.
Kondratiev, A. and Jak, E., Modeling of viscosities of the partly crystallized slags in the Al2O3–CaO–FeO–SiO2 system, Metall. Mater. Trans. B, 2001, vol. 32, pp. 1027–1032.
Liu, Z., Pandelaers, L., Blanpain, B., and Guo, M., Viscosity of heterogeneous silicate melts: A review, Metall. Mater. Trans. B, 2018, vol. 49, pp. 2469–2486.
Wang, L., Zhang, C., Cai, D., Zhang, J., Sasaki, Y., and Ostrovski, O., Effect of CaO/SiO2 ratio and Na2O content on melting properties and viscosity of SiO2–CaO–Al2O3–B2O3–Na2O mold fluxes, Metall. Mater. Trans. B, 2017, vol. 48, pp. 516–526.
Wright, S., Zhang, L., Sun, S., and Jahanshahi, S., Viscosity of a CaO–MgO–Al2O3–SiO2 melt containing spinel particles at 1646 K, Metall. Mater. Trans. B, 2000, vol. 31, pp. 97–104.
Bale, C.W., Bélisle, E., Chartrand, P., Decterov, S.A., Eriksson, G., Gheribi, A.E., Hack, K., Jung, I.H., Kang, Y.B., Melançon, J., Pelton, A.D., Petersen, S., Robelin, C., Sangster, J., and van Ende, M.-A., FactSage thermochemical software and databases, 2010–2016, CALPHAD, 2016, vol. 54, pp. 35–53.
Mishra, P.R., Sahu, R., and Chakravarty, S., Viscosity analysis of indian origin coal by using factsage at different temperatures, Trans. Indian Inst. Met., 2020, vol. 73, pp. 207–214.
Chaidez-Felix, J., Romero-Serrano, A., Hernandez-Ramirez, A., Perez-Labra, M., Almaguer-Guzman, I., Benavides-Perez, R., and Flores-Favela, M., Effect of copper, sulfur, arsenic and antimony on silver distribution in phases of lead blast furnace, Trans. Nonferr. Met. Soc. China, 2014, vol. 24, pp. 1202–1209.
Zhen, Y.-L., Zhang, G.-H., and Chou, K.-C., Viscosity of CaO–MgO–Al2O3–SiO2–TiO2 melts containing TiC particles, Metall. Mater. Trans. B, 2015, vol. 46, pp. 155–161.
Fujino, S., Hwang, C., and Morinaga, H., Density, Surface tension, and viscosity of PbO–B2O3–SiO2 glass melts, J. Am. Ceram. Soc., 2004, vol. 87, pp. 10–16.
Penttilä, K., Modelling of thermophysical properties in EAF-process and steelmaking, Research Report VTT-R-00514-14, Technical Research Centre of Finland, 2013.
Mills, K.C., Kargadde, S., Lee, P.D., Yuan, L., and Shahbazian, F., Calculation of physical properties for use in models of continuous casting process, Part 1: Mould slags, ISIJ Int., 2016, vol. 56, pp. 264–273.
Von Marcel Potuzak, V., Physico-chemical properties of silicate melts, Ph.D. Thesis, München: München Univ., 2006.
Blander, M. and Pelton, A.D., Thermodynamic analysis of binary liquid slags and prediction of ternary slag properties by modified quasichemical equations, Geochim. Cosmochim. Acta, 1987, vol. 51, pp. 85–95.
Park, H.-S., Park, S.-S., and Sohn, I., The viscous behavior of FeOt–Al2O3–SiO2 copper smelting slags, Metall. Mater. Trans. B, 2011, vol. 42, pp. 692–699.
Osugi, T., Sukenaga, S., Inatomi, Y., Gonfa, Y., Saito, N., and Nakashima, K., Effect of oxidation state of iron ions on the viscosity of alkali silicate melt, ISIJ Int., 2013, vol. 53, pp. 185–190.
ACKNOWLEDGMENTS
The authors wish to thank the company Servicios Administrativos Peñoles S.A. de C.V., and the institutions National Council for Science and Technology (CONACYT) and National Polytechnic Institute for the support of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Manuel Flores-Favela, Pelaez-Ramirez, H., López-Rodriguez, J. et al. Effect of CaO/SiO2 and Fe/SiO2 Ratios on the Viscosity at 1300°C of Partly Crystallized Silicate Slags. Glass Phys Chem 47, 75–82 (2021). https://doi.org/10.1134/S1087659621020048
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659621020048