Abstract
Controlled rolling followed by accelerated cooling was carried out in-house to study the microstructure and mechanical properties of a low carbon dual-phase steel. The objective of the study described here was to explore the effect of cooling schedule, such as air cooling temperature and coiling temperature, on the final microstructure and mechanical properties of dual-phase steels. Furthermore, the precipitation behavior and yield ratio are discussed. The study demonstrates that it is possible to obtain tensile strength and elongation of 780 MPa and 22 pct, respectively, at the two cooling schedules investigated. The microstructure consists of 90 pct ferrite and 10 pct martensite when subjected to moderate air cooling and low temperature coiling, such that the yield ratio is a low 0.69. The microstructure consists of 75 pct ferrite and 25 pct granular bainite with a high yield ratio of 0.84 when the steel is directly cooled to the coiling temperature. Compared to the conventional dual-phase steels, the high yield strength is attributed to precipitation hardening induced by nanoscale TiC particles and solid solution strengthening by high Si content. The interphase precipitates form at a suitable ledge mobility, and the row spacing changes with the rate of ferrite transformation. There are different orientations of the rows in the same grain because of the different growth directions of the ferrite grain boundaries, and the interface of the two colonies is devoid of precipitates because of the competitive mechanisms of the two orientations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
High-strength low-alloy (HSLA) steels are widely used for automotive applications, primarily from the viewpoint of fuel efficiency and weight saving. The high strength is mainly attributed to microalloying additions (e.g., Nb, V, and Ti) and thermo-mechanical controlled processing (TMCP). Recently, HSLA steels with tensile strength of 780 MPa have been used for vehicle components.[1–7] It is realized as an important branch of HSLA steels that advanced high-strength steels (AHSS) such as ferrite/martensite dual-phase (FMDP) steel and ferrite/bainite dual-phase (FBDP) steel can be cost effective with a good combination of strength and ductility.[8,9] The dual-phase (DP) steels are ductile because of the presence of a soft ferrite phase and the strength is attributed to hard martensite or bainite constituent. The conventional ferrite/martensite dual-phase steels are characterized by low yield ratio (yield strength/tensile strength) because of the significant difference in hardness of the two phases. The low yield ratio means high resistance to deformation from yielding to plastic instability. However, newer engineering specifications require high yield strength.[10] Thus, it is important to obtain both high yield strength and low yield ratio.[11] The application area of soft ferrite and hard martensite DP steels is limited because the interface of the two phases is identified as the site for nucleation of voids during ductile fracture. The martensite–ferrite interface strength can approach 2.4 to 2.5 GPa.[12,13] Nanoscale TiC particles play a significant role in precipitation hardening and consequently influence yield strength of DP steels.[14] Moreover, high Si addition is also considered to increase the yield strength by substitutional solid solution strengthening and promoting TiC precipitation.[9,15] From the perspective of obtaining a different yield ratio, it is meaningful to develop a new low carbon high-strength DP steel and study the variation of yield ratio in steels subjected to different cooling schedules. This aspect has not been studied to the best of our understanding.
Currently, there is strong interest in using as-hot-rolled DP steels in the place of intercritically heat-treated DP steels because of cost and energy considerations and inherent metallurgical disadvantages, such as residual stresses, quench cracking, and distortion.[6] In this study, we describe the microstructure and mechanical properties of low cost high yield strength as-hot-rolled DP strip steels. These steels with different yield ratios were successfully processed at the laboratory scale using different cooling practices. Furthermore, the microstructural evolution, precipitation behavior, and the resultant yield ratio are discussed.
2 Experimental
2.1 Materials and Thermo-mechanical Processing Condition
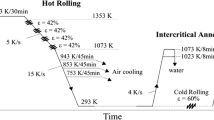
The experimental steel was melted in a vacuum induction furnace and cast into 150-kg ingots. The chemical composition in wt pct was 0.08 C, 1.5 Si, 1.7 Mn, 0.03 Al, 0.1 Ti, and balance Fe. The excellent welding performance is insured because of low carbon content. The 40-mm-thick slabs were heated to 1473 K (1200 °C) for 1 hour to dissolve the microalloying elements and then air cooled to 1223 K (950 °C). The slabs were hot rolled into steel plates with 6 mm thickness after seven passes on the Φ450-mm rolling mill. The end temperature of the finish rolling was controlled to 1123 K (850 °C). The plates were subjected to three different cooling practices. Steels A and B were water cooled to the air cooling temperatures and then held for 10 seconds on the run-out table in the air (air cooling rate: ~5 K/s (°C/s)). Next, the plates were accelerated cooled to the coiling temperature (Table I). Finally, they were cooled slowly to room temperature in an insulated blanket. Steel C was directly water cooled to the coiling temperature without the air cooling process. The schematic diagram illustrating the TMCP and the procedure of specific cooling practice are presented in Figure 1 and Table I, respectively.
2.2 Tensile Test
The tensile tests were conducted at room temperature using a computerized tensile testing system. Steel sheets were cut into dog bone-shaped specimens (dimensions: 50 mm gage length and 12.5 mm width), and the crosshead speed was 3 mm/min.
2.3 Microstructural Characterization
Metallographic specimens were polished and etched with 4 pct nital before investigation using a Leica DMIRM optical microscope (OM) and an FEI Quanta 600 scanning electron microscope (SEM). Transmission electron microscopy (TEM) studies were conducted on 3-mm-diameter thin foils, which were electropolished using a solution of 8 pct perchloric acid alcohol, and examined by an FEI Tecnai G2 F20 TEM at an accelerating voltage of 200 kV. The precipitates were characterized by energy-dispersive X-ray spectroscopy (EDX).
3 Results
3.1 Microstructural Evolution
The OM, SEM, and TEM micrographs of steel A are presented in Figure 2. The microstructure consists of ~10 to 15 μm coarse polygonal ferrite (PF), ~3 to 5 μm fine-grained PF, and granular bainite (GB). The fraction of PF and GB was ~80 and ~20 pct, respectively, as estimated from Figures 2(a) and (b). Figures 2(c) through (e) are TEM micrographs of fine-grained PF. Some ferrite grains contained a high density of dislocations. The granular bainite-ferrite (BF) laths and martensite/austenite (M/A) constituents are presented in Figure 2(f). The width of BF is ~0.5 μm and the size of M/A constituent is ~0.5 to 1 μm.
Figure 3 shows precipitates in steel A. A majority of interphase precipitates in rows are ~5 nm in size and the interphase spacing is ~10 to 20 nm. The random precipitates in the ferrite matrix are smaller than ~10 nm. There are, however, some interphase precipitates of size ~30 nm with large spacing. The coarse precipitates of 20 nm are assumed to be formed at the sub-boundaries. A representative 12-nm precipitate analyzed by EDX was TiC (Figure 3(e)). Figure 2(e) shows the interphase precipitates present among a high density of dislocations. During examination by TEM, the row formation of the interphase precipitates is observed only when the precipitates’ rows are oriented approximately parallel to the incident electron beam. In all other orientations, interphase particles will exhibit an apparently random distribution. Random particle distribution should be observed more frequently than the events of row formation.[16] Moreover, the orientation relationship of TiC interphase precipitates disappears at longer times during isothermal transformation.[17]
The OM, SEM, and TEM micrographs of steel B are shown in Figure 4. The microstructure consists of ~90 pct PF and ~10 pct martensite, as shown in Figures 4(a) and (b). Compared to steel A, finer ferrite is obtained at the lower air cooling temperature of 943 K (670 °C). The start temperature of martensite transformation (M s) was calculated to be 716 K (443 °C) using empirical Eq. [1].[18] Thus, the martensite forms at the low coiling temperature of 623 K (350 °C). The morphology of ferrite and high density of dislocations are presented in Figures 4(c) and (d). The martensitic laths in Figures 4(e) and (f) have a width of ~200 to 300 nm. The morphologies of precipitates in steel B are shown in Figure 5. There is a high volume fraction of precipitates in part of the ferritic matrix, while a lower volume fraction of precipitates in the rest of the matrix.
The OM, SEM, and TEM micrographs of steel C are presented in Figure 6. The microstructure consists of ~75 pct ferrite and ~25 pct GB. Compared to steels A and B, the ferrite is significantly refined because of higher supercooling. Moreover, a higher proportion of GB forms due to the absence of the air cooling process, as shown in Figures 6(a) and (b). Figures 6(c) and (d) illustrate the morphology of PF and GB. In general, the M/A constituent consists of single martensite, single retained austenite, or the alternative layers of retained austenite and martensite, which was systematically studied and characterized by Misra and co-workers.[19] As shown in Figures 6(e) and (f), the M/A constituent is ~1 μm in size. During the slow cooling process, the undercooled austenite first transforms to ferrite and the untransformed austenite becomes C-enriched. On further decreasing the transformation temperature, part of the untransformed austenite transforms to BF, and the balance is stabilized with high C content. When C-enriched austenite is cooled to a temperature below the M s, it is partially or totally transformed to martensite and forms the M/A constituent.
Figure 7 shows the TEM micrographs of precipitates in steel C. The precipitates in steel C are ~3 to 5 nm and the interphase spacing is significantly reduced. Part of the region has a low volume fraction of random precipitates (Figures 7(a) and (b)). The interphase spacing varies from 5 to 20 nm, as shown in Figure 7(c). The orientation of interphase precipitates is different in the same ferrite grain, and the interphase spacing is different in each precipitation colony of the same orientation. Moreover, the interface of the two colonies is devoid of precipitates (Figure 7(d)).
3.2 Mechanical Properties
The yield strength, tensile strength, elongation after fracture, and yield ratio obtained from tensile tests of high-strength DP steels are listed in Table II. In steel A, the yield strength, tensile strength, elongation, and yield ratio are 635 MPa, 765 MPa, 21.6 pct, and 0.83, respectively; in steel B are 540 MPa, 785 MPa, 22 pct, and 0.69, respectively; and in steel C are 659 MPa, 788 MPa, 22.6 pct, and 0.84, respectively. The varied yield ratio is obtained when steels are subjected to different cooling schedules. It can be seen that a good combination of strength and ductility is obtained.
4 Discussion
4.1 Microstructure
The microstructures of steels A, B, and C were characterized by 80 pct PF + 20 pct GB, 90 pct PF + 10 pct M, and 75 pct PF + 25 pct GB, respectively. The microstructural constituents, morphology, and fraction are governed by the cooling practice. A schematic diagram summarizing the microstructural evolution for three different cooling practices is presented in Figure 8. The start temperature of water cooling is 1123 K (850 °C), and the microstructure is pancaked austenite due to heavy deformation in the austenitic non-crystallization zone. During high temperature air cooling from 1003 K (730 °C) of steel A, the coarse PF forms due to the lack of supercooling and high grain coarsening rate. The formation of PF accompanies diffusion of C atom, and the nearby untransformed austenite is gradually enriched with carbon as PF forms. The untransformed austenite is stabilized due to high C content. The 3 to 5 μm fine-grained PF and GB form during the slow cooling process, and a large fraction of untransformed austenite is retained at room temperature, as shown in Figure 2(f). Compared to steel A, the PF is refined due to lower air cooling temperature in steel B. On water cooling to the coiling temperature of 623 K (350 °C), martensite forms. In steel C, without the air cooling process, the stored energy and defects generated by deformation are preserved until the coiling process. Furthermore, more transformation nucleation sites are generated because of high supercooling, and consequently the grain size is refined. The M/A constituent is predominately composed of alternative M/RA or M because of low C content (Figures 6(e) and (f)).
4.2 Precipitation Behavior
There are two types of precipitates in the experimental steels, namely, the interphase precipitates and random precipitates. The precipitation of TiC particles can occur either randomly in ferrite in the wake of the migrating austenite–ferrite (γ/α) interface (random precipitation) or by developing the sheets of particles parallel to the γ/α interface formed repeatedly (interphase precipitation).[20] Honeycombe and Mehl[21] suggested a ledge mechanism for the planar type of interphase precipitation. Ricks and Howell[22] stated that the precipitates can be arranged in periodical but non-planar sheets (curved). The quasi-ledge mechanism for the non-planar type of interphase precipitation was suggested. Although the mechanism of the interphase precipitation is controversial, the experimental evidence reveals that it nucleates at the γ/α interphase.[23,24] The realization of interphase precipitation requires suitable mobility of the transforming γ/α interphase, which is determined by the driving force of precipitation and diffusion coefficient of microalloyed atoms. It is expected that in the high temperature region, when the chemical driving force for precipitation is low, the γ/α interphase is energetically favorable for nucleation.[20] The mobility of the γ/α interphase is slow because of low supercooling. Furthermore, the high diffusion coefficient of the microalloyed substitutional atoms enables precipitation to occur in a short time. At low temperature, the mobility of the γ/α interphase increases due to high supercooling and the diffusion coefficient is significantly reduced. Random precipitation occurs in place of the interphase precipitation in the supersaturated ferrite during the coiling process.
In steel A, the interphase precipitation occurs during the air cooling process at 1003 K (730 °C), and random precipitates form during coiling at 803 K (530 °C). In general, the interphase precipitation occurs in part of the grain starting at high transformation temperature and does not undergo completion such that the general precipitation occurs in the remaining part of the grain at lower temperatures. If interphase precipitation occurs throughout the grain, then there is limited thermodynamic potential in the same grain for random precipitation to occur. Moreover, these nanoscale precipitates are recognized to be stable and highly resistant to coarsening because of low surface energy.[25] A majority of the interphase precipitates are within 5 nm, and the interphase spacing is 10 to 20 nm, while some interphase spacing is 30 nm due to the slow ferritic growth rate at high air cooling temperature. It is well recognized that the dislocations and sub-boundary are heterogeneous nucleation sites for random precipitates. The interphase precipitation occurs with austenite-to-ferrite transformation, and the dislocations are generated during austenite-to-ferrite transformation by the transformation stress, which is derived from the lattice structural change from face-centered cubic to body-centered cubic. The formation of interphase precipitates and generation of dislocations are simultaneous. Therefore, the effect of the dislocations on the interphase precipitates is complex and should be quite different from random precipitates. However, the random precipitates can nucleate at dislocations and sub-boundaries, and these defects are the preferential nucleation sites for random precipitates because they are the diffusion pipelines of the atoms, as shown in Figure 3(d).
In steel B, the precipitates form at 943 K (670 °C) during the air cooling process. Precipitation rarely occurs at 623 K (350 °C) during coiling process because of the low diffusion coefficient of the substitutional atom, Ti. Therefore, a large fraction of precipitates forms within 10 seconds facilitated by high Si addition. This phenomenon is attributed to increase in the activity of interstitial atoms (C and N) and diffusivity of microalloyed atoms, Ti.[15,26] However, some regions were devoid of precipitates (Figure 5(a)).
In steel C, the interphase precipitates are finer and the sheet spacing was reduced because of the larger ledge mobility of γ/α interphase at higher supercooling. The growth of allotriomorphic ferrite occurs following the parabolic growth law, and the thickness of the allotriomorphic ferrite during isothermal growth is proportional to t 1/2, where t is time required to initiate growth. The total amount of solute partitioned during growth increases with time. Consequently, the diffusion distance increases with time, and the growth rate slows down with the ferrite growth.[27,28] Thus, during the initiation of the ferrite transformation, the interphase precipitates are unable to form due to the large ledge mobility of the γ/α interphase, where the concentration of the solute atoms is insufficient. As the ferritic transformation rate slows down, interphase precipitates with small interphase spacing are generated. By further decreasing the transformation rate, the interphase spacing is enlarged gradually, as shown in Figure 7(c). The different row orientations of the interphase precipitates in the same grain are assumed to be due to the different growing directions of the polygonal ferrite grain boundaries. The different spacing widths in each precipitation colony are attributed to the different growing rates of the polygonal ferrite grain boundaries. The region devoid of precipitates is a result of competition mechanism between the two colonies, as shown in Figure 7(d). It is assumed that some rows at the interface of two colonies are distorted due to the interaction of the two ledges with different orientations. In the experimental steel, both the planar and curved interphase precipitates were observed, and the morphology seems to mainly depend on the migrating γ/α interphase, while the interaction of the two colonies with different orientations could also have an effect on the morphology of the interphase precipitates.
4.3 Mechanical Properties
In general, for the FMDP and FBDP steels, the ferrite is the soft phase and the bainite or martensite is the hard phase. The soft phase provides good ductility, and the hard phase is the load-bearing phase. In the tensile test, plastic yielding starts in the soft ferrite, and the hard bainite or martensite remains in the elastic state. During the plastic deformation of the ferrite, stress is transferred to the bainite or martensite. Internal stresses at the interface of ferrite/bainite or ferrite/martensite are built up due to the plastic strain incompatibility. At this stage, the strain-hardening rate is very high as a consequence of rapid dislocation multiplication and the back stresses resulting from the strain incompatibility. When the transferred stress is large enough to reach the elastic limit of the bainite or martensite, it starts to deform plastically. In our DP steels, the low strength difference between two phases promotes plastic deformation of the hard phase, and the yield ratio increases.[29–31] In the conventional DP steel devoid of precipitates in ferrite, the yield strength mainly depends on the soft phase and the tensile strength is dominated by the joint effect of the two phases. On the other hand, in the modern FBDP steel,[6] the ferrite is strengthened by multiple mechanisms, such as solid solution strengthening from substitutional atoms Si and Mn, precipitation hardening from the nanoscale carbide particles, effective grain boundary strengthening, and dislocation hardening. Consequently, the hardness difference of the ferrite and GB is significantly reduced. Therefore, plastic yielding starts in the hardened ferrite and part of GB due to the compatible deformation of the two phases. The bainitic transformation strengthening comes from the M/A constituent and bainitic laths play additional roles in enhancing the yield strength.
In steel A, the microstructure is coarse-grained ferrite and GB. There is a high volume fraction of the interphase precipitates and random precipitates in the matrix of ferrite formed during the air cooling process and coiling process, respectively, and the precipitates are nanoscale and with ~10- to 20-nm interparticle spacing. Therefore, the precipitation hardening plays a significant role on yield strength. The high yield strength is also attributed to the dislocation strengthening and GB transformation strengthening, while the M/A constituent in steel A is mainly composed of retained austenite, which is thought to decrease the yield strength. Moreover, the large ferritic grain size provides a weak effective grain boundary strengthening effect. Therefore, the yield strength of steel A is medium among the three steels. In steel B, the microstructure consists of ferrite and martensite. The volume fraction of precipitates is low due to low coiling temperature. Because of the large hardness difference between the ferrite and martensite phase, in the tensile test, the plastic yielding starts in soft ferrite and martensite remains in the elastic state. Moreover, a high density of mobile dislocations is generated during austenite-to-martensite transformation.[32] The yield strength is significantly reduced. After plastic deformation of ferrite, the martensite deforms elastically and the tensile strength increases. Thus, low yield ratio is obtained. The yield strength is still higher than the conventional FMDP steel because of the effect of precipitation strengthening, effective grain boundary strengthening, and solid solution strengthening. In steel C, the microstructure is fine-grained ferrite and GB. The grain size of ferrite is significantly refined. The interphase precipitates are ~3 to 5 nm with ~5- to 20-nm interphase spacing. Furthermore, the M/A constituent is changed into the alternative M/RA or M. Thus, the increased effective grain boundary strengthening, precipitation hardening, and bainitic transformation strengthening contribute to the highest yield strength.
In summary, the cooling schedules have a significant effect on the microstructure and mechanical properties of the experimental steels. In steel B and C, the tensile strength and elongation are ~780 MPa and ~22 pct, respectively. In order to obtain low yield ratio, steels should be subjected to the cooling schedule of moderate air cooling and low temperature coiling, and the microstructure consists of polygonal ferrite and martensite. To achieve the high yield ratio, steel should be subjected to the cooling schedule of moderate coiling without air cooling, and the microstructure is composed of PF ferrite and GB. Compared to the conventional dual-phase steel, the high yield strength is due to the precipitation hardening of nanoscale TiC particles and solid solution strengthening of high Si. The yield ratio of the experimental steels is lower than that of the conventional 780 MPa grade HSLA steel.
5 Conclusions
We have studied here the microstructure and mechanical properties of the as-hot-rolled Ti microalloyed DP steels for three different cooling practices and the variation in the yield ratio is discussed. The conclusions are as follows:
-
(1)
The microstructure of the experimental steel subjected to moderate air cooling temperature of 943 K (670 °C) and low coiling temperature of 623 K (350 °C) consists of ~90 pct polygonal ferrite and ~10 pct martensite. The yield strength, tensile strength, and elongation are 540 MPa, 785MPa, and 22 pct, respectively. The low yield ratio of 0.69 is attributed to the low volume fraction of precipitates with large interparticle spacing, the incompatible elastic deformation ability of the ferrite/martensite, and the mobile dislocation at the interface generated by the austenite-to-martensite transformation.
-
(2)
The microstructure of the experimental steel subjected to moderate coiling temperature of 893 K (620 °C) and without the air cooling process consists of ~75 pct polygonal ferrite and ~25 pct granular bainite. The yield strength, tensile strength, and elongation are 659 MPa, 788 MPa, and ~23 pct, respectively. The high yield ratio of 0.84 is attributed to the high volume fraction of precipitates with small interparticle spacing and the compatible elastic deformation ability of the ferrite/granular bainite.
-
(3)
The microstructure of the experimental steel subjected to high air cooling temperature of 1003 K (730 °C) and moderate coiling temperature of 803 K (530 °C) consists of ~80 pct coarse-grained polygonal ferrite and ~20 pct granular bainite. This steel possesses similar yield ratio of 0.83, but inferior tensile properties compared to the steel subjected to direct coiling.
-
(4)
In comparison to the conventional dual-phase steel, the high yield strength is due to the precipitation hardening of nanoscale TiC particles and solid solution strengthening of high Si.
-
(5)
The precipitation rate of TiC particles increases because of high Si addition. The interphase precipitates form at suitable ledge mobility, and the row spacing changes with the ferritic transformation rate. There are different orientations of the rows in the same grain because of the different growing directions of the ferritic grain boundaries, and the interface of the two colonies is devoid of precipitates because of the competitive mechanisms of the two orientations.
References
T. Senuma: ISIJ Int., 2001, vol. 41, p. 520-32.
Y. Funakawa, T. Shiozaki, K. Tomita, T. Yamamoto, and E. Maeda: ISIJ Int., 2004, vol. 44, p. 1945-51.
C.P. Reip, S. Shanmugam, and R.D.K. Misra: Mater. Sci. Eng. A, 2006, vol. 424, p. 307-17.
C.Y. Chen, H.W. Yen, F.H. Kao, W.C. Li, C.Y. Huang, J.R. Yang, and S.H. Wang: Mater. Sci. Eng. A, 2009, vol. 499, p. 162-66.
I.A. Yakubtsov and J.D. Boyd: Mater. Sci. Technol., 2008, vol. 24, p. 221-27.
R.D.K. Misra, H. Nathani, J.E. Hartmann, and F. Siciliano: Mater. Sci. Eng. A, 2005, vol. 394, p. 339-52.
N. Nakata and M. Militzer: ISIJ Int., 2005, vol.45, p. 82-90.
A. Kumar, S.B. Singh, and K.K. Ray: ISIJ Int., 2008, vol. 48, p. 1285-92.
M.H. Cai, H. Ding, Y.K. Lee, Z.Y. Tang and J.S Zhang: ISIJ Int., 2011, vol. 51, p. 476-81.
Y.M. Kim, S.K. Kim, Y.J. Lim, and N.J. Kim: ISIJ Int., 2002, vol. 42, p. 1571-77.
A. Guo, R.D.K. Misra, J.Q. Xu, B. Guo, and S.G. Jansto: Mater. Sci. Eng. A, 2010, vol. 527, p. 3886-92.
P. Poruks, I. Yakubtsov, and J.D. Boyd: Scripta Mater., 2006, vol. 54, p. 41-45.
I.A. Yakubtsov, P. Poruks, and J.D. Boyd: Mater. Sci. Eng. A, 2008, vol. 480, p. 109-16.
R. Soto, W. Saikaly, X. Bano, C. Issartel, G. Rigaut, and A. Charai: Acta Mater., 1999, vol. 47, p. 3475-81.
J.X. Dong, F. Siciliano Jr, J.J. Jonas, W.J. Liu, and E. Essadiqi: ISIJ Int., 2000, vol. 40, p. 613-18.
H.J. Kestenbach, S.S. Campos, and E.V. Morales: Mater. Sci. Technol., 2006, vol. 22, p. 615-26.
H.W. Yen, C.Y. Chen, T.Y. Wang, C.Y. Huang, and J.R. Yang: Mater. Sci. Technol., 2010, vol. 26, p. 421-30.
J. Mahieu, J. Maki, B.C. De Cooman, and S. Claessens: Metall. Mater. Trans. A, 2002, vol. 33A, pp. 2573-80.
S. Shanmugam, N.K. Ramisetti, R.D.K. Misra, J. Hartmann, and S.G. Jansto: Mater. Sci. Eng. A, 2008, vol. 478, p. 26-37.
R. Lagneborg and S. Zajac: Metall. Mater. Trans. A, 2000, vol. 31A, p. 1-12.
R.W.K. Honeycombe and R.F. Mehl: Metall. Trans. A, 1976, vol. 7A, p. 915-36.
R.A. Ricks and P.R. Howell: Acta Metall., 1983, vol. 31, p. 853-61.
F.A. Khalid and D.V. Edmonds: Mater. Sci. Technol., 1993, vol. 9, p. 384-96.
H.W. Yen, C.Y. Huang, and J.R. Yang: Scripta Mater., 2009, vol. 61, p. 616-19.
S.S. Nayak, R.D.K. Misra, J. Hartmann, F. Siciliano, and J.M. Gray: Mater. Sci. Eng. A, 2008, vol. 494, p. 456-63.
S. Kurokawa, J.E. Ruzzante, A.M. Hey, and F. Dyment: Metal Sci., 1983, vol. 17, p. 433-38.
C. Zener: J. Appl. Phys., 1949, vol. 20, p. 950-53.
H.K.D.H. Bhadeshia: Prog. Mater. Sci., 1985, vol. 29, p. 321-86.
P. Movahed, S. Kolahgar, S.P.H. Marashi, M. Pouranvari, and N. Parvin: Mater. Sci. Eng. A, 2009, vol. 518, p. 1-6.
M. Calcagnotto, Y. Adachi, D. Ponge, and D. Raabe: Acta Mater., 2011, vol. 59, p. 658-70.
M. R. Akbarpour, and A. Ekrami: Mater. Sci. Eng. A, 2008, vol. 477, p. 306-10.
R.G. Davies: Metall. Trans. A, 1978, vol. 9A, p. 41-52.
Acknowledgments
This work was carried out with financial support from the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (No. 2011BAE13B03), the Fundamental Research Funds for the Central Universities (No. N110402002), and the Natural Science Foundation of Liaoning Province of China (No. 201202062).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted June 27, 2012.
Rights and permissions
About this article
Cite this article
Hu, J., Du, LX., Wang, JJ. et al. Microstructures and Mechanical Properties of a New As-Hot-Rolled High-Strength DP Steel Subjected to Different Cooling Schedules. Metall Mater Trans A 44, 4937–4947 (2013). https://doi.org/10.1007/s11661-013-1839-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-013-1839-z