Abstract
In order to remove impurity AlCl3 from LiCl-KCl melts before Li electrolysis, the Al3+ reduction potential on a tungsten electrode and the relation between Al3+ reduction peak current and AlCl3 concentration in LiCl-KCl-AlCl3 melts were determined by cyclic voltammetry (CV). Constant potential electrolysis at –1.6 V vs Cl2/Cl– on both solid Fe and liquid Zn cathodes was performed to remove AlCl3 impurity from the LiCl-KCl-AlCl3 melts. The removal rate of Al3+ from the melts was analyzed by both electrochemical methods and inductively coupled plasma–atomic emission spectrometry (ICP-AES) analysis. The results showed that 96.11 wt pct of Al were removed on a Fe cathode and 99.90 wt pct on a Zn cathode through 10 hours electrolysis, respectively. While stirring the melts by argon gas, 99.21 wt pct of Al3+ was separated from the melts by 4 hours of electrolysis at 723 K (450 °C), which effectively expedited the Al3+ electrochemical reduction rate and shortened the electrolysis time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Currently, the only way to produce primary lithium in industry is by molten salt electrolysis from KCl-LiCl melts at 693 to 703 K (420 to 430 °C).[1–6] Usually, the KCl and LiCl used in industry have a purity of 98 to 99 wt pct containing an amount of impurities, such as NaCl, AlCl3, MgCl2, and CaCl2. These impurities can be deposited as the corresponding metals during the Li electrolytic process due to a lower theoretical decomposition voltage than that of LiCl; therefore, the primary Li contains a purity of 98 to 99 wt pct with 0.1 to 0.8 wt pct Na, 1 wt pct K, 0.03 wt pct Al, and 0.01 to 0.05 wt pct Ca.[7] These impurities have to be further removed by vacuum distillation in a stainless steel reactor at temperatures from 873 to 1073 K (600 to 800 °C), and sometimes followed by zone melting,[8–10] to satisfy the purity requirement for use in batteries, alloys, and nuclear power generation. About 52 kWh/kg Li has to be used to purify the primary lithium from 98.5 to 99.9 wt pct accompanied with a serious corrosion of the reactor. Hence, it is necessary to explore an economically feasible method to remove these impurities instead of the traditional one.
Lou et al.[11] and Gulens et al.[12] reported that a kind of lithium ion conductive material Li1.3Ti1.7Al0.3(PO4)3 was used to remove Na+ ions from LiCl solution to purify LiCl salt. Jin et al.[13] suggested an alcohol extraction method to prepare a highly purified LiCl salt with lower Na and K content. In our laboratory, electrochemical methods are proposed to separate those impurities that have lower theoretical decomposition voltages than LiCl from the LiCl-KCl melts prior to Li electrolysis. As one of the impurities in LiCl-KCl melts, AlCl3 presents a much lower theoretical decomposition voltage than LiCl; it is supposed to be deposited and separated from the AlCl3-KCl-LiCl melts by potentiostatic electrolysis prior to Li electrochemical reduction. Usually, the Al3+ ion concentration in the KCl-LiCl melts is low and gradually gets lower as the electrolysis proceeds. Since the kinetics of aluminum deposition in chloride melts by electrochemical techniques is controlled by ion diffusion according to Yan et al.,[14] Bouteillon and Marguier,[15] Gabčo et al.,[16] and Zhang et al.,[17] the Al3+ electrochemical reaction rate will become lower and lower. The main challenges during the removal of Al3+ ions from the KCl-LiCl melt are if Al3+ ions can be completely removed, how long the process will take, and how to improve the electrochemical reaction kinetics of Al deposition in the melts.
In this article, cyclic voltammetry (CV) is employed to determine Al3+ reduction potentials on a tungsten electrode in the LiCl-KCl-AlCl3 melt at 723 K (450 °C) and the relation of Al3+ reduction peak current with AlCl3 concentration. Then, a solid Fe and a liquid Zn electrode are used as cathodes, respectively, to remove Al3+ ions from the melts by constant potential electrolysis. The removal rate of Al3+ from the melts is studied by both electrochemical methods and ICP-AES analysis. Then, stirring the melts by argon gas is performed during potentiostatic electrolysis, and the effect of stirring on the removal rate of Al3+ from the melts is also examined.
2 Experimental
2.1 Chemicals and Preparation of Melts
The chemicals used in the experiments were composed of anhydrous LiCl (AR, >99 wt pct, Shanghai, China), anhydrous KCl (AR, >99.5 wt pct, Shanghai, China), and AlCl3 (AR, >99.5 wt pct, Shanghai, China). Before experiments, LiCl, KCl, and AlCl3 were dried at 473 K (200 °C) for 4 hours under vacuum and stored in a glove box to avoid moisture for use. A certain stoichiometric amount of electrolyte consisting of LiCl-KCl (1:1 mol) with various concentrations of AlCl3 was loaded to an Al2O3 crucible located in a stainless-steel vessel with an airtight seal. The electrolyte was heated to 473 K (200 °C) and maintained for 4 hours under vacuum, and then heated to the required temperature under argon gas.
2.2 Electrodes
In all electrochemical experiments, the working electrode used was a 1-mm tungsten wire welding on a 1-mm nickel-cadmium wire shrouded in an alumina tube with 1 to 2 cm left exposed. The counter electrode used was a 6-mm spectral pure graphite shielded by an alumina tube with 3 cm left exposed. During electrolysis experiments, Fe and Zn plates were used as cathodes, respectively, and spectral pure graphite was employed as an anode. The reference electrode was a Ag/AgCl (4 mol pct) in LiCl-KCl (1:1 mol) melt contained in a porcelain tube fabricated according to Qi.[18] All the potentials in this article will be measured with respect to this reference electrode and then referred to the Cl2/Cl– standard potential for reporting an accurate potential.
2.3 Analytical Techniques
All electrochemical measurements were performed using a PARSTAT2273 (PAR-Ametek Co., Ltd., Oak Ridge, TN) with a powersuite software package. After several hours of potentiostatic electrolysis, the cathode products were examined by energy dispersive spectrometry (EDS, FALCON-60S, EDAX Inc., Mahwah, NJ), and the content of Al in the remaining melts was analyzed by inductively coupled plasma–atomic emission spectrometry (ICP-AES, Varian 710-ES, Varian Inc., Santa Clara, CA).
3 Results and Discussion
3.1 Al3+ Electrochemical Behavior
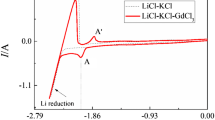
Figure 1 shows typical CVs obtained on a tungsten electrode before and after addition of 0.247 mol L−1 AlCl3 in LiCl-KCl melts at 723 K (450 °C). The dotted curve represents the cyclic voltammogram before addition of AlCl3. Only one couple of cathodic/anodic signals B and B′ is observed, which corresponds to the deposition and dissolution of liquid Li. The solid curve shows the cyclic voltammogram after addition of AlCl3. Since the deposition potential of Al is more positive than that of Li in a chloride system,[19] the peak A at the potential –1.55 V vs Cl2/Cl– in the cathodic scan is ascribed to Al3+ ion reduction.[14] In the reverse scan, in addition to the anodic peak corresponding to oxidation of liquid Li, the profile of peak A’ clearly indicates the dissolution of deposited Al.
Figure 2 shows the deposition and dissolution of Al in LiCl-KCl-AlCl3 (0.247 mol L−1) on a tungsten electrode with different scan rates at 723 K (450 °C). The sharp oxidation peak A′ implies an insoluble reduction product since the ratio of \( i_{A} /i_{{A^{\prime } }} \) is smaller than 1. The plot of the cathodic peak current density i A vs v 1/2 after subtracting the background current, as shown in Figure 3, gives a good linear relation and passes through the origin. This indicates that the electrode process of Al3+ ions is controlled by ion diffusion. For a soluble-insoluble system,[20] the diffusion coefficient of the Al3+ ions in the melts can be calculated according to the Berzins–Delahay equation in Eq. [1]:
where S is the electrode surface area in cm2, C 0 is the solute concentration in mol cm−3, D is the diffusion coefficient in cm2 s−1, F is Faraday’s constant (96,485 C mol−1), R is the universal gas constant, n is the number of exchanged electrons, v is the potential sweep rate in V s−1, and T is the absolute temperature in Kelvin.
Equation [1] yields \( D_{{{\text{Al}}^{3 + } }} = 3.1 \times 10^{ - 5} {\text{cm}}^{2} \,{\text{s}}^{ - 1} . \) For a reversible electrode reaction involving the deposition of an insoluble substance, \( \left| {E_{p} - E_{p/2} } \right| \) (where E p/2 is the half-peak potential) should have a value of 2.29 RT/nF or 0.0475 V for a three-electron reaction at 723 K (450 °C).[21] In this case, \( \left| {E_{p} - E_{p/2} } \right| = 0.046 \) is found almost equal to the value 0.0475 V. These observations suggest the deposition/dissolution reaction of Al3+ ion is reversible, which is in good agreement with the before mentioned references.[14–17]
At a given temperature of 723 K (450 °C), from the obtained CVs, the current density of the reduction peak for Al3+ ions has a linear relation with AlCl3 concentration at the scan rate 0.1 V s−1, as shown in Figure 4. This plot also can be used to determine the concentration of Al3+ ions present in the melts after several hours of electrolysis.
3.2 Feasibility of Electrochemical Removal of Aluminum
Al can form solid solutions with Fe at 723 K (450 °C), but existing in the solid state according to Figure 5. At the same time, Al can form uniform liquid mixtures with liquid Zn at this temperature, as shown in Figure 6. Therefore, according to this principle, it is feasible to remove impurity Al3+ by potentiostatic electrolysis at its reduction potential on these cathode materials to form alloys. In order to compare the effects of electrode materials on the removal rate and the electrochemical reaction kinetics of Al3+, solid Fe and liquid Zn electrodes[22–25] at the temperature of 723 K (450 °C) are selected as the cathodes, respectively.
Potentiostatic electrolysis is carried out on both solid Fe and liquid Zn electrodes at Al3+ ion reduction potentials, and the responses of the current vs electrolysis time are recorded. After electrolysis, the cathode deposits with metallic luster are dissolved into alcohol to remove adherent melts on cathode and then analyzed by EDS. The content of Al in LiCl-KCl (1:1 mol) melts before and after several hours electrolysis is analyzed by ICP-AES. The theoretical removal amount of Al can be calculated using Faraday law, shown in Eq. [2], as follows:
where A is the Al electrochemical equivalent of 0.336 in g A−1 h−1, I is the current in A; t is the electrolysis time in seconds, and Q is the consumed electricity quantity obtained from the chronoamperograms in C.
3.2.1 Electrochemical removal of aluminum using a solid Fe electrode
Figure 7(a) gives the current response vs electrolysis time on a solid Fe electrode at –1.6 V vs Cl2/Cl– in LiCl-KCl-AlCl3 (0.247 mol L−1) melts. The current decreases from –0.55 to –0.15 A in the first 2 hours of electrolysis, and further decreases to –0.07 A for the successive 2 hours of electrolysis, and finally the current is lower than –0.005 A for another 5 hours of electrolysis. Accordingly, the consumed electricity quantities for the three stages are equal to 1272 C, 652 C, and 138 C, respectively, as shown in Figure 7(b). The theoretical Al removal rates are 61.76, 93.42, and 100.12 wt pct for 2, 4, and 10 hours electrolysis, respectively, which are a little different from the results obtained by the ICP analysis (Table I).
Obviously, since Al3+ electrochemical reduction is controlled by ion diffusion rate, as explained previously, the electrochemical reaction current for the third stage is decreased with the lower Al3+ ions diffusion rate, which results in a very low removal rate. Therefore, enhancing the Al3+ ions diffusion rate is required to increase the reduction current and further increase the removal rate of Al3+ ions. If stirring is applied in the melts during electrolysis, it will accelerate the Al3+ ions diffusion in the melt. This will be used to remove Al3+ ions in Section II.
As mentioned previously, AlCl3 concentration in the melts is proportional to the reduction peak current density in the CVs. Therefore, the Al3+ ions concentration remaining in the melts after several hours electrolysis can be gained from the reduction peak current density in the corresponding CV curves. Figure 8 shows CVs of LiCl-KCl-AlCl3 (0.247 mol L−1) melts before and after 2, 4, and 10 hours electrolysis at –1.6 V vs Cl2/Cl– (curves 1 through 4), respectively. Obviously, the reduction peak current density of Al3+ decreases to 0.0129 A cm−2 after 4 hours electrolysis, even no distinct reduction peak can be seen in curve 4 after 10 hours electrolysis, which indicates that most of the Al3+ impurity was removed by electrolysis on the Fe cathode. According to the linear relationship of the Al3+ ions reduction current density with the AlCl3 concentration shown in Figure 4, the Al3+ contents remaining in the melts are 0.62 × 10−1 mol L−1 and 0.97 × 10−2 mol L−1, respectively, for 4 and 10 hours electrolysis, while ICP-AES analysis gives 0.20 × 10−1 mol L−1 and 0.96 × 10−2 mol L−1 Al3+ contents in the melts, respectively, which correspond to 91.77 and 96.11 wt pct removal rates after 4 and 10 hours electrolysis, as shown in Table I. Obviously, the electrochemical method gives a higher value than the ICP-AES method. The cathodic product contains 7.00 wt pct Al and 93.00 wt pct Fe analyzed by EDS
3.2.2 Electrochemical removal of aluminium using a liquid Zn electrode
Another choice is to use liquid Zn as a cathode; the advantage is maintaining the cathode in the liquid state during the entire electrolysis process at 723 K (450 °C). The amount of the Zn used is 4.03 g, as listed in Table II, placed in the bottom hole of the Al2O3 crucible (6.5 cm2), which is connected to the current collector.
Figure 9 shows the relations between current (a) and electricity quantity variation (b) with time during electrolysis on a liquid Zn electrode at –1.6 V vs Cl2/Cl– for the first 2 hours, and another 2 and 5 hours.
Relation of (a) current with time and (b) consumed total electricity quantity with time (1: the first stage, 2: the second stage, and 3: the third stage) in LiCl-KCl-AlCl3 (0.247 mol L−1) melts on a liquid Zn cathode at 723 K (450 °C). WE: Zn (S = 6.5 cm2), CE: spectral pure graphite, and RE: Ag/AgCl
The Al theoretical removal rates are calculated to be 74.81, 97.61, and 105.83 wt pct, respectively, for 2, 4, and 10 hours electrolysis. Compared with the solid Fe cathode, the liquid Zn cathode theoretically accelerates the Al3+ electrochemical reduction reaction in the first 2 hours electrolysis, though the Zn cathode area is smaller than the Fe cathode. This may be the reason a fresh Zn surface is always maintained during the electrolysis process and can easily form solid solution with the deposited Al, which results in a successive under-potential deposition of Al3+ ions on the liquid Zn electrode. Due to a larger amount of Al3+ ions being reduced in the first 2 hours electrolysis, the reduction current and electricity quantity are distinctly decreased in the following electrolysis stages: 2 and 5 hours of electrolysis. So, it is necessary to stir the melts to increase the Al3+ ions diffusion rate in the melt and enhance the removal rate of Al3+ ions. After 10 hours of electrolysis, the removal rate of Al on a liquid Zn cathode reaches 99.90 wt pct. The cathode product is composed of Zn 67.80 wt pct and Al 32.20 wt pct, as detected by EDS analysis, and a partly solid solution and a liquid phase are formed.[26] Compared with the solid Fe electrode, it is obvious that the liquid Zn electrode effectively removes the impurity of Al3+ from the KCl-LiCl-AlCl3 melt.
3.3 Stirring Effects
In order to expedite the Al3+ ions diffusion rate in the melts, a stirring installation, shown in Figure 10, is placed in the bottom of the crucible during electrolysis and 0.3 L min−1 argon gas continuously flows from the hole (2-mm i.d.) to the electrolyte surface for efficient mixing of the electrolyte. Under the preceding conditions, potentiostatic electrolysis is carried out on a Fe electrode at –1.6 V vs Cl2/Cl– in LiCl-KCl-AlCl3 (0.247 mol L−1) melts for 3 and 4 hours, respectively. The current variation with electrolysis time is given in Figure 11(a).
The reduction current observably decreases from –0.88 to –0.05 A for 3 hours of electrolysis and gradually decreases to 0.01 A after 4 hours of electrolysis, and the electricity quantities consumed for 3 and 4 hours of electrolysis are 2001 C and 2151 C, respectively, as shown in Figure 11(b), corresponding to 97.16 and 104.44 wt pct of theoretical Al removal rates, according to Eq. [2] (the 104.44 wt pct may be caused by the trace of other impurities). The reduction current and the consumed electricity quantities in Figure 11(b) are far greater than for the Fe electrode without stirring within the short electrolysis time.
From the CVs in Figure 12, no prominent reduction peak current for Al3+ is observed after 4 hours of electrolysis; meanwhile, the oxidation current is also very small and can be neglected. The AlCl3 concentration in the remaining melts is 1.95 × 10−3 mol L−1 by ICP-AES analysis, so more than 99.00 wt pct Al3+ was removed within 4 hours of electrolysis under stirring, which means stirring the melts can accelerate the removal rate of the Al3+ ions and shorten the electrolysis time.
Typical CVs of the LiCl-KCl-AlCl3 (0.247 mol L−1) melts on a tungsten electrode (0.1963 cm2) before and after electrolysis on a solid Fe cathode at 723 K (450 °C) under stirring. Scan rate: 0.1 V s−1, stirring with flow rate of 0.3 L min−1, WE: W (S = 0.1963 cm2), CE: spectral pure graphite, and RE: Ag/AgCl
Compared with the results with and without stirring in Table III, Al3+ ions concentration in the melts can be reduced to 0.0016 wt pct by 4 hours of electrolysis at –1.6 V vs Cl2/Cl– on a solid Fe electrode under stirring, which excel one distillation effect applied in industry. Electrochemical potentiostatic electrolysis is a low cost and convenient operation method to remove impurity AlCl3 manipulation before Li electrolysis.
4 Conclusions
The Al3+ reduction potential is –1.55 V vs Cl2/Cl– obtained by CV in LiCl-KCl-AlCl3 melts at 723 K (450 °C). The electrochemical reduction process of Al3+ is controlled by ion diffusion. The relation between Al3+ reduction current density and the AlCl3 concentration is linear and can be used to determine the Al3+ content remaining in the melts after several hours of electrolysis. By potentiostatic electrolysis at –1.6 V vs Cl2/Cl– in LiCl-KCl-AlCl3 (0.247 mol L−1) melts on a solid Fe electrode, the Al3+ theoretical removal rates are 93.42 and 100.12 wt pct for 4 and 10 hours electrolysis, respectively, calculated from the Faraday law, while 91.77 and 96.11 wt pct of removal rates are achieved after 4 and 10 hours electrolysis analyzed by ICP-AES. Compared with the solid Fe electrode, the liquid Zn electrode can effectively remove the 99.90 wt pct of impurity Al3+ from KCl-LiCl-AlCl3 melts. Al3+ concentration in the melts can be reduced 99.21 wt pct by 4 hours electrolysis at –1.6 V vs Cl2/Cl– on a Fe cathode under stirring the melts, which means stirring distinctly accelerates the removal rate and shortens the electrolysis time.
References
W.A. Averill and D.L. Olson: Int. J. Energy Res., 1978, vol. 3 (3), pp. 305–13.
P.A. Mahi, A.J. Smeets, D.J. Fray, and J.A. Charles: J. Met., 1986, vol. 38 (11), pp. 20–26.
W.E. Cowley: Molten Salt Technology, D.G. Lovering, ed., Plenum Press, New York, NY, 1982, pp. 215–18.
P.E. Landolt: Rare Metals Handbook, C.A. Hampel Reinhold Press, New York, NY, 1954, pp. 136–38.
C.L. Mantel: Electrochemical Engineering, McGraw-Hill Press, New York, NY, 1960, pp. 56–59.
G.T. Motock: Electrochem. Technol., 1963, vol. 1, pp. 122–28.
S. Shen: Molten Salts Electrochemical Theory Foundation, China Industrial Press, Beijing, 1963, pp. 98–102.
W.L. Chen, L.Y. Chai, X.B. Min, B. Yang, Y.N. Dai, X. Yu, and C.F. Zhang: Trans. Nonferrous Met. Soc. China, 2002, vol. 1, pp. 152–55.
W.L. Chen, L.Y. Chai, X.B. Min, B. Yang, Y.N. Dai, X. Yu, and C.F. Zhang: Trans. Nonferrous Met. Soc. China, 2001, vol. 6, pp. 937–41.
H. Lan: Xinjiang Youse Jinshu, 1996, vol. 8, pp. 55–57.
T.P. Lou, D.G. Li, R. Pan, and H.P. Zhang: Acta Phys. Chim. Sinica, 2003, vol. 19, pp. 839–43.
J. Gulens, B.W. Hildebrandt, J.D. Canaday, A.K. Kuriakose, T.A. Weat, and A. Ahmad: Solid State Ionics, 1989, vol. 35 (1–2), pp. 45–49.
Z.N. Jin, X.M. Li, W.J. Lan, and X.R. Liu: J. Northeast. Univ. (Nat. Sci.), 2006, vol. 27 (11), pp. 1251–54.
Y.D. Yan, M.L. Zhang, Y.X.W. Han, D.X. Cao, and L.Y. He: J. Appl. Electrochem., 2009, vol. 39, pp. 455–46.
J. Bouteillon and A. Marguier: Surf. Coat. Technol., 1984, vol. 22 (3), pp. 205–17.
M. Gabčo, P. Fellner, and Ž. Lubyová: Electrochim. Acta, 1984, vol. 29 (3), pp. 397–401.
Y.J. Zhang, A. Bjørgum, U. Erikson, R. Tunold, and R. Ødegård: J. Electroanal. Chem. Interface, 1986, vol. 210 (1), pp. 127–36.
X. Qi and H.M. Zhu: Basic Study of Electrochemical Co-Deposition of Mg-Al Alloy in Alkali Chloride Melt, University of Science and Technology Beijing Press, Beijing, 2004, pp. 26–27.
Q.Q. Yang, B.L. Fang, and Y.X. Tong: J. Appl. Electrochem., 2nd ed., Zhongshan University Press, Guangzhou, 2005, pp. 49–55.
T. Berzins and P. Delahay: J. Am. Chem. Soc., 1953, vol. 75 (3), pp. 555–59.
R. Ødegard, A. Bjørgum, A. Sterten, J. Thonstad, and R. Tunold: Electrochim. Acta, 1982, vol. 27 (11), pp. 1595–98.
R.P. Elliot: Phase Diagram, McGraw-Hill Press, New York, NY, 1965, pp. 381–88.
J.Q. Yu, W.Z. Yi, B.D. Chen, and H.J. Chen: Binary Alloy Phase-Diagrams, Shanghai Science and Technology Press, Shanghai, 1983, pp. 140–63.
A.E. Herrera-Erazo, H. Habazaki, K. Shimizu, P. Skeldon, and G.E. Thompson: Corros. Sci., 2000, vol. 42 (10), pp. 1823–30.
M. Jafarian, F. Forouzandeh, I. Danaee, F. Gobal, and M.G. Mahjani: J. Solid State Electrochem., 2009, vol. 13 (8), pp. 1171–79.
Z.X. Qiu: Principle and Application of Aluminum Electrolysis, Chinese Mining Industrial University Press, Suzhou, 1998, pp. 567–689.
Acknowledgments
This work was financially supported by the 863 projects of the Ministry of Science and Technology of China (Grant No. 2009AA06Z102), the Key Program of the National Natural Science Foundation (Grant No. 50934001), and the National Natural Science Foundation (Grant No. 51054004); we also appreciate the support of the Key Laboratory of Chemical Engineering, Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted September 5, 2010.
Rights and permissions
About this article
Cite this article
Shen, M., Li, B., Li, S.Z. et al. Electrochemical Removal of AlCl3 from LiCl-KCl Melts. Metall Mater Trans A 43, 1662–1669 (2012). https://doi.org/10.1007/s11661-011-0982-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-011-0982-7