Abstract
Molten magnesium and its alloy are easily oxidized during processing. SF6 in conjunction with dilute gas is usually used to protect molten magnesium. The cover gas protects the melt by reacting with the metal to form a protective film on the melt surface. This article describes the investigation of the microstructure and composition of the surface film formed in a controlled atmosphere. The results showed that the film contained only MgO and MgF2. Both Auger electron spectroscopy (AES) depth profiling and scanning electron microscopy (SEM) observation indicated the film thickness to be in the range of 0.3 to 2 μm, and the composition of the film was uniform in depth. Particles containing magnesium fluoride formed on the interface between the oxide film and bulk magnesium, which corresponded to the concave areas from the surface observation by SEM. The results also showed that the film thickness increased with the increase of holding time and melting temperature. High concentration of SF6 or high temperature will accelerate the reaction between magnesium and fluoride.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Molten magnesium oxidizes rapidly during casting and handling. Because of this, a cover gas is required, and both SO2 and SF6, mixed with N2, CO2, or dry air are the most common methods used to protect molten magnesium. Recently, the use of SF6 has become more and more restricted, because of its expensive price and environmental issues. Studying the protective mechanism of SF6 is very important for finding replacements. Some investigations have been started, but the protective mechanism of SF6 is still not very clear and requires further study. Couling[1,2] studied the protection of pure magnesium and the AZ91B alloy in atmospheres containing SF6. He found that SF6 was consumed during the experiments and attributed this to the adsorption of SF6 to an MgO surface or reaction to form MgF2.
Recently, Cashion[3–5] used several kinds of techniques to study the surface films formed during controlled exposure of molten magnesium. He found that the films consisted of MgO and MgF2; the amount of fluoride increased with increasing concentration of SF6 in the range of 0.1 to 1 pct. He also found that the thickness of the films increases with the increase of the protective time. These results were partly confirmed by Pettersen et al.[6] In Pettersen’s experiments, little sulfur was found when the melt was protected by SO2, while fluoride was the only element detected in the oxide when SF6 or other fluoride-containing gases were used for protection.
Aarstad[7] used various techniques to study the surface of magnesium protected by SF6. He found that the thickness of the film varied between 0.3 and 1 μm, increasing with the increase of the melting temperature and holding time. Large spots containing MgF2 may form on the interface between the oxide film and the bulk magnesium. These spots grew until they covered 25 to 50 pct of the total surface area.
Experimental methods
Apparatus
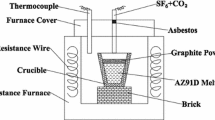
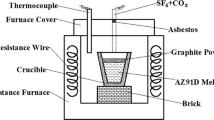
Figure 1 shows a schematics diagram of the apparatus. A stainless steel melting pot was located in the furnace. A silicon control temperature controller was used to adjust the temperature of the melt (error band <= 3 °C). Several mass flow meters were used to control the composition and flow rate of the protective gas. A silica-glass viewing port was located at the top of the furnace and was used to observe the protective effect.
Experimental Procedures
Procedures for preparing samples
Film samples were produced under cover gas (90 pct CO2 + 10 pct air) mixtures containing 0.1, 0.3, 0.5, 0.75, 1, and 1.5 pct SF6. First, high-purity AZ91D alloy was added to the crucible, producing mixed gas, which was controlled by mass flow meters. Twenty minutes later, heat was applied until a constant temperature was reached, and then the scraper was pulled out so that a fresh melt surface could be obtained. The melt was left in the furnace for a certain period. The crucible was then pulled out of the furnace using the handles and cooled rapidly (50 °C/min). Specimens (Figure 2) for X-ray diffraction (XRD), scanning electron microscopy (SEM), and scanning Auger microscopy (SAM) analyses were cut from the samples with a saw. Specimens for side observation were coated with epoxy resin before grinding and polishing.
Analysis methods
The chemical characterization of the surface films was performed using XRD. The SEM was used for surface and side observation of protective films, and energy dispersive spectroscopy (EDS) in conjunction with SEM was used to analyze the elements. The element concentrations of films were investigated using SAM in conjunction with ion-beam etching. Both SEM and SAM were used to measure the film thickness with different methods. In this experiment, a PHI-610/SAM analyzer (Perkin-Elmer, Wellesley, MA) and a LEO1530 SEM (Zeiss, Germany) were used for observation and composition analysis.
Results and discussion
Film Appearance and Structure
Figure 3 is a cross-sectional view of the sample with 0.3 pct SF6 held at 650 °C for 10 minutes. It indicates that there is a regular film on the surface of the alloy, and the film seems to be uniform in thickness. Further observation showed that the thickness of the film was between 0.3 and 2 μm. In addition, some semicircular particles were found at the interface between the bulk magnesium alloy and the uniform film. These particles were several microns in diameter and were embedded in the bulk magnesium. Further observations proved that this was the typical structure of protective films formed by SF6 mixtures.
Figure 4 shows the surface appearance of the 0.3 pct SF6 sample held at 650 °C for 10 minutes. The SEM images indicated that there were many concavities on the surface film. These concavities were commonly several microns in diameter. Closer observation showed that the grain size inside the concavities was smaller compared to other flat areas.
Composition of the Surface Film
Particle composition
The special size and shape of the particles between the uniform surface film and bulk magnesium indicated the particles might also have a special composition. The EDS analysis was used to identify the elemental composition of the particles. Figure 5 shows the EDS mapping results of the cross section of the particles. It seemed that the concentration of fluoride in the particle area was very high, so fluoride is the dominant element in the particles. Using the point analysis function of EDS, we could determine the precise composition of the particles. Table I lists the EDS results of points 1 and 2. Only a small concentration of O could be detected, which could be attributed to the inaccuracy of EDS analysis.
Figure 6 shows the surface appearance of the sample held at 650 °C for 10 minutes with 0.3 pct SF6. The distinct appearance of the concavities also indicated the presence of a distinguishing composition compared to the other areas. In order to identify the difference between the concavity and the other areas, EDS analysis was used. Table II gives the results of the EDS analysis. Points 1 through 6 correspond to the associated positions on the figure to the left of the table; the column labeled “Macro” gives the results of EDS analysis applied to a 4-mm2 area, which indicates the average composition of the sample. According to the results, the concentration of fluoride in the concave area was apparently higher than in the other areas, and the ratio of F/Mg in atoms was close to 2. This shows that the concave area was full of MgF2, and the film thickness in the white area was apparently thinner than the concave area, because the concentrations of magnesium in these areas were significantly high, indicating that EDS analysis had penetrated through the surface film so that bulk magnesium could also have contributed to the results.
Comparing the particles in Figure 5 and the concavities in Figure 6, we can find that they share the same size. More importantly, both of them are full of MgF2. With careful observation, obvious concavities were found at the bottom side of the particles (Figure 5). Therefore, particles under the surface films must be located in the concave areas.
Film composition
To study the depth characteristic of films, the film thickness was analyzed by depth profiling, using ion-beam etching in conjunction with AES. These techniques were chosen due to the fact that only the excited atomic species produced near the surface of the sample have a chance of escaping the sample and being collected by the detector. Consequently, the chemical information obtained is restricted to the outermost layers of the sample, making these techniques truly surface sensitive. The AES analysis showed that magnesium, oxygen, fluoride, and carbon were main elements in the surface films (Figure 7(a)). With changes in depth, relative concentration changes of magnesium, oxygen, fluoride, and carbon were detected as the film was sputtered away. The sharp change of element concentrations corresponded to the film/bulk magnesium interface.
Figure 8 shows the AES result for the sample produced under a cover gas containing 0.3 pct SF6 at 650 °C for 10 minutes. Depth analysis showed that the concentration of each element remained almost constant from the outer surface of the films to the interface between the films and bulk metal. However, the concentration near the surface or interface was varied because of the diffusion of elements, inaccuracy of AES, and the contamination by air and moisture as well. This indicates that the composition of the films was uniform in depth.
According to Figure 8, we could find that the concentration of carbon was very high at the outer surface of the protective film and then decreased sharply to a very low level (<5 pct), which could be attributed to the inaccuracy of the AES analysis. The AES surveys at different depths (Figure 7) also indicated that carbon only appeared at the outer surface of the films. There was no carbon peak appearing after 5 minutes of sputtering, whereas the AES survey on the outer surface showed a strong carbon peak. Therefore, the high concentration of carbon at the outer surface of the films could be attributed to the reaction between the sample and ambient atmosphere. This was particularly prevalent with magnesium, due to its high reactivity with air, even at room temperature, with MgCO3 being a common corrosion product of magnesium.
Figure 8 clearly shows that there was a sharp rise in magnesium concentration after about 18 minutes’ sputtering, denoting the film/bulk magnesium interface. When using the ion-beam sputtering technique to profile through a film, the thickness is calculated by multiplying the sputtering time by the sputter rate. The sputter rate in this experiment was 30 nm/min. The thickness of this sample was about 0.5 μm.
The EDS analysis applied on a large area (2 × 2 mm) of surface films could provide the general information of the elements’ concentration. In this protection experiment, the compositions of cover gas, melting temperature, and holding time were the three most important factors, which could visibly affect the characterization of films. By keeping two of the three parameters constant and changing the other one in the experiments, we could obtain the trend of the reaction.
Figure 9(a) shows the changes in element concentration of film with increasing SF6 concentration. Samples were produced under cover gas containing different percentages of SF6 at 650 °C for 10 minutes. The results show that concentrations of Mg and O elements decreased with the increase of SF6 content, whereas the concentration of F increased visibly. These results strongly illustrate that film composition is relevant to SF6 content; high SF6 concentration in the cover gas will accelerate the reaction between magnesium and fluoride and lead to an increase of MgF2 concentration in surface films.
Figure 9(b) shows the change in element concentrations of film with variation in holding time. Samples were produced under a cover gas containing 0.3 pct SF6 at 650 °C. The results show that concentrations of Mg and O decreased with the increase of holding time, whereas the concentration of F increased. Careful observation showed that the main reaction occurred at 30 to 60 minutes. These results indicate that the composition of films will not change after the film has reached a certain composition.
Figure 9(c) shows the variation in element concentrations of film varies with increasing temperature. Samples were produced under a cover gas containing 0.3 pct SF6 at various temperatures for 10 minutes. The result shows that the concentration of Mg and O decreased with the increase of the melting temperature, whereas the concentration of F clearly increased. This indicates that high temperature will accelerate the reaction between magnesium and fluoride.
Film Thickness
The SEM analysis was used to measure the film thickness. The film thickness of the sample produced under a cover gas containing 0.3 pct SF6 at 650 °C for 10 minutes was 0.46 μm according to SEM analysis, which was close to the results determined by the AES analysis (0.5 μm), as shown in Figure 8. Therefore, this method could be used to measure the film thickness of all the samples.
Figures 10(b) and (c) clearly show that the film thickness increased with the increase of the holding time and the melting temperature, but it seemed that the SF6 concentration had little effect on film thickness (Figure 10(a)).
Experiment conditions’ influence on film thickness. (a) The thickness of the film as a function of SF6 concentration (holding time: 10 min, temperature: 650 °C). (b) The thickness of the film as a function of holding time (SF6 concentration: 0.3 pct, temperature: 650 °C). (c) The thickness of the film as a function of temperature (SF6 concentration: 0.3 pct, holding time: 10 min)
Although SF6 concentration had little effect on film thickness, a large number of observations showed that the quantity and diameter of MgF2 particles increased with the increase of SF6 concentration.
Therefore, the experimental results showed that the composition of cover gas, the melting temperature, and the holding time were the three most important factors in the current experiments. The influences of these parameters on the protective effect, the film thickness, and the density of MgF2 particles are listed in Table III.
Conclusions
A dense cohesive film was found from the magnesium surface when molten AZ91D alloy was protected by the SF6 mixture. A large number of MgF2 particles were found at the interface of the surface film and bulk magnesium, which corresponded to the concave areas on the surface observed by SEM. These particles were several microns in diameter and were embedded in the bulk magnesium.
The protective film consisted of MgO and MgF2, and the concentrations of these elements were affected by the SF6 concentration. It seemed that MgO was the main element in the surface films, whereas MgF2 particles were mainly found at the interface. The depth profile of the film showed that the elemental concentrations were constant at different depths. The film thickness increased with the increase of the holding time and the melting temperature, whereas the SF6 concentration had little effect on it.
The size and quantity of MgF2 particles increased with the increase of the holding time, the melting temperature, and the SF6 concentration. A high SF6 concentration or long holding time even led to a coherence of MgF2 particles.
References
S.L. Couling, F.C. Bennett, and T.E. Leontis: Light Met. Age, 1977, no. 1, pp. 12–21
S.L. Couling: Proc. 36th Annual World Conf. on Magnesium, Oslo, Norway, 1979, p. 54–57
S.P. Cashion: Ph.D. Thesis, University of Queensland, Queensland, Australia, 1998, pp. 145–47
S.P. Cashion, N.J. Ricketts, P.C. Hayes: J. Light Met., 2002, vol. 2, pp. 37–42
S.P. Cashion, N.J. Ricketts, P.C. Hayes: J. Light Met., 2002, vol. 2, pp. 43–47
G. Pettersen, E. Øvrelid, G. Tranell, J. Fenstad, H. Gjestland: Mater. Sci. Eng., 2002, vol. A332, pp. 285–94
K Aarstad, G Tranell, G Pettersen: Magnesium Technology 2003, TMS, Warrendale, PA, 2003, pp. 5–10
Acknowledgments
This research work was financially supported by the Tsinghua-Toyo R&D Center of Magnesium and Aluminum Alloys Processing Technology, jointly established between Tsinghua University and Toyo Machinery & Metal Co., Ltd. The study was also supported by the Program for New Century Fund for Excellent University Talents.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted ...
Rights and permissions
About this article
Cite this article
Xiong, SM., Liu, XL. Microstructure, Composition, and Depth Analysis of Surface Films Formed on Molten AZ91D Alloy under Protection of SF6 Mixtures. Metall Mater Trans A 38, 428–434 (2007). https://doi.org/10.1007/s11661-006-9035-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-006-9035-z