Abstract
Graphite powder was adopted to prevent AZ91D magnesium alloy from oxidizing during the melting and casting process. The microstructure of the resultant surface films formed on the molten alloy protected by 0, 2.7, 5.4, 8.1, and 10.8 g dm−2 graphite powder at 973 K (700 °C) for holding time of 30 minutes was investigated by scanning electron microscopy, energy dispersive spectrometer, X-ray diffraction, and the thermodynamic method. The results indicated that the surface films were composed of a protective layer and the underneath MgF2 particles with different morphology. The protective layer was continuous with a thickness range from 200 to 550 nm consisting of magnesium, oxygen, fluorine, carbon, and a small amount of aluminium, possibly existing in the form of MgO, MgF2, C, and MgAl2O4. The surface films were the result of the interaction between the graphite powder, the melt, and the ambient atmosphere. The unevenness of the micro surface morphology and the number and size of the underneath MgF2 particles increased with graphite powder amount. The mechanism of the effect of graphite powder amount on the resultant surface films was also discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnesium alloys are widely used in many industry fields due to their excellent intrinsic properties such as low density, high specific strength, and rigidity. However, the oxidation of the magnesium alloys during the melting and casting process limits their application in industry. To combat the oxidation problem, certain protective methods are used during the melting and casting process such as the addition of flux to the melt, protective gasses, alloying with ignition proof materials, and vacuum protection.[1,2]
In the early stage of oxidation prevention, fluxes were widely used for protecting the magnesium alloys due to exhibiting effective protection and ease of application. However, fluxes release harmful gasses at high temperatures, which will not only pose a serious threat to human health but also corrode equipment.[2] Recently, gasses such as SO2,[3,4] SF6,[5] HFC-134a,[6] and HFC125[7,8] have been used to protect molten magnesium alloys. Unfortunately, the gasses bring about environmental problems because they can cause the greenhouse effect when released into the atmosphere.[9,10] Regarding the alloying method, the addition of the alloying elements significantly deteriorates the mechanical properties of the magnesium alloys.[11] Vacuum protection has no harmful side effects to the environment but the process raises the cost of magnesium production. The goal, therefore, is to find a cost-effective environmentally friendly solution to prevent the magnesium oxidation.
Research has shown that CO2 can protect molten magnesium in different forms and can be used as a substitute for protective gasses not only because CO2 is not toxic or corrosive, cheaper than SF6 and SO2 but also because it has a lower degree of the greenhouse effect than SF6.[12] Fruehling[13] claimed that an atmosphere of pure CO2 gas could effectively protect the magnesium melt. Emami et al.[12] used CO2/air gas to protect molten magnesium from oxidation. Yang and Lin[14] used CO2 snow to develop a highly effective method for protecting magnesium melts.
Because graphite can react with oxygen to produce CO2 at high temperatures,[9,15,16] which is expected to act as barrier for molten magnesium from the atmosphere, the graphite powder is a potential medium for protecting the molten magnesium alloys from oxidation during melting and casting. Surface films formed on molten magnesium under protection of CO2 was composed of MgO and C. The resultant carbon fills the interstices of MgO grains and then makes the surface films dense, thus preventing oxygen from reaching the surface of the molten magnesium.[12] Our previous results[17] showed that graphite powder could protect AZ91D melt and the protection ability was affected by graphite powder amount. However, the microstructure of the surface films formed on molten magnesium alloy protected by graphite powder requires further investigation to understand the protection mechanism.
Surface films, formed statically or dynamically,[18,19,20,21,22,23] are products of reactions between molten magnesium alloys and a protective medium. For example, Al2O3, MgAl2O4, and MgO were the products of the oxidation of Al alloys containing 0.3 to 4.5 wt pct magnesium in an atmosphere with a very low oxygen partial pressure.[24] And alumina and calcium aluminate were produced when entrapped air bubbles reacted with the aluminium-calcium alloys.[25] The thermodynamic method to phase formation can indicate whether a reaction will occur. The reactions between graphite powder, the melt, and the ambient atmosphere, of which the products may be the phases of the surface films formed on the AZ91D melt protected by graphite powder, also requires further analysis.
The amount or the concentration of a certain protective medium is one of the important parameters that affect the formation of the surface films. With an increase in CO2 concentration in air, the protection behavior of molten magnesium is enhanced and the protective MgO-C layer increases.[12] Furthermore, when molten magnesium was protected by SF6 in conjunction with dilute gas, the coherence as well as the size and quantity of MgF2 particles also increases with the SF6 concentration of cover gas.[26] Therefore, the graphite powder amount might affect the formation of the surface films, which also needs to be understood.
The purpose of the present work is to understand the protection mechanism of graphite powder for molten magnesium alloy and to find an optimal graphite powder amount that can be used in industry. Experiments were carried out to study the effect of graphite powder amount on the surface films and the mechanism was discussed. Reactions between graphite powder, the melt, and the ambient atmosphere were analyzed by the thermodynamic approach. The results are thought to provide the theoretical support for an industrial application of graphite powder as a protective medium of magnesium alloy melts.
Experimentation
Commercial AZ91D alloy ingots with the chemical composition in weight percent of 9.1 pct Al, 0.67 pct Zn, 0.16 pct Mn, 0.02 pct Si, 0.003 pct Fe, 0.01 pct Cu, 0.002 pct Ni, and Mg balance were employed for the melting process. The graphite powder was chemically pure with carbon content of no less than 99.85 wt pct and particle size of no more than 30 μm.
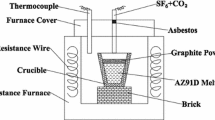
The ingots were melted in an electrical resistance furnace (Figure 1) and the surface films were thereby formed. The main components of the crucible were graphite and silicon carbide. Thermocouples were used to measure the furnace’s atmospheric temperature. The furnace atmosphere temperature was assumed to be the same as the melt temperature. The experimental process was as follows:
-
(1)
The alloy ingots with a weight of approximately 500 g and the crucible were placed inside of the furnace for the preheating process.
-
(2)
Protective gas consisting of 0.5 vol pct SF6 and 99.5 vol pct CO2 was introduced when the temperature reached 673 K (400 °C).
-
(3)
When the temperature stabilized at 973 K (700 °C), the protective gas pipe was removed and the crucible was placed outside of the furnace.
-
(4)
The old surface film was removed to expose the underneath melt. The newly exposed melt was protected by the protective gas for 1 minute, and then graphite powder was uniformly distributed on the melt surface. Note that the newly exposed melt rapidly oxidized with cauliflower-like oxides appearing as soon as the fresh surface was exposed. Therefore, the fresh surface needed to be protected by the gas before the application of graphite powder.
-
(5)
After being held at 973 K (700 °C) for 30 minutes in the furnace, the crucible was moved out and then allowed to be cooled in air. The graphite powder amount was designed to be 0, 2.7, 5.4, 8.1, and 10.8 g dm−2. The hole in the furnace cover for the protective gas pipe was blocked by asbestos during the formation process of the surface films.
-
(6)
The graphite powder was easily removed to expose the casting surfaces shown in Figure 2. The surface was fully covered with cauliflower-like oxides when graphite powder was not used (Figure 2(a)), while few cauliflower-like oxides appeared when graphite powder used (Figures 2(b) through (e)), indicating that the graphite powder can protect the molten alloy and further indicating that the protection of the gas for the fresh surface before the application of graphite powder had little effect on the present results.
Two surface film samples with dimension of approximately 10 mm × 10 mm were cut off from each casting surface, and then were ultrasonically cleaned. One was used to observe the surface morphology, and the other was used to prepare for the sample to observe the cross section. The cross section sample was mounted, polished, and etched in 0.5 wt pct oxalic acid solution at room temperature for 15 seconds.
The surface and cross section morphology of the surface films was examined by EVO MA 10 scanning electron microscopy (SEM) with Inca X-Max 50 energy dispersive spectroscopy (EDS). The phase of the surface films was determined by a D8 ADVANCE X-ray diffraction (XRD) with Mo Kα radiation. The samples for phase analysis by XRD were those used for surface morphology observation.
Results and discussion
Effects of Graphite Powder Amount on Surface Morphology of Surface Films
Figure 3 presents the surface morphology of the surface films with 2.7, 5.4, 8.1, and 10.8 g dm−2 graphite powder at 973 K (700 °C) for holding time of 30 minutes, showing many folds on the surface film. Mirak et al.[27] classified these folds into two different types. The first type, micro-sized folds, is considered as wrinkles, the formation of which is similar to those appearing on the dynamically formed surface films, which probably formed as the result of the contraction stress exerted on the surface film as the contraction coefficient was different between the surface film and the molten alloy.
The other type with the macro-sized features can be classified as folds, of which the formation was different from those that appeared on the dynamically formed surface films. Literature indicates that the formation of the folds was the result of turbulence of the molten alloy during casting.[27] However, the surface films in the present work were formed under static conditions and thus the folds could not be the result of turbulence of the molten AZ91D alloy.
The formation of the folds is most likely related to the solidification process. During solidification, the contraction of solidified and molten AZ91D alloy is different in volume, resulting in larger volume shrinkage of molten alloy than that of the solid alloy. Therefore, some sites at which the alloy solidified earlier became the peaks of the folds, while other sites at which the alloy solidified later became the valleys.
The folds rather than the wrinkles, of which the height indicating the distance between the peak and the valley makes casting surfaces uneven, affect the surface quality. Therefore, the variation of the folds with graphite powder amount needs to be understood. When 2.7 g dm−2 graphite powder was used, the height of the folds was short (Figure 3(a)), which made the surface smooth. With the increase in graphite powder amount, the surfaces became uneven due to the increased height of the folds (Figures 3(b) and (c)). When the graphite amount increased to 10.8 g dm−2, the surface was very uneven due to the further increased height of the folds (Figure 3(d)). The results indicate that increasing the graphite powder amount increased the height of the folds and then the surface quality deteriorated.
As mentioned previously, the formation of the folds is most likely related to the solidification process, indicating that some sites at which the alloy solidified earlier became the peaks of the folds, while other sites at which the alloy solidified later became the valleys. Generally, solidification contraction depends on several factors including chemical composition, super heat, mold temperature, gas porosity, applied stress, and solidification range. Obviously, the applied stress exerted by the graphite powder on the underneath melt solidified later was different when different amount of graphite powder was used. The applied stress increased by increasing the graphite powder amount, leading to an increase of the melt in solidification contraction and then that of the folds in height.
Effects of Graphite Powder Amount on Compositions of Surface Films
The chemical composition of the surface films formed with different graphite powder amount was determined using EDS at an acceleration voltage of 5 kV. The composition determination was carried out at three different selected areas with 2.301 mm ×1.732 mm with the results showing that the chemical composition of the surface films mainly consisted of magnesium, oxygen, fluorine, carbon, and a small amount of aluminium.
Figure 4 presents the variation of the chemical composition of the surface films with graphite powder amount, showing the variation of the magnesium content with graphite powder amount was opposite to that of the carbon content and the total content of the two elements was approximately 41 at. pct. However, the variation of the oxygen content with graphite powder amount was opposite to that of the fluorine content and the total content of the two elements was approximately 58 at. pct.
Figure 5 presents the phase composition of the surface films formed at different graphite powder amount, showing the appearance of Mg, MgO, and MgF2 in all surface films. Obviously, Mg came from the bulk alloy. C appeared only in the surface film formed with 8.1 g dm−2 graphite powder, while MgAl2O4 appeared in the surface film formed with 2.7 g dm−2 graphite powder.
Reaction [1] shown below can readily occur due to the high affinity of magnesium to oxygen and the resultant MgO was an important phase of the surface films. However, the resultant MgO is porous with a Pilling-Bedworth ratio (PBR) of 0.81 at 298 K (20 °C),[12] not preventing the molten alloy from oxidation and volatilization.
The graphite powder on the molten alloy surface can be removed easily and the surface film samples were ultrasonically cleaned before observation. Therefore, the carbon phase detected out by XRD (Figure 5) was one phase of the surface films, not coming from the graphite powder used to protect the molten alloy. However, the carbon phase was detected out only from the surface film formed with 8.1 g dm−2 graphite powder and was not detected out from those formed at other graphite powder amount (Figure 5), which is most likely related to the carbon content in the surface films as shown in Figure 4. Therefore, the graphite powder on the molten surface is believed to undergo some reactions during the holding process to generate the carbon phase in the surface film.
The graphite powder can react directly with oxygen in the atmosphere to produce gaseous carbon dioxide[16] by the following chemical equation:
The oxidation of graphite belongs to the in-pore diffusion controlled regime between 873 K and 1173 K (600 °C and 900 °C),[28] and the resultant carbon dioxide could continue to react with magnesium according to the following chemical reaction[12,29]:
The MgO produced by Reaction [1] was highly porous, providing easy paths for CO2 to react with Mg2+ ions diffusing from the molten alloy to the melt surface by Reaction [3], and then the resultant carbon phase filled the interstices of MgO grains to form a protective layer of MgO-C.[12]
The generation of MgF2 was related to the SF6 in the protective gas because SF6 can react with Mg or MgO to produce MgF2 between the molten alloy or MgO layer as seen below[26,30,31,32]:
Furthermore, the residual fluorine also could continue to produce MgF2 after the supply of the protective gas was stopped.[33] The resultant MgF2 (PBR = 1.29) increased the PBR of the surface film, preventing the molten alloy from oxidation and volatilization.[34]
MgAl2O4 are the product of gradual oxidation,[35] which may occur according to the following chemical reaction:
Aluminium is soluble in magnesium and can thus diffuse out to form MgAl2O4 at the interfacial layer between the oxide and the substrate, which may affect Mg diffusion from the substrate, preventing the melt from oxidizing.[36] MgAl2O4 was detected out only from the surface film formed with 2.7 g dm−2 graphite powder and was not detected out from those formed at other graphite powder amount (Figure 5), which is most likely related to the aluminium content in the surface films as shown in Figure 4.
That the effect of graphite powder amount on the reactions resulted in the variation of the surface films in composition with graphite powder amount is certain. However, how the graphite powder amount affects the reactions remains unknown, which may be our future work. Our future works may also include knowing the reason why the total content of oxygen and fluorine or that of magnesium and carbon of the surface films formed with different graphite powder amount could be stable at 58 or 41 at. pct.
Effects of Graphite Powder Amount on Structure of Surface Films
The cross sections were observed to understand the structure of the surface films formed with different graphite powder amount at 973 K (700 °C) for holding time of 30 minutes. Figure 6 presents the observation result of the cross section of the surface film formed with 8.1 g dm−2 graphite powder, indicating that the surface film was composed of a continuous and compact layer and the underneath regular particles. The layer was protective and the protective layer may be contributively preventing the melt from oxidizing.
As mentioned previously, carbon was thought to be one phase of the surface films. However, the mounting materials contain a lot of carbon. When the electron beam of SEM is very close to boundary of mounting materials and the sample, the carbon signal may come from the mounting materials, leading to wrong analytic results. In order to separate the mounting materials and the samples, a gold layer was deposited on the sample surface before mounting by K 570-type sputter coater system with sputtering time of 200 seconds.
The chemical composition of the cross section of the surface film formed with 8.1 g dm−2 graphite powder was tested using EDS at an acceleration voltage of 5 kV to understand the distribution of the above mentioned phases and the results were also shown in Figure 6. The chemical composition of the protective layer at Point 1 in Figure 6(a) consisted of C, F, O, and Mg (Figure 6(b)), and the phases were thought to be MgF2, MgO, and C. The chemical composition of the regular particles at Point 2 (Figure 6(a)) mainly consisted of F and Mg (Figure 6(c)), and the phase was thought to be MgF2.
The element Al in the surface film frequently appeared at the interface between the protective layer and the bulk alloy. For example, the chemical composition of the protective layer at Point 3 in Figure 6(a) consisted of C, F, O, Mg, and a small amount of Al (Figure 6(d)), and the Al-containing phase was thought to be MgAl2O4. The lower Gibbs free energy than the MgO may attribute to the formation of MgAl2O4. During the melting process of Mg-Al alloys, MgAl2O4 could easily form at the interfaces between the MgO/ or Mg17Al12 and the matrix due to the reaction of Al diffusing to the interface to react with oxygen and magnesia film.[37]
Through the above results, the surface films formed on the molten AZ91D alloy protected by graphite powder composed of a protective layer and the underneath regular MgF2 particles with the schematic illustration shown in Figure 7. The phase of the continuous and compact protective layer mainly consisted of MgO, MgF2, C, and MgAl2O4.
The cross section morphology of the surface films formed with different graphite powder amount is shown in Figures 8 and 9, being observed at high magnification and low magnification, respectively. The thickness of the protective layer attributed to preventing the melt from oxidizing was measured and the underneath particles were not included in the measurement. For example in Figure 7(b), the large white particle would not be measured as a part of the protective layer.
When 2.7 g dm−2 graphite powder was used, the protective layer was loose with thickness of approximately 536 nm (Figure 8(a)), partially protecting the underneath molten alloy (Figure 2(b)). A small amount of MgF2 particles with small size existed at the interface between the protective layer and the bulk alloy (Figure 9(a)).
When the graphite powder amount increased to 5.4 g dm−2, the thickness of the protective layer decreased to approximately 201 nm (Figure 8(b)), while the number and size of MgF2 particles increased compared to those of the surface film formed with 2.7 g dm−2 graphite powder (Figure 9(b)). However, the protective layer was compact and increased the protective ability of the graphite powder. Therefore, only a few cauliflower-like oxides with small size appeared on the casting surface (Figure 2(c)).
When 8.1 g dm−2 graphite powder was used, the protective layer was relatively compact with thickness of approximately 469 nm (Figure 8(c)). However, the number of the large underneath MgF2 particles was so numerous that many particles were closely arranged with no distinct interface between them (Figure 9(c)).
Though the thickness of the protective layer of the surface film formed with 10.8 g dm−2 graphite powder was approximately 279 nm and less than that of the surface film formed with 8.1 g dm−2 graphite powder, the protective layer was also relatively compact (Figure 8(d)), completely protecting the underneath alloy melt (Figures 2(d) and (e)). The number and size of MgF2 particles of the surface film formed with 10.8 g dm−2 graphite powder was also large (Figure 9(d)), similar to that of the surface film formed with 8.1 g dm−2 graphite powder.
From the above results, the surface films formed with different amount of graphite powder consisted of a protective layer and the underneath MgF2 particles, the structure of which varied with graphite powder amount. The number and size of the underneath MgF2 particles increased with graphite powder amount, while the thickness of the protective layer ranging from 200 to 550 nm shows no trend with relation to graphite powder amount.
However, the variation of the thickness of the protective layer with the graphite powder amount does not contradict to the fact that the surface films grow thicker with increasing the reactants. The protective layers consisted of MgO, MgF2, C, and MgAl2O4, which were the products of the previously mentioned reactions. With increasing the graphite powder amount, the reaction area of Reaction [2] increased, leading to more resultant CO2, and then more resultant carbon phase in surface films, which was a benefit for the protective layer to increase in thickness. Furthermore, the thickness growth of the protective layer completed with the generation of the underneath MgF2 particles, which made the growth of the protective layer in thickness unstable. However, the growth of the thickness of the protective layer was related not only to the previously mentioned reactions but also to the compactness of the layer, which also enhanced the instability of the thickness growth of the protective layer.
The graphite powder amount also affected the number and size of the underneath MgF2 particles. As mentioned previously, the newly exposed melt was protected for 1 minute by the protective gas consisting of 0.5 vol pct SF6 and 99.5 vol pct CO2 before the application of graphite powder. When graphite powder covered AZ91D alloy melt, a part of SF6 absorbed on the melt surface continued to react with Mg or MgO by Reactions [4] or [5] to produce MgF2 particles, while the other part might diffuse into the atmosphere through the graphite powder above, with the understandable fact that the diffusion resistance of SF6 increases with increasing the graphite powder amount. Therefore, more SF6 was absorbed on the melt surface to form more MgF2 particles, leading to an increase of MgF2 particles in number, and size with graphite powder amount.
Graphite powder was adopted to protect molten magnesium alloy and the effect of holding time, melt temperature, and graphite powder amount on protection ability,[17] and the resultant surface films has been studied. Based on the results, the protection mechanism was thought to be that the protective layer, the products of the reactions between the graphite powder, AZ91D alloy melt, and the ambient atmosphere, might contribute to preventing the melt from oxidizing.
However, the protection of graphite powder for molten magnesium alloy might be due to other protection mechanisms such as the effect of gas phase CO2 that formed by graphite oxidation shown in Reaction [2]. The gas layer over the magnesium surface separated air and magnesium, which may lead to lower reaction rate than that occurring when molten magnesium alloy directly exposed to air. As an evidence, Figure 2(b) shows that several spots of the cauliflower-like oxides appeared at the edge. The temperature at the edge is typically lower that at the center. Air flows from the edge to center and pushes CO2 protective gas away from the edge. Therefore, the concentration of oxygen at the edge will be higher than at the edge, which will lead to fast oxidation and form the cauliflower-like oxides at the edges.
Although, the graphite powder could protected the molten magnesium alloy at melt temperature from 933 K to 1053 K (660 °C to 780 °C) under certain conditions, the protection method still exists many problems to be solved such as whether CO forms by graphite oxidation or forms in the reaction of Mg with CO2. However, CO is possible to be produced by the thermodynamic method according to the following reaction:
In order to realize the industrial application of graphite powder as a protective medium of magnesium alloy melts, future study should be focused on the existing problems including what is the effect of the resultant CO on the process to protect magnesium from oxidation, whether the CO content in the system reaches to the level over which the CO gas will harm human health and how the harmful side effect can be avoided.
Conclusions
-
1.
The morphology of the surface films formed on molten AZ91D alloy protected by graphite powder with different amounts were full of folds and wrinkles. The unevenness of the micro surface morphology caused by the folds increased with graphite powder amount, which may be due to the increased applied stress exerted on the melt during solidification.
-
2.
The composition of the surface films consisted of magnesium, oxygen, fluorine, carbon, and a small amount of aluminium, possibly existing in the form of MgO, MgF2, C, and MgAl2O4. The variation of the oxygen content with graphite powder amount was opposite to that of fluorine, while that of magnesium was opposite to that of carbon.
-
3.
The surface films were composed of a protective layer and the underneath MgF2 particles. The protective layer, of which the thickness range was from 200 to 550 nm, contributed to the effective prevention of the molten alloy from oxidizing. The number and size of the underneath MgF2 particles increased with graphite powder amount. The graphite powder amount may affect the composition and the structure of the surface films by affecting the reactions between the graphite powder, AZ91D alloy melt and the ambient atmosphere.
References
V. Fournier, P. Marcus and I. Olefjord: Surf. Interface Anal., 2002, 34, pp. 494-97.
A. Karger, F. W. Bach and C. Pelz: Mater. Sci. Forum, 2005, 488-489, pp. 4.
X. F. Wang and S. M. Xiong: J. Mater. Sci. Technol., 2014, 30, pp. 353-58.
X. F. Wang and S. M. Xiong: Corros. Sci., 2013, 66, pp. 300-07.
A. Mirak, C. J. Davidson and J. A. Taylor: Corros. Sci., 2010, 52, pp. 1992-2000.
H. K. Chen and Z. F. Gong: Trans. Nonferrous. Met. Soc., 2012, 22, pp. 2898-905.
X. Zhang, G. You, J. Zha and S. Long: Rare Metal Mat. Eng., 2011, 40, pp. 1496-99.
G. Q. You, S. Y. Long and R. F. Li: Mater. Sci. Forum, 2007, 546-549, pp. 119- 22.
W. Ha, J. I. Youn and Y. J. Kim: Mater. Sci. Forum, 2006, 510-511, pp. 806-09.
H. Won, J. E. Lee and Y. J. Kim: Mater. Sci. Forum, 2005, 475-479, pp. 2543-46.
S. L. Cheng, G. C. Yang, J. F. Fan, Y. J. Li and Y. H. Zhou: Trans. Nonferrous. Met. Soc., 2009, 19, pp. 299-304.
S. Emami and H. Y. Sohn: Metall. Mater. Trans. B, 2014, 46, pp. 226-34.
J.W. Fruehling: Ph.D. Thesis, University of Michgan, Ann Arbor, Michigan, 1970.
S. C. Yang and Y. C. Lin: J. Clean. Prod., 2013, 41, pp. 74-81.
W. M. Guo, H. N. Xiao and G. J. Zhang: Corros. Sci., 2008, 50, pp. 2007-11.
H. Badenhorst: Chem. Eng. Sci., 2013, 104, pp. 117-24.
W. H. Li, J. X. Zhou, B. C. Ma, J. H. Wu, J. W. Wang, H. H. Zhuang, Y. S. Yang and X. H. Huang: Mater. Sci. Forum, 2017, 898, pp. 111-17.
D. Dispinar and J. Campbell: Int. J. Cast Met. Res., 2006, 19, pp. 5-17.
J. Campbell and M. Tiryakioglu: Aluminium Alloys 2006, Pts 1 And 2, 2006, vols. 519–521, pp. 1453–60.
M. Divandari and J. Campbell: Int. J. Cast Met. Res., 2005, 18, pp. 187-92.
M. Divandari and J. Campbell: Int. J. Cast Met. Res., 2004, 17, pp. 182-87.
R. Raiszadeh and W. D. Griffiths: Metall. Mater. Trans. B, 2006, 37, pp. 865-71.
S. A. Azarmehr, M. Divandari and H. Arabi: Mater. Sci. Technol., 2012, 28, pp. 1295-300.
S. Amirinejhad, R. Raiszadeh and H. Doostmohammadi: J. Therm. Anal. Calorim., 2013, 113, pp. 769-77.
B. Nayebi, A. Bahmani, M. S. Asl, A. Rasooli, M. G. Kakroudi and M. Shokouhimehr: J. Alloys Compd., 2016, 655, pp. 433-41.
S. M. Xiong and X. L. Liu: Metall. Mater. Trans. A, 2007, 38, pp. 428-34.
A. R. Mirak, M. Divandari, S. M. A. Boutorabi and J. Campbell: Int. J. Cast Met. Res., 2007, 20, pp. 215-20.
L. Xiaowei, R. Jean-Charles and Y. Suyuan: Nucl. Eng. Des., 2004, 227, pp. 273-80.
L. Zhang, J. L. Tang and D. B. Zeng: Foundry Technol., 2005, 26, pp. 930-34.
S. H. Nie, S. M. Xiong and Z. Liu: Rare Metal Mat. Eng., 2007, 36, pp. 21-25.
S.H. Nie, X.L. Liu, S.M. Xiong and B.C. Liu: J. Mater. Eng., 2005, vol. 6, pp. 3–6, 26.
S. Emami, H. Y. Sohn and H. G. Kim: Metall. Mater. Trans. B, 2014, 45, pp. 1370-79.
S. H. Nie, S. M. Xiong and B. C. Liu: Mater. Sci. Eng., A, 2006, 422, pp. 346–51.
W. Ha and Y. J. Kim: J. Alloys Compd., 2006, 422, pp. 208-13.
F. Czerwinski: Acta Mater., 2002, 50, pp. 2639-54.
T. S. Shih, J. B. Liu and P. S. Wei: Mater. Chem. Phys., 2007, 104, pp. 497–504.
Y. J. Chen and P. S. Wei: Mater. Trans., 2007, 48, pp. 3181-89.
Acknowledgments
Funding for this work was provided by the National Key Research and Development Program of China (No. 2016YFB0701202 and No. 2016YFB0301105). This work was also supported by the Natural Science Foundation of Shandong Province (No. ZR2016EMB11 and ZR2015YL007) and by the Youth Foundation of Shandong Academy of Sciences (No. 2014QN024). We greatly appreciate the help of Ian R. McAdams and Dr. Feng Gao for revising the English manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 16, 2017.
Rights and permissions
About this article
Cite this article
Li, W., Zhou, J., Ma, B. et al. Effect of Graphite Powder Amount on Surface Films Formed on Molten AZ91D Alloy. Metall Mater Trans B 48, 2564–2573 (2017). https://doi.org/10.1007/s11663-017-1062-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-017-1062-1