Abstract
Background
Previous studies have reported that the fracture risk related to sarcopenic obesity (SO) may be influenced by the distribution of fat mass. Therefore, it is useful to explore a body component suitable for defining obesity when predicting fracture risk. This study was an attempt to explore the contribution of SO defined by visceral adiposity on the incidence of osteoporotic fracture.
Methods
We enrolled 736 Chinese patients aged > 60 years in this prospective study. Sarcopenia was defined as low skeletal muscle index (SMI) with muscle strength or low SMI with low physical performance. Obesity was categorized as follows: (1) android to gynoid ratio (A/G ratio, men > 0.82, women > 0.65) as an indicator of visceral adiposity; (2) body fat percentage (men > 27.8%; women > 34.5%); and (3) body mass index (≥ 25 kg/m2). A Cox proportional hazard model was used to determine the association between SO and the risk of osteoporotic fracture.
Results
The incidence of SO was 8.7%; 9.0% in females and 8.1% in males. Of 223 (30.2%) patients with self-reported fractures. SO classified by A/G was associated with an increased risk of osteoporotic vertebral fracture (HR: 1.71, 95% CI: 1.07–2.72). High SMI was associated with a reduced risk of osteoporotic vertebral fracture (HR: 0.82, 95% CI: 0.72–0.93), higher BMI was associated with a higher risk vertebral fracture (HR: 1.12, 95% CI: 0.94–1.63), and higher A/G ratio was associated with a higher risk of any fracture (HR: 1.28, 95% CI: 1.14–1.43) and osteoporotic vertebral fracture (HR: 1.19, 95% CI: 1.05–1.36).
Conclusions
Our findings suggest that SO, defined by visceral adiposity, was associated with the risk of osteoporotic vertebral fracture. Moreover, low SMI, low muscle strength and visceral adiposity were independently associated with osteoporotic fracture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcopenic obesity, characterized by loss of muscle mass, low physical performance with body fat accumulation with age, is related to a higher risk of falls, fractures, and metabolic diseases in older adults[1]. This further results in declining quality of life and increased risk of death, which are associated with heavy social and economic burdens[2].
Sarcopenia produces a negative impact on bone mass and leads to a decrease in BMD, which is significantly associated with osteoporosis[3]. Elderly individuals with sarcopenia are at a high risk of falls and fragility fractures, and women with sarcopenia are 2.7 times more likely to sustain osteoporotic fracture (vertebral fractures and non-vertebral fractures) than their normal cohorts[4]. In addition to sarcopenia, obesity has been reported to be site-specific regarding fractures[5]; however, the relationship between obesity and fractures remains controversial. Traditionally, low body mass index (BMI) is related to an increased risk of fracture, and obesity was identified as a protective factor[6]. However, recent studies have shown that individuals with obesity have a higher risk of osteoporotic fractures[7]. Moreover, some studies have shown that obesity according to BMI is associated with a reduced risk of fracture, whereas those who are obese according to body fat percentage (BF%) have an increased risk of fracture[8], which is likely to be related to the different body components used to define obesity.
The link between obesity and fracture risk may be influenced by the distribution of fat mass, and BMI is not a great marker of adiposity particularly in older adults[9]. Although higher BMI is beneficial for bone mass owing to the large mechanical load, different fat distribution can alter this relationship. A study involving 54,934 participants, the Nurses’ Health Study, showed that larger waist circumference was related to higher vertebral fracture risk after adjusting for BMI [10]. Another study found that increased fat content and BF% were positively associated with BMD [11], and abdominal fat (A/G ratio) was negatively associated with BMD [12] and increased fracture risk. Thus, the type of obesity, for example, abdominal obesity, and total and visceral adiposity, increase the likelihood of osteoporosis-related fractures. A longitudinal study found an adverse effect of visceral adiposity on peripheral bone strength [13], and Machado et al. [14] reported that visceral adiposity evaluated by DXA scan was associated with the risk of non-spine fragility fractures in elderly women in the community. These findings presumably further suggest that visceral adiposity in abdominal obesity potentially plays a crucial role in the incidence of osteoporotic fractures.
While computed tomography (CT) is considered the gold criteria of visceral adipose tissue measurement, however, CT has shortcomings in clinical use (such as expense and radiation exposure). Whole-body DXA is an inexpensive and convenient measure for assessing visceral fat that also accurately measures regional fat and muscle mass[15]. Some previous studies have demonstrated that total and visceral adiposity obtained using whole-body DXA were related to the prevalence of vertebral fracture in women[16], and the A/G ratio measured by DXA was a good indicator of central adipose distribution. To our knowledge, no studies have compared the predictive power of total and visceral fat based on whole-body DXA for examining the association between SO and osteoporotic fracture.
We conducted a prospective study to assess the contributing of sarcopenic obesity defined by different body composition measurements on the incidence of osteoporotic fracture, and to determine which indicator of obesity measured by DXA is appropriate for diagnosing sarcopenic obesity to predict osteoporotic fractures. Furthermore, we analyzed the relationship between SO components and the risk of osteoporotic fracture.
Methods
Participants
This prospective study involved patients aged over 60 years who regularly visited the Spinal Surgery Department of Shanghai East Hospital between 1 May 2013 and 31 May 2018. Patients were enrolled if they were diagnosed with osteoporosis according to the criteria of the guidelines for osteoporosis[17]. Exclusion criteria were as follows: (1) the presence of metabolic bone disease other than osteoporosis (Cushing’s disease, hyperthyroidism, hyperparathyroidism, thyroid cysts, or hypothyroidism); (2) incomplete medical records, imaging data, and other physical measurements; (3) patients who subsequently died owing to an accident or other diseases; (4) pathological fracture caused by cancer, infection, inflammatory disease, or high-energy trauma; (5) patients who underwent surgery (percutaneous vertebroplasty, percutaneous kyphoplasty) after an initial fracture; and (6) patients treated with glucocorticoids and/or hormone replacement therapy. This study was approved by the ethics committee of Shanghai East Hospital. All participating individuals agreed to the use of their clinical data for the present study and provided signed informed consent. All enrolled individuals with osteoporosis were routinely treated with vitamin D, calcium tablets, and bisphosphonates.
Baseline clinical variables and anthropometrics
Demographic status, health status, and lifestyle data for all patients were recorded using a structured comprehensive questionnaire. Age, sex, smoking status (current smoker), alcohol intake, number of comorbidities (such as cardiovascular disease, diabetes, and other major illness), chronic pain (the presence of pain in different parts of the body), history of falls, and prior fracture information were collected from the patient questionnaires, and a history of antiosteoporosis drug use was recorded as regular use of bisphosphonates or teriparatide. Physical disability was assessed by 7 items from a modified version of the Katz activities of daily living (ADL) scale, ADL disability was defined as needing help with one or more activities[18, 19].
At baseline, height and weight were obtained using an electronic scale. BMI was calculated as weight (kg) divided by height in meters squared (kg/m2), waist circumference (WC) was measured at the mid-point between the lowest rib and the iliac crest, and BMI and WC were measured by two trained nurses. Serum 25(OH) vitamin D concentration was measured using an enzyme-linked immunosorbent assay method at enrollment. Whole-body DXA (Prodigy GE Healthcare, Chicago, IL) was performed to measure body composition and BMD. Appendicular skeletal muscle mass (ASM) was calculated according to the total of the lean mass of the upper and lower extremities (kg), and SMI was calculated as ASM divided by height in meters squared (kg/m2). Femoral neck (FN) BMD, total fat mass, BF%, and regional fat mass (android fat mass and gynoid fat mass) were obtained using whole-body scans, as previously described [20]. The A/G ratio was calculated as android fat mass divided by gynoid fat mass, and was used to assess visceral adiposity.
Hand grip strength and physical performance
A digital hand dynamometer (EH101; KYTO, Shanghai Province, China) was used to measure HGS in each participant. The patients were asked to sit with their back in a chair, and HGS was measured by elbow flexion to 90 degrees with maximum force on each hand [21]. HGS was tested three times, and the highest value for each hand was used for analysis.
Gait speed was used to assess physical performance (PP). Patients walked in a straight line at their usual speed in a sufficiently long hallway, and each patient underwent standardized training before measuring gait speed. All participants were asked to walk 6 meters without deceleration, and the time was recorded by two trained doctors. Every participant was tested twice, and the averaged measurement was used (accurate to 0.01 m/s).
Definitions of sarcopenic obesity
We categorized the patients into four groups (non-sarcopenic, non-obese; non-sarcopenic, obese; sarcopenic, non-obese; and sarcopenic, obese) according to the diagnostic standards for sarcopenia and obesity. Sarcopenia was defined according to the AWGS 2019 diagnostic criteria[22] as follows: low SMI with low muscle strength (MS) or low SMI with low PP. SMI < 7.26 kg/m2 for men and SMI < 5.5 kg/m2 for women was considered low muscle mass. Low MS was defined according to MS < 28 kg for men and MS < 18 kg for women, and low PP was determined by gait speed ≤ 1.0 m/s in all patients.
Obesity was defined according to different body components in this study, using several indicators of obesity, as follows[8]: (1) according to the WHO definition of obesity, individuals with BMI < 25 kg/m2 were considered “non-obese,” and those with a BMI ≥ 25 kg/m2 were considered “obese;” (2) obesity was also defined according to BF% (men > 27.8%; women > 34.5%) higher than the 60th percentile of the present study cohort; (3) in addition, A/G, as an indicator of central adipose accumulation, was used to evaluate the association between obesity and fracture. An A/G higher than the 60th percentile of the present study cohort was considered obese (men > 0.82, women > 0.65).
Fracture evaluation
Following the baseline assessment, participants were followed-up according to self-reported fractures in the outpatient clinic and were evaluated at approximately 1 year and 3–5 years. All patients were asked to answer the question, “Have you had any fractures since you were enrolled in the study?” Once the history of fracture was reported, the specific time and location of the fractures were recorded from the hospital medical records and radiology imaging data or were obtained through patient self-reporting; only fragility fractures were recorded. Pathological, high-energy trauma fractures, and some site-specific fractures, such as those in the hands, fingers, feet, toes, and the skull, were excluded from the data collection. Fractures were categorized as any fracture, vertebral fracture, and non-vertebral fracture.
Statistical analyses
Categorical variables are presented as percentage and were analyzed using Pearson’s chi-square test or Fisher’s exact test. Continuous variables are shown as mean ± standard deviation, and differences between the four categories were analyzed by one-way ANOVA. Bonferroni post hoc tests were used for these analyses. The survival curve was created using the Kaplan–Meier method, and a log-rank test was used to compare the survival time in each group.
The association between baseline SO categories according to the A/G ratio, SO components, and the occurrence of any fracture (non-vertebral and vertebral fracture) was analyzed with the Cox proportional hazard model, and the outcomes considered in the models were fracture-related. All potential risk factors were incorporated into a univariate model with non-sarcopenic and non-obese groups as the reference. In the multivariate model, age, current smoker, number of comorbidities, ADL disability, history of falls, prior fracture, history of anti-osteoporosis drug use, and BMD were adjusted in the multivariate model with a stepwise procedure. We also calculated the HRs of SO when obesity was defined by BF% or BMI. Next, the associations of muscle mass and strength with osteoporotic fracture were also explored using another multivariate model, and potential body composition risk factors were incorporated into the model. A restricted cubic spline (smooth curve) was used to assess nonlinear relationships between visceral fat (A/G ratio) and osteoporotic vertebral fracture (OVF) risk. Sensitivity analyses included the following: (1) considering for possible vertebral biomechanical changes, we analyzed whether the association would change if only individuals who underwent surgery (percutaneous vertebroplasty, percutaneous kyphoplasty) after an initial vertebral fracture at baseline; (2) considering the effect of glucocorticoids, hormone replacement therapy on bone metabolism, we reanalyzed the association between SO and osteoporotic fracture by excluding participants who were treated with glucocorticoids and/or hormone replacement therapy in the follow-up period. In the sensitivity analysis, the results did not change substantially.
All statistical analyses were performed using the R statistical package, version 3.0.1 for Windows (The R Project for Statistical Computing, Vienna, Austria). The level of statistical significance was set at p < 0.05.
Results
Data for 736 patients were analyzed; 48.9% (360), 30.4% (224), 12.0% (88), and 8.7% (64) of the patients were categorized as follows: (1) non-sarcopenic, non-obese, (2) non-sarcopenic, obese, (3) sarcopenic, non-obese, and (4) sarcopenic, obese. The baseline characteristics of the four groups of participants classified by visceral fat are presented in Table 1. Patients with SO were older than patients in the non-sarcopenic, non-obese and non-sarcopenic, obese groups. The proportion of men with SO was significantly higher compared with non-sarcopenic, obese patients. Patients with SO were significantly more likely to smoke, fall, and have higher ADL disability compared with patients in the non-sarcopenic, non-obese group and non-sarcopenic, obese group. Compared with the non-sarcopenic, non-obese group, those with SO had a significantly higher number of comorbidities, prior fractures, and anti-osteoporosis drug use. The SO group had significantly higher BMI, A/G ratio, and trunk fat than the non-sarcopenic, non-obese group and sarcopenic, non-obese group. Body fat, android fat mass, and handgrip strength were significantly higher in participants with SO than in the other groups. Participants with SO had significantly higher ALM, SMI, and gait speed compared with the non-sarcopenic, non-obese group and non-sarcopenic, obese group, and BMD was significantly lower than in non-sarcopenic, non-obese patients.
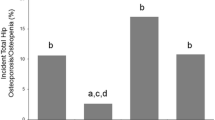
During the 5-year follow-up, 223 (30.2%) patients self-reported fractures; 186 (25.2%) sustained a vertebral fracture, and 73 (9.9%) sustained a non-vertebral fracture. Figure 1 shows the prevalence of fractures according to the groups classified by visceral fat. Hazard ratios for osteoporotic fractures in the groups classified by different body composition are shown in Table 2.
SO defined by visceral adiposity (A/G): in the unadjusted models, SO was related to a greater risk of any fracture (HR: 1.53, 95% CI: 1.03–2.26) and vertebral fracture (HR: 1.98, 95% CI: 1.28–3.07). Adjustment for other variables did not alter the association with vertebral fracture (HR: 1.71, 95% CI: 1.07–2.72); “sarcopenic, non-obese,” was also associated with an increased risk of vertebral fracture (HR: 1.55, 95% CI: 0.94-2.55). The survival curve is shown in Fig. 2.
SO defined by body fat percentage and BMI: no significant association was observed in patients with SO; “sarcopenic, non-obese,” was also associated with an increased risk of any fracture (BF%: HR: 1.78, 95% CI: 1.17–2.72; BMI: HR: 1.71, 95% CI: 1.13–2.59) and vertebral fracture (BF%: HR: 2.42, 95% CI: 1.51–3.85; BMI: HR: 2.21, 95% CI: 1.39–3.53). The relationship between sarcopenic, non-obese and the risk of any fracture (BF%: HR: 1.91, 95% CI: 1.23-2.98; BMI: HR: 1.73, 95% CI: 1.12-2.69) and vertebral fracture (BF%: HR: 2.36, 95% CI: 1.47-3.78; BMI: HR: 2.16, 95% CI: 1.35-3.46) remained significant when adjusting the variables.
The associations of the sarcopenic obesity components (muscle mass, muscle strength, BMI, BF%, A/G) as categorical variables with osteoporotic fracture are shown in Table S1. Higher SMI was related to a lower risk of vertebral fracture (HR: 0.82, 95% CI: 0.72–0.93). Greater grip strength was associated with a lower risk of any fracture (HR: 0.94, 95% CI: 0.92–0.96) and vertebral fracture (HR: 0.92, 95% CI: 0.91–0.93). Higher BMI was associated with a higher risk vertebral fracture (HR: 1.12, 95% CI: 0.94-1.63). Higher A/G ratio was associated with a higher risk of any fracture (HR: 1.28, 95% CI: 1.14–1.43) and vertebral fracture (HR: 1.19, 95% CI: 1.05–1.36). More importantly, we observed a nonlinear relationship between A/G ratio and vertebral fracture risk, with a J-shaped curve (Figure S1). In the sensitivity analysis that excluded individuals who underwent surgery after an initial vertebral fracture at baseline and treated with glucocorticoids and/or hormone replacement therapy in the follow-up period, the results did not change substantially (Table S2).
Discussion
This prospective study is the first to demonstrate that sarcopenic obesity determined according to visceral adiposity (A/G ratio) was related to an increased risk of OVF. There was no significant association between sarcopenic obesity defined by BMI or the BF% and the risk of osteoporotic fracture. In addition, low ASM, low MS, and visceral adiposity were independently associated with osteoporotic fracture.
Our results suggest that sarcopenic obesity diagnosed using visceral adiposity was associated with an increased risk of OVF. The influence of obesity on bone is most dependent on the site in which fat mass accumulates. Numerous recent studies have focused on the association between abdominal obesity and fracture risk. In the Tasmanian Older Adult Cohort study, the incidence of vertebral fracture was positively associated with WC in women [23]. According to the current literature, WC is negatively related to BMD and might increase the risk of vertebral fracture [24]. Moreover, Paik et al. reported that larger WC was independently related to higher vertebral fracture risk [10]. However, a study involving 1126 participants aged over 50 years found a significant association between abdominal fat and lower fracture risk in women, where each 1 SD decrease in abdominal fat mass was related to a 50% increased risk of low-level-trauma-related fracture and nonpathological fracture [25]. Thus, the impacts of abdominal obesity on the risk of osteoporotic fracture are complex, and the possible explanation is that visceral adipose tissue and subcutaneous adipose tissue have different effects on bone tissue. In contrast, many studies have proposed the opposite effects of visceral adipose tissue and subcutaneous adipose tissue on bone structure and strength [26], stating that visceral adiposity has a potential adverse impact on bone density, structure, and quality, and that subcutaneous fat may be beneficial [14, 27].
Differences in traditional measures of abdominal obesity are likely to have a contradictory impact on bone mass according to previous studies [28], such as WC and waist-hip ratio (WHR), owing to the lack of quantitative measurements of visceral fat. Given these findings, it is conceivable that a direct measurement of visceral adipose might be more helpful for predicting fracture risk. Android fat, as measured by DXA, represents visceral adipose tissue, and gynoid fat represents subcutaneous adipose tissue[29]. The A/G ratio may be a valuable indicator of visceral fat accumulation. A cross-sectional study involving 5749 Asian people showed that A/G was negatively associated with BMD in women; large amounts of visceral fat (visceral/subcutaneous) were negatively associated with the trabecular bone microarchitecture when adjusting for age [30]. These findings could explain why sarcopenic obesity diagnosed using visceral adipose tissue was associated with a greater risk of OVF, in the present study. Moreover, in a study of 352 elderly patients aged 62 years, greater (+1 SD) visceral adiposity increased the odds of any grade of vertebral fractures (OR = 2.50), and our results showed that visceral adiposity defined by A/G was independently associated with OVF (HR: 1.21)[16]. Therefore, visceral adiposity should be considered when assessing OVF risk in women.
It is somewhat surprising that SO, defined by BMI and BF%, was not associated with osteoporotic fractures at any site. Previous studies have shown that patients with SO (obesity was defined as BF%) had a higher incidence of non-vertebral fracture compared with the normal control group. Additionally, compared with obesity alone, SO was also associated with higher fracture rates over 10 years in men, but the result was not statistically significant after BMD adjustment in women [31]. Another study involving community-dwelling older men showed that the prevalence of fracture for SO (defined as BF%) was not significantly different compared with non-sarcopenic, non-obese older men after adjusting the variables [19], consistent with our findings. These heterogeneous results show that SO may be associated with an increased risk of fracture, but the effects are likely to be influenced by sex-specific and diverse methods of measuring body composition. The definition of SO according to different diagnostic criteria may result in different predictions of fracture risk. A prospective cohort study suggested that obesity defined according to BF% was associated with an increased prevalence of fracture, whereas when obesity was defined by BMI, obesity was related to lower risk of fracture. It is possible that high BMI is related to higher areal BMD. BF% was negatively associated with BMD and was identified as a risk factor for vertebral fractures; however, the relationship between fracture risk and obesity was not observed when obesity was defined by lower BF% cut-offs [8]; moreover, we found that high BMI (as continuous variables) was independently associated with osteoporotic vertebral fracture. Indeed, there is no universal definition of obesity according to BF% or BMI, and the use of different boundaries to define obesity may have different effects on fracture risk assessment. These heterogeneous findings may support our results. More research is needed to determine more accurate cut-off values for SO.
The benefit of obesity on bone strength and the cushioning impact of fat mass are insufficient to compensate for greater fall impact forces [32], although higher mechanical load can activate bone cells and promote the expression of insulin-like growth factor-1 and osteocalcin [33]. However, studies have found an inverse association between hip fracture risk and BMI, and WC was associated with hip fracture risk [34, 35]. This suggests that fat distribution may weaken the positive effect of obesity on bone. In our study, obesity defined by BMI was associated with a decreased risk of OVF, and we found the opposite result when visceral fat was used to assess obesity. In addition, A/G ratio was independently associated with OVF and any fracture. Increased visceral fat leads to increased inflammatory cytokines and oxidative stress in obese individuals, which can adversely affect bone metabolism by stimulating osteoclast activity [36]. Possible mechanisms underlying visceral fat and osteoporotic fracture risk may include different effects of mechanical stress, inflammatory factors, and the effect of adipocyte secretions on bone metabolism.
Sarcopenia is a potential risk factor for falls and fractures in older people [37], and our findings support this. Muscle mass and strength, as two different sarcopenia components, might have independent roles in health-related outcomes [38]. Studies have shown that the relationship between muscle strength and BMD may be explained by muscle mass [39]; however, it is unclear whether muscle mass and strength are independently related to osteoporotic fracture. In the present study, we found that low ASM and low MS were independently associated with osteoporotic fracture. Therefore, in addition to intervention measures for obesity, it is also important to pay attention to muscle mass and strength in older people. Our outcomes are consistent with previous studies showing that reduced muscle mass and lower HGS were associated with OVF in women [40].
The major strengths of this study are that we used different body components to define SO and assess fracture risk, and we also investigated the associations of osteoporotic fracture with muscle mass and strength. Moreover, traditional measures of abdominal obesity, such as WC and WHR, were not used to evaluate the relationship between SO and fracture risk. However, WC and WHR have not been recognized as good indicators of visceral fat; therefore, we used A/G to more accurately evaluate visceral fat. Additionally, we used DXA to measure body composition, which may be more accurate.
There are several limitations to this prospective study. First, changes in body composition with age were not evaluated, and increases or decreases in body composition may affect outcome events. The primary purpose of our study was to explore the impact of body composition at baseline on osteoporotic fracture, and more prospective studies are required to clarify the association between changes in body composition and fracture risk in SO patients. Second, we used only gait speed to assess PP for each patient, and some patients were excluded because they could not complete the gait speed test for specific reasons, which may have caused bias. Finally, gender sensitivity analysis was not used in the present study. Gender differences may exist in the relationship between SO and fracture risk[31]. However, we were unable to establish a valid statistical model for each gender because of the limited sample size and the number of women (only 10 women with SO sustained OVF) reaching the endpoint of osteoporotic events was relatively small. Therefore, studies involving large samples are required to demonstrate whether SO is related to fracture events in both men and women.
In conclusion, sarcopenic obesity, defined by high visceral fat, was associated with the risk of OVF, and whole-body DXA is considered a more appropriate assessment of SO. Meanwhile, muscle mass and muscle strength, as two different sarcopenia components, were independently associated with osteoporotic fracture. Our study highlights that individual with sarcopenic obesity require closer monitoring of bone health with age. Specific interventions should focus on reducing abdominal fat while improving muscle strength and muscle mass. The relationship between visceral fat and osteoporotic fracture warrants further investigation.
References
Batsis JA, Villareal DT (2018) Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 14:513–537
Arango-Lopera VE, Arroyo P, Gutiérrez-Robledo LM, Pérez-Zepeda MU, Cesari M (2013) Mortality as an adverse outcome of sarcopenia. J Nutr Health Aging 17:259–262
Walsh MC, Hunter GR, Livingstone MB (2006) Sarcopenia in premenopausal and postmenopausal women with osteopenia, osteoporosis and normal bone mineral density. Osteoporos Int 17:61–67
Sjöblom S, Suuronen J, Rikkonen T, Honkanen R, Kröger H, Sirola J (2013) Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas 75:175–180
Mpalaris V, Anagnostis P, Goulis DG, Iakovou I (2015) Complex association between body weight and fracture risk in postmenopausal women. Obes Rev 16:225–233
Compston J (2015) Obesity and fractures in postmenopausal women. Curr Opin Rheumatol 27:414–419
Johansson H, Kanis JA, Odén A et al (2014) A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res 29:223–233
Gandham A, Zengin A, Bonham MP et al (2020) Incidence and predictors of fractures in older adults with and without obesity defined by body mass index versus body fat percentage. Bone 140:115546
Bahat G, Kilic C, Topcu Y, Aydin K, Karan MA (2020) Fat percentage cutoff values to define obesity and prevalence of sarcopenic obesity in community-dwelling older adults in Turkey. Aging Male 23:477–482
Paik JM, Rosen HN, Katz JN, Rosner BA, Rimm EB, Gordon CM, Curhan GC (2019) BMI, waist circumference, and risk of incident vertebral fracture in women. Obesity (Silver Spring) 27:1513–1519
Meyer HE, Willett WC, Flint AJ, Feskanich D (2016) Abdominal obesity and hip fracture: results from the Nurses' Health Study and the Health Professionals Follow-up Study. Osteoporos Int 27:2127–2136
Zhang J, Jin Y, Xu S, Zheng J, Zhang Q, Chen J, Huang Y, Shao H, Yang D, Ying Q (2015) Associations of fat mass and fat distribution with bone mineral density in Chinese obese population. J Clin Densitom 18:44–49
Glass NA, Torner JC, Letuchy EM, Burns TL, Janz KF, Eichenberger Gilmore JM, Schlechte JA, Levy SM (2018) Does visceral or subcutaneous fat influence peripheral cortical bone strength during adolescence? A longitudinal study. J Bone Miner Res 33:580–588
Machado LG, Domiciano DS, Figueiredo CP, Caparbo VF, Takayama L, Oliveira RM, Lopes JB, Menezes PR, Pereira RM (2016) Visceral fat measured by DXA is associated with increased risk of non-spine fractures in nonobese elderly women: a population-based prospective cohort analysis from the São Paulo Ageing & Health (SPAH) Study. Osteoporos Int 27:3525–3533
Direk K, Cecelja M, Astle W, Chowienczyk P, Spector TD, Falchi M, Andrew T (2013) The relationship between DXA-based and anthropometric measures of visceral fat and morbidity in women. BMC Cardiovasc Disord 13:25
Hind K, Pearce M, Birrell F (2017) Total and visceral adiposity are associated with prevalent vertebral fracture in women but not men at age 62 years: the Newcastle Thousand Families Study. J Bone Miner Res 32:1109–1115
Ensrud KE, Crandall CJ (2017) Osteoporosis. Ann Intern Med 167:Itc17-itc32
Washburn RA, Smith KW, Jette AM, Janney CA (1993) The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46:153–162
Scott D, Seibel M, Cumming R, Naganathan V, Blyth F, Le Couteur DG, Handelsman DJ, Waite LM, Hirani V (2017) Sarcopenic obesity and its temporal associations with changes in bone mineral density, incident falls, and fractures in older men: the concord health and ageing in men project. J Bone Miner Res 32:575–583
Xiao Z, Tan Z, Shang J, Cheng Y, Tang Y, Guo B, Gong J, Xu H (2020) Sex-specific and age-specific characteristics of body composition and its effect on bone mineral density in adults in southern China: a cross-sectional study. BMJ Open 10:e032268
Zhang SB, Chen H, Xu HW, Yi YY, Wang SJ, Wu DS (2020) Association between handgrip strength and subsequent vertebral-fracture risk following percutaneous vertebral augmentation. J Bone Miner Metab
Kim M, Won CW (2020) Sarcopenia in Korean community-dwelling adults aged 70 years and older: application of screening and diagnostic tools from the Asian working group for sarcopenia 2019 update. J Am Med Dir Assoc 21:752–758
Laslett LL, Just Nee Foley SJ, Quinn SJ, Winzenberg TM, Jones G (2012) Excess body fat is associated with higher risk of vertebral deformities in older women but not in men: a cross-sectional study. Osteoporos Int 23:67–74
Kim KC, Shin DH, Lee SY, Im JA, Lee DC (2010) Relation between obesity and bone mineral density and vertebral fractures in Korean postmenopausal women. Yonsei Med J 51:857–863
Yang S, Nguyen ND, Center JR, Eisman JA, Nguyen TV (2013) Association between abdominal obesity and fracture risk: a prospective study. J Clin Endocrinol Metab 98:2478–2483
Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD (2009) Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab 94:3387–3393
Russell M, Mendes N, Miller KK, Rosen CJ, Lee H, Klibanski A, Misra M (2010) Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab 95:1247–1255
Høiberg MP, Rubin KH, Gram J, Hermann AP, Brixen K, Haugeberg G (2015) Risk factors for osteoporosis and factors related to the use of DXA in Norway. Arch Osteoporos 10:16
Kang SM, Yoon JW, Ahn HY, Kim SY, Lee KH, Shin H, Choi SH, Park KS, Jang HC, Lim S (2011) Android fat depot is more closely associated with metabolic syndrome than abdominal visceral fat in elderly people. PLoS One 6:e27694
Kim JH, Choi HJ, Ku EJ, Hong AR, Kim KM, Kim SW, Cho NH, Shin CS (2016) Regional body fat depots differently affect bone microarchitecture in postmenopausal Korean women. Osteoporos Int 27:1161–1168
Scott D, Chandrasekara SD, Laslett LL, Cicuttini F, Ebeling PR, Jones G (2016) Associations of sarcopenic obesity and dynapenic obesity with bone mineral density and incident fractures over 5-10 years in community-dwelling older adults. Calcif Tissue Int 99:30–42
Ishii S, Cauley JA, Greendale GA, Nielsen C, Karvonen-Gutierrez C, Ruppert K, Karlamangla AS (2014) Pleiotropic effects of obesity on fracture risk: the Study of Women's Health Across the Nation. J Bone Miner Res 29:2561–2570
Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, Deng HW (2008) Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res 23:17–29
Ofir O, Buch A, Rouach V, Goldsmith R, Stern N, Monsonego-Ornan E (2020) Association between abdominal obesity and fragility fractures among elderly Israeli women. Aging Clin Exp Res 32:1459–1467
Liao Q, Zheng Z, Xiu S, Chan P (2018) Waist circumference is a better predictor of risk for frailty than BMI in the community-dwelling elderly in Beijing. Aging Clin Exp Res 30:1319–1325
Kawai M, de Paula FJ, Rosen CJ (2012) New insights into osteoporosis: the bone-fat connection. J Intern Med 272:317–329
Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, Maier AB (2019) Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 10:485–500
Gan D, Wang L, Jia M et al (2020) Low muscle mass and low muscle strength associate with nonalcoholic fatty liver disease. Clin Nutr 39:1124–1130
Segal NA, Torner JC, Yang M, Curtis JR, Felson DT, Nevitt MC (2008) Muscle mass is more strongly related to hip bone mineral density than is quadriceps strength or lower activity level in adults over age 50 year. J Clin Densitom 11:503–510
Eguchi Y, Toyoguchi T, Orita S et al (2019) Reduced leg muscle mass and lower grip strength in women are associated with osteoporotic vertebral compression fractures. Arch Osteoporos 14:112
Funding
This work was funded by the Aging and Health of Women and Children Research Project of Shanghai Municipal Health Commission (2020YJZX0116), Science and Technology project of Jiangxi Provincial Health Commission (202140997).
Author information
Authors and Affiliations
Contributions
SJW and DSW initiated the idea, did the data analysis; HC YYY and ZSB wrote the assay. YYY supervised and reviewed the manuscript. XHW, XYF, and TH gathered the data and helped with the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hao Chen and Yu-Yang Yi are co-first authors.
Supplementary information
Figure S1.
Hazard ratios for osteoporotic vertebral fracture incidence according to A/G ratio, using restrictive cubic spline analysis (PNG 224 kb)
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Chen, H., Yi, YY., Zhang, SB. et al. Sarcopenic obesity defined by visceral adiposity was associated with osteoporotic vertebral fracture. Arch Osteoporos 17, 41 (2022). https://doi.org/10.1007/s11657-022-01087-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-022-01087-9