Abstract
To assess the effects of river damming on dissolved inorganic carbon in the Jialing River, a total of 40 water samples, including inflow, outflow, and stratified water in four cascade reservoirs (Tingzikou, Xinzheng, Dongxiguan, Caojie) were collected in January and July, 2016. The major cations, anions, and δ13CDIC values were analyzed. It was found that the dissolved compositions are dominated by carbonate weathering, while sulfuric acids may play a relatively important role during carbonate weathering and increasing DIC concentration. Different reservoirs had variable characteristics of water physiochemical stratification. The DIC concentrations of reservoir water were lower in summer than those in winter due to the dilute effects and intensive aquatic photosynthesis, as well as imported tributaries. The δ13CDIC values in Tingzikou Reservoir were higher during summer than those in winter, which indicated that intensive photosynthesis increased the δ13CDIC values in residual water, but a similar trend was not obvious in other reservoirs. Except for in Xinzheng Reservoir, the δ13CDIC values in inflow and outflow reservoir water were lower than those in the surface water of stratified sampling in summer. For stratified sampling, it could be found that, in summer, the Tingzikou Reservoir δ13CDIC values significantly decreased with water depth due to the anaerobic breakdown of organic matter. The significant correlation (p < 0.01 or 0.05) between the DIC concentrations, the δ13CDIC values and anthropogenic species (Na++K+, Cl–, \({\text{SO}}_{4}^{2 - }\)and \({\text{NO}}_{3}^{ - }\)) showed that the isotope composition of DIC can be a useful tracer of contaminants. In total, Tingzikou Reservoir showed lacustrine features, Xinzheng Reservoir and Dongxiguan Reservoir had “transitional” features, and Caojie Reservoir had a total of “fluvial” features. Generally, cascade reservoirs in the Jialing River exhibited natural river features rather than typical lake features due to characteristics of reservoir water in physiochemical stratification, spatiotemporal variations of DIC concentrations and isotopic compositions. It is evident that the dissolved inorganic carbon dynamics of natural rivers had been partly remolded by dam building.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A river acts as a bridge that connects the material circulation of the continents and oceans. This transportation of biogenic elements (C, N, P, Si, etc.) has an important impact on marine and riverine aquatic ecosystems (Meybeck 1982; Ittekkot 1988; Liu et al. 2009). However, interception and storage of hydropower dams had greatly changed the characteristics and quantities of the river-to-ocean material transportation system in past few decades due to needs of economic development, resulting in a lot of dams building (Humborg et al. 1997; Milliman 1997; Klaver et al. 2007). Originally, hydrodynamic conditions had been changed because of the dam effect; with appearance of “limnology effects” such as thermal stratification, carbon cycle process will be altered due to the alterations of aquatic organism distribution and metabolic direction/strength (Wang et al. 2012; Bonnet et al. 2000; Becker et al. 2008; Liu et al. 2014). Until now, there were many studies focused on the “limnology effect” of reservoirs, including environment, aquatic ecology, hydraulics, hydrodynamics and geochemistry, which included studies on hydrology and reservoir management (Tufford and Mckellar 1999; Casamithjana et al. 2003; Ducan 1990; Bayley 1991), physiochemical or biological stratifications in reservoirs (Ma et al. 2013; Wang et al. 2005); activation and release of C, N, P, Si, Hg etc. in water–sediment interfaces (Louchouarn et al. 1993; Jossette et al. 1999; Wang et al. 2000); interception effect of nutrients in dammed rivers (Humborg et al. 1997, 2000; Jossette et al. 1999; Ran et al. 2009); source-sink effects of C in reservoir areas (Giles 2006; Barros et al. 2011; Knoll et al. 2013) and variation of aquatic ecosystems (Xie 2003; Wang et al. 2008).
As an important biogenic element, carbon has close relationships with the circulation of other nutrients, energy flow, CO2 dynamics and trophic states in the “River-Reservoir-River” system (Li et al. 2015). The DIC concentrations and isotope compositions can reflect the geochemical behaviors and biogeochemical characteristics of carbon (Liu 2007). Present studies mainly focus on source, migration or transformation of carbon in natural lakes (Quay et al. 1986; Herczeg 1987; Wachniew and Rόżński 1996), meanwhile there were a few studies on reservoir DIC cycling, for example, Jędrysek et al. (2006) studied diurnal variations of δ13CDIC compositions in the Sulejów reservoir. Wang et al. (2011) studied the DIC and δ13CDIC changes in two reservoirs in the Wujiang River and Yu et al. (2008) studied the DIC concentrations and isotope compositions in cascade reservoirs in the Wujiang drainage basin. The studies of carbon cycling in reservoir showed that the DIC concentrations of reservoir water decreased from the tail to the front area of dam, while the δ13CDIC values were more positive in summer and autumn than in winter and spring, and that the DIC concentrations increased but δ13CDIC values decreased in profiled water. Liu et al. (2014) studied on the features of DIC in the Wulixia reservoir in Guangxi and found similar results with those from the Wujiang River. However, Wu et al. (2012) found there were no significant differences in DIC concentrations and δ13CDIC values in water column. Besides, Zhang et al. (2014) described spatiotemporal distribution of DIC and DOC in main stem of the Yangtze River and effects of the Three Gorges Reservoir; Yu et al. (2009) discussed spatiotemporal characteristics of DIC and its isotopic compositions in the Hongjiadu Reservoir in the Wujiang River drainage basin, etc.
However, most of this relevant research is focused on a single reservoir or several independent reservoirs in different river drainage basins. Some researches studied on the DIC concentrations and δ13CDIC values in cascade reservoirs in the same drainage basin. The Jialing River is an important tributary of the Yangtze River and there were many reservoirs constructed along the Jialing River but very few studies related to the DIC concentrations and isotope compositions in this drainage basin (Li et al. 2015). Thus, four typical reservoirs in middle-lower reach of the Jialing River were selected, while spatiotemporal characteristics of the DIC concentrations and δ13CDIC values were analyzed. Then, source, migration, transformation and controlling factors of DIC in different reservoir types will be discussed.
2 Study area
2.1 Topography and geology
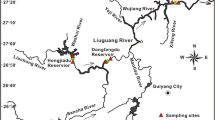
The Jialing River, a major tributary to the upper reaches of the Yangtze River, originated from the Qinling Mountains in the Shanxi Province, and carves southward through hilly lands in the middle Sichuan Basin, cutting across the Paralleled Ridge-Valley of East Sichuan. The main stream is 1120 km long, with a drainage area of 1.6 × 104 km2 and mean annual runoff discharge of 704 × 108 m3. The middle and lower reaches of the Jialing River are distributed in the Mesozoic and Cenozoic Sichuan Syneclise. The Jialing River itself has four major tributaries, the Xihanshui River, the Bailongjiang River, the Qujiang River and the Fujiang River, which import into the main channel at Lveyang, Zhaohua and Hechuan (Qujiang and Fujiang River), respectively (Fig. 1).
In middle and lower courses of the Jialing River, the Sichuan Basin comprises a relatively un-deformed part of the Yangtze platform. And there is a lower terrain with an elevation of 250–600 m. From Guangyuan to the south, the formation lithology is composed of Cretaceous continental clastic sedimentary rocks, Jurassic purplish red sand-mudstone, Quaternary loose sediment accumulation of glacier and ice-water and also scattered outcrops of Triassic carbonate rocks of the lower reaches (Fig. 1).
2.2 Climate and soil
The Jialing River basin has a subtropical monsoon humid climate. Mean annual precipitation is 1010–1250 mm in middle and lower Jialing River zones, mostly from May to October, accounting for about 85% of the total annual precipitation (Changjiang Water Resource Commission, 2003). The Jialing River had an average annual runoff of 696 × 108 m3 in the same period of 1956–1979, and consequently, water discharge is also highly seasonal (Water conservancy and electric power department of Sichuan Province, 1991). The most widely distributed soils are calcareous purple soils, neutral purple soils, yellow soil and alluvial soils. There are other soil types such as red soil, brown soil (Bureau of Geology and Mineral Resources of Sichuan Province, 1991).
The Jialing River basin is located in center of the Sichuan Basin, and acid rain pollution is aggravating because of rapid economic growth and sulfur-content coal combustion in recent decades. A series of reservoirs have been built and put into use since 1970s, and original hydrological situation and characteristics of water environment have been greatly changed. To a certain extent, natural chemical weathering processes have also been affected by the development of agriculture and industry, indirectly.
3 Sample collection and analyses
3.1 Field sampling and data collection
Four reservoirs were selected from the Jialing catchment (Tingzikou Reservoir, Xinzheng Reservoir, Dongxiguan Reservoir and Caojie Reservoir from midstream to downstream, respectively), See Table 1 for more details. A total of 80 samples were collected in Jan (low flow season, dry season) and Jul (high flow season, rainy season) from midstream to downstream, include samples of inflow (surface water), outflow (surface water) and reservoir area (profiled water). Sampling depths were chosen based on the structure of thermal profiles and water depths in all reservoirs (ex: 0, 5, 10, 15, 30 and 60 m for water depths around 60 m). The sampling sites for surface and stratified water are shown in Fig. 1. JL1, JL5, JL9 and JL13 were inflow samples, and JL4, JL8, JL12 and JL17 were outlets of reservoirs, and JL3, JL4, JL6, JL7, JL10, JL11, JL14, JL15 and JL16 sampled from water column inside reservoirs.
Water temperature, dissolved oxygen and pH were measured in situ using an YSI 6920, which had been calibrated. Alkalinity (counted as \({\text{HCO}}_{3}^{ - }\)) was determined by Gran titration using 0.02 mol HCl within 8 h. All HDPE bottles used for collecting water samples were acid-washed and cleaned with distilled water in laboratory previously. All samples were filtered in field using 0.45 µm Millipore nitrocellulose filter and 15 mL of these samples were stored for measuring anions, while another 15 mL were acidified with ultra-pure HNO3 to pH < 2 for cationic determination. Both anionic and cationic samples were stored at 4 °C in refrigerator. The other water samples (60 mL) were filtered by pressure filtration through 0.45 µm cellulose acetate filter and stored in previously acid-washed HDPE bottles with air-tight caps, and then conserved in refrigerator at 4 °C for δ13CDIC measuring. Vials for δ13CDIC measuring were injected with HgCl2 solution to poison the samples.
3.2 Analysis
Major cations (K+, Na+, Ca2+, Mg2+) and Si concentrations were determined with ICP-OES, and anions (\({\text{SO}}_{4}^{2 - }\), \({\text{NO}}_{3}^{ - }\) and Cl–) were analyzed using a Dionex ICS90 (with a SE < 5%). Generally speaking, a CBE within ±5% is acceptable (Li et al. 2011). In this study, the extent of Tz+–Tz− charge characterized by the normalized inorganic charge balance (NICB = (Tz+–Tz−)/Tz+) ranged from 2.6% to 4.99%, which was considered acceptable.
Then, CO2 was collected using the off-line vacuum system (Atekwana and Krishnamurthy 1998), 40 mL water samples was injected by syringe into glass bottles that were pre-filled with 1 mL 85% phosphoric acid. The CO2 was extracted and purified after cryogenic removal of H2O using a liquid nitrogen-ethanol trap; finally, CO2 was transferred cryogenically into a tube for isotope measurement (Li et al. 2008a, 2008b). Carbon isotope ratios of the DIC that determined on a Finnigan MAT252 mass spectrometer are reported in δ notation relative to PDB (‰), and have a precision of 0.1‰. All analyses were conducted at the Institute of Geochemistry, Chinese Academy of Science.
4 Results and discussion
4.1 Chemical composition of surface water
Variations of major ion compositions in surface water samples are shown in the anion and cation ternary diagrams (Fig. 2a and b). Total cationic charge (Tz+= Na++K++2Ca2++2 Mg2+) ranged from 3.67 (Jul) to 4.02 (Jan) with an average of 3.85 meq/L, three times more than the world rivers’ average of 1.25 meq/L (Meybeck 1981), and higher than the average of the Yangtze River (2.80 meq/L; Han and Liu 2004). The total anionic charge (Tz− = \({\text{HCO}}_{3}^{ - }\) + Cl− + \({\text{NO}}_{3}^{ - }\) + 2 \({\text{SO}}_{4}^{2 - }\)) ranged between 3.49(Jul) and 3.91(Jan) with an average of 3.70 meq/L. Calcium is the dominant cation with proportions from 61.1% to 63.2%, magnesium from 25.6% to 27.1%, and Na++K+ from 11.3% to 11.7% of the total cations, respectively. Silicon in study area is negligible. Bicarbonate is the dominant anion with proportions from 69% to 71% of total anions. The second major anion is \({\text{SO}}_{4}^{2 - }\), next \({\text{NO}}_{3}^{ - }\) + Cl–, proportioning from 21% to 24% and from 7% to 8% of total anions, respectively.
On a short time scale, carbonate minerals are actively involved in global carbon cycle due to high solubility (Yuan 1997). It has been proven that carbonate minerals are the main controlling factor of riverine DIC pool, even with a small quantity (Barth et al. 2003). According to mass balance considerations, the water body can be mainly characterized as \({\text{HCO}}_{3}^{ - }\) − Ca2+·Mg2+ type. Combined with lithologic distribution (Fig. 1) and water chemistry ternary diagram (Fig. 2), the solute compositions are dominated by carbonate weathering (include limestone and dolomite). The sodium excess over chloride (Na:Cl = 1.81–1.97) requires input from aluminosilicate weathering, but is negligible (Hu et al. 1982). The low silica value may be an artefact of removal by diatoms in the reservoirs upstream from sampling site (Hu et al. 1982). Besides, co-variation of the equivalent ratios of [Ca2++Mg2+] and [\({\text{SO}}_{4}^{2 - }\) + \({\text{HCO}}_{3}^{ - }\)] indicate that significant additional \({\text{SO}}_{4}^{2 - }\) is required to achieve ionic balance (Fig. 3a and b), which implied that sulfuric might play a relatively important role in carbonate weathering in studied area.
Hu et al. (1982) investigated major ion chemistry in the main stream of Changjiang River and found Ca2+, Mg2+, Na+, K+, Cl– and \({\text{SO}}_{4}^{2 - }\) concentrations of 2.17, 0.68, 0.3, 0.04, 0.18 and 0.62 (meq/L), respectively. Chen et al. (2002) reported major ion compositions of Jialing River as follows: 2.06, 0.78, 2.35, 0.55, 0.13 and 0.097 (meq/L) for Ca2+, Mg2+, \({\text{HCO}}_{3}^{ - }\) \({\text{SO}}_{4}^{2 - }\), Cl– and SiO2, respectively. Chetelat et al. (2008) reported main ion compositions of the Jialing River mouth in Chongqing as follows: 2.096, 0.78, 0.22, 0.06 and 0.89 (meq/L) for Ca2+, Mg2+, Cl–, \({\text{NO}}_{3}^{ - }\) and \({\text{SO}}_{4}^{2 - }\), respectively. However, major ion equivalent concentrations of this study are higher than that of previous studies (Table 2), indicating the accumulation effect due to the increasing intensity of rocky weathering and anthropogenic inputs over time. Interestingly, the outflow of the Dongxiguan Reservoir had relatively high contents of Cl++ \({\text{NO}}_{3}^{ - }\) + \({\text{SO}}_{4}^{2 - }\) and Na+ + K+ (site 12; Fig. 2a and b), which might have resulted from domestic sewages.
4.2 Characteristics of seasonal physiochemical stratification
Thermal stratification of water is one of the most important characteristics of lakes and reservoirs that differs from rivers, and is the most significant control factor of many chemical-biological processes in deep water reservoirs (Liu et al. 2009). In this study area, most of reservoirs belonged to polymictic or oligomictic types (Liu et al. 2014). For example, there was an obvious thermal stratification in Tingzikou Reservoir during summer, with an average water temperature of 27.56 and 8.81 °C in top and hypolimnetic water, respectively, and the temperature difference was up to 18.75 °C (Fig. 4). However, weak thermal stratification was observed in Xinzheng Reservoir and Dongxiguan Reservoir, with average temperatures of 26.44 and 29.11 °C in top water and of 25.82 and 28.1 °C in bottom water, respectively (Fig. 4). Additional, there was no obvious thermal stratification of profiled water in Caojie Reservoir, while thermal stratification disappeared in all reservoirs in the winter (Fig. 4).
The variation characteristics of DO contents in profiled water were similar to those of T (°C), and vertical exchanges of waters were effectively limited by thermal stratification in summer. Then, aerobic and anaerobic environments were generated in the epilimnion and hypolimnion, respectively. Variable characteristics of DO contents in the water column were observed in different reservoirs (Fig. 4). During summer, the DO contents in Tingzikou Reservoir decreased continuously with water depth, and ranged from 9.26 mg/L (top water) to 6.21 mg/L (bottom water). The DO contents in the top water near the water–air interface were close to saturation of local climatic condition due to direct connection with air and wind mixing, while the restoration of oxygen tended to become more difficult along with increase of water depth, and disappearance of photosynthesis in the hypolimnion combined with degradation of organic matter in sediments lead to oxygen depletion. Therefore, the DO contents of profiled water usually decreased linearly with water depth in summer (Ma et al. 2013). In Xinzheng Reservoir and Dongxiguan Reservoir, the average DO contents in water column slightly decreased with water depth increases, which ranged from 7.78 mg/L (top water) to 6.98 mg/L (bottom water) and from 6.52 mg/L (top water) to 5.42 mg/L (bottom water), respectively (Fig. 4). There was also a small difference (0.17 mg/L) between surface water and hypolimnetic water in Caojie Reservoir (Fig. 4). In winter, profile variations of DO contents were not obvious (Fig. 4), while the average DO contents were higher in winter than that in summer due to disappearance of thermal stratification, frequently vertical exchange of water column and consumption mechanism of hypolimnetic oxygen was compensated by oxygen renewal (Ma et al. 2013). Besides, lower water temperature (°C) would result in reduction of photosynthesis and consumption of DO.
In thermal stratification period, change trends of pH values in water column were similar to that of DO contents and temperature (°C), thus pH values decrease with water depth on the whole, especially in Tingzikou Reservoir (Fig. 4). In rainy season, average pH values of Tingzikou reservoir were 8.42 (top water) and 7.1 (bottom water), respectively (Fig. 4), and this obvious acidification trend was resulted from the anaerobic decomposition of organic matter in water column and sediments. Moreover, surface waters in reservoir area were affected by the inflow of upstream significantly, while river section was dominated by carbonate weathering and usually had high pH values. The average pH values in water column of Xinzheng Reservoir and Dongxiguan Reservoir ranged from 7.87 (top water) to 7.5 (bottom water) and from 7.77 (top water) to 7.54 (bottom water), respectively (Fig. 4), but the differences were not significant. There were no obvious profile variations of pH values in Caojie Reservoir in summer, while there were also no evident profile changes of pH values in all of the reservoirs in winter (Fig. 4).
Generally, stability of thermal stratification in summer was affected by water turbulent kinetic energies, vertical diffusions and hydrodynamic conditions, which were under influences of reservoir types, capacities, water depths and regulation ways (Table 1). Based on the physiochemical stratification characteristics, Tingzikou Reservoir exhibited the features of a natural lake rather than a river, while Xinzheng Reservoir and Dongxiguan Reservoir belonged to a “Transitional type” between river–lake and Caojie Reservoir belonged to a total “Fluvial type”.
4.3 Spatiotemporal change characteristics of DIC concentrations
The pH values in study area ranged from 7 to 8.4, with an average of 7.61 (n = 80). The dissolved inorganic carbon in water body was dominated by \({\text{HCO}}_{3}^{ - }\), which accounts for about 90% of the total DIC (CO2aq, H2CO3, \({\text{HCO}}_{3}^{ - }\) and \({\text{CO}}_{3}^{2 - }\)). Thus \({\text{HCO}}_{3}^{ - }\) can be used to represent DIC in the aquatic system (Yu et al. 2008).
Different from riverine DIC, which are mainly affected by exogenous sources (soil CO2, weathering of carbonate minerals and atmospheric CO2), the DIC in reservoir water are affected by both exogenous and endogenous sources. The exogenous sources include riverine DIC and decompositions of organic matter which are carried by rivers, dissolution of atmospheric CO2, etc., while endogenous sources include aquatic biological respiration, decomposition of biogenic organic matters, etc. The mainly lose ways of DIC in reservoir water include CO2 degassing, carbonate precipitation or transfer to organic matters via photosynthesis, etc. (Yang et al. 1996a, 1996b; Ludwig et al. 1996; Liu et al. 2014).
4.3.1 Seasonal variation of DIC
The DIC concentrations were usually higher in winter than that in summer (except site12), both in surface and profiled water (Figs. 5a and 6). The DIC concentrations of surface water ranged from 1.81 to 2.65 mmol/L with an average of 2.35 mmol/L in summer, and ranged from 2.35 to 2.95 mmol/L with an average of 2.76 mmol/L in winter (Table 2). This result was consistent with other relevant research (Yu et al. 2008; Li et al. 2008a, 2008b, 2009), which can be explained by intensive dilution effects through the increase of water flow in summer (including precipitation, tributaries, surface and underground runoff). Besides, reduction of DIC concentration resulted from high temperatures, increasing light intensity and photosynthesis in summer. In winter, disappearance of thermal stratification lead to an adequately vertical exchange of water column, while hypolimnetic water with high \({\text{HCO}}_{3}^{ - }\) concentrations that were generated by organic matter decompositions and biological respiration were upwelling.
4.3.2 Spatial variation of DIC
Variation along the direction of flow In summer, the DIC concentrations of surface water decreased with tributaries imported, and inflow of Tingzikou Reservoir had minimum DIC concentration of 1.81 mmol/L (site 1) due to water dilution from the Bailong River (Fig. 5a). Then, the average concentration of DIC in surface water was increased to 2.19 mmol/L in Tingzikou Reservoir area due to the interception effect, and the outflow had maximum DIC concentration of 2.65 mmol/L (Fig. 5a). The DIC concentration in the inflow (2.58 mmol/L; Site 5) of Xinzheng Reservoir was slightly lower than the surface water in reservoir area (Average: 2.61 mmol/L), and continuously increased to 2.64 mmol/L of outflow (Fig. 5a). In summer, the DIC concentrations of surface water declined continuously from Dongxiguan Reservoir to Caojie Reservoir due to the obvious dilution effects of the Qujiang River and Fujiang River (Fig. 5a). The DIC concentration in the outflow of all reservoirs were higher than the surface water in front of the dam due to the released water generally keeping the signal of respiration C in hypolimnion (Fig. 5a).
In winter, inflow of Tingzikou Reservoir had maximum DIC concentrations of 2.95 mmol/L due to Bailong River imported (Fig. 5a). After that, the DIC concentrations of surface water changed slightly from Xinzheng Reservoir to Dongxiguan Reservoir, while outflow of Dongxiguan Reservoir had lowest DIC concentration of 2.35 mmol/L due to domestic wastewater (Fig. 5a). The DIC concentrations of surface water significantly increased from Dongxiguan Reservoir to Caojie Reservoir due to the Qujiang River and Fujiang River imported (Fig. 5a).
Variation along the water depth In summer, the DIC concentrations usually increased with water depth increasing, especially in Tingzikou Reservoir (Fig. 6). The average DIC concentrations of surface water (2.19 mmol/L) were lower than those of bottom water (2.92 mmol/L) in Tingzikou Reservoir during summer (Fig. 6). This can be explained by CO2aqu (aqueous CO2) absorption, which resulted from intense photosynthesis in euphotic zone of th epilimnion, whereas CO2 released by decomposition of organic matters in water column and water–sediment interface would lead to higher DIC concentrations in hypolimnion. A similar trend was not obvious in Xinzheng Reservoir and Dongxiguan Reservoir due to shallow water depth (average depth: 17.5 m) and enhanced vertical exchanges of profiled water, which resulted from wide distributions of sand quarry activities and daily regulation way (Fig. 6). There were no obvious profile variations of DIC concentrations in Caojie Reservoir, which was also due to intensive turbulent kinetic energy and vertical diffusions that resulted from maximum inflow (tributaries and upstream water imported) and a shorter water residence time (daily/weekly regulation way). There were no significant profile variations of DIC concentrations in winter due to disappearance of thermal stratification and sufficient vertical exchanges of water column (Fig. 6).
4.4 Spatiotemporal variation characteristics of δ13CDIC values
4.4.1 Seasonal variation of δ13CDIC
As a whole, higher δ13CDIC values of surface water in all studied reservoir (Tingzikou Reservoir excluded) were in winter (average:−7.05‰) than that in summer (average:−10.18‰), indicating more DIC were derived from soil CO2 in rainy season due to high temperature and precipitations (Fig. 5b). The average δ13CDIC values of surface water in Tingzikou Reservoir were lower in winter (−6.86‰) than that in summer (−4.5‰), indicating that photosynthesis prevails in epilimnion of Tingzikou Reservoir in summer and enhancement of primary productivity which results in higher δ13CDIC values (Fig. 5b). This phenomenon was similar to reservoirs which had lacustrine features and natural lake. Such as the DIC concentrations of surface water in front of Hongjiadu dam which situated in upper reach of the Wujiang River ranged from 1.84 mmol/L (summer) to 2.28 mmol/L (winter) and the δ13CDIC values ranged from −8.3‰ in winter to −4.5‰ in summer (Yu et al. 2009). Yu et al. (2008) reported the average DIC concentrations of surface and profile water in cascade reservoirs in the Wujiang River were higher in winter (2.39 mmol/L) than those in summer (2.21 mmol/L), and the average δ13CDIC values were lower in winter (−9.08‰) than those in summer (−8.1‰). Li et al. (2009) reported the DIC concentrations in cascade reservoirs in the Maotiao River and found relatively high DIC concentration (2.67 mmol/L) combined with relatively negative δ13CDIC value (−9‰) in autumn, and relatively low DIC concentration (2.12 mmol/L) combined with positive δ13CDIC value (−8.6‰)in summer. Similar results also reported by Mybro and Shapley (2006) of the Jones Lake in Montana group lakes in US. Generally, the δ13CDIC values of natural rivers tend to become more negative in summer than those in winter (Liu 2007; Jiao et al. 2008).
4.4.2 Spatial variation of δ13CDIC
In summer, the δ13CDIC values of surface water tend to become more negative along the flow direction. This can be explained by the outflow water from the upstream reservoirs, as water with more negative δ13CDIC values will pour into the epilimnion of the downstream reservoirs, and this kind of spatial cumulative effect would cause the δ13CDIC values to become more negative and the DIC concentrations to become higher (Yu et al. 2008). The inflows of Tingzikou Reservoir and Caojie Reservoir had more negative δ13CDIC values of −12.32‰ and −13.51‰ in summer, respectively (Fig. 5b), indicating that inflows of Tingzikou Reservoir and Caojie Reservoir were influenced by the Bailong River, Fujiang River and Qujiang River water imported (Figs. 1, 5b). The similar variation characteristics appeared in the St. Lawrence River, while upstream and downstream of the St. Lawrence River were fed by Great Lakes and tributaries, respectively, and the downstream water had more negative δ13CDIC values rather than those upstream (Yang et al. 1996a, 1996b; Hélie et al 2002). In summer, surface water of Tingzikou Reservoir had maximum average δ13CDIC value of −4.15‰ (Fig. 5b), which could be attributed to photosynthesis of phytoplankton that utilized 12C preferentially. 20‰–23‰ fractionation was produced in the synthesis process of organic matter by CO2aqu (aqueous CO2), which makes the δ13CDIC values of the body of water more positive (Buhl et al. 1991; Pawellek and Veizer. 1994; Hélie et al. 2002; Yu et al. 2008). Decomposition of organic matter in sediments will be accompanied with the extensive release of 12CO2 into water column and then lead to δ13CDIC values decreasing. Thus, outflow came from hypolimnetic waters usually had more negative δ13CDIC values in summer, such as outflow of Tingzikou Reservoir, Dongxiguan Reservoir and Caojie Reservoir with a relatively negative δ13CDIC values of −7.24‰, −11.46‰ and −12.74‰, respectively (Fig. 5b). Except for Xinzheng Reservoir, inflow and outflow of reservoirs had more negative δ13CDIC values rather than surface water in reservoir area (Fig. 5b). Under the influence of outflow of Tingzikou Reservoir which had a relatively positive δ13CDIC value (−7.24‰, site 4), inflow of the Xinzheng Reservoir had a relatively positive δ13CDIC value (−7.76‰, site 5) rather than the surface water (average: −9.96‰) in reservoir area (Fig. 5b). Outflow of Xinzheng Reservoir had a relatively positive δ13CDIC value of −9.01‰ (Fig. 5b). There was no water draining of Xinzheng Reservoir during sampling period, while the downstream water was shallow and clear and intensive photosynthesis would result in higher δ13CDIC values.
In a reservoir showing a lacustrine feature, the δ13CDIC values usually become more negative along with increasing water depth (Yu et al. 2008, 2009; Liu et al. 2014; Myrbo and Shaply 2006; Li et al. 2009), but there were different variation characteristics in different kinds of reservoirs in study area (Fig. 7). In rainy season, the average δ13CDIC value of surface water and bottom water samples collected from water column in Tingzikou Reservoir were −4.15‰ and −8.4‰ (Fig. 7); see first paragraph of Text 4.4.2 for specific reasons. The δ13CDIC values in front of Tingzikou dam (site 3) decreased firstly and lastly, increasing intermediately, while the δ13CDIC values in center of Tingzikou Reservoir (site 2) increased after decreasing firstly with water depth (Fig. 7). This may be attributed to the niche differentiation of different phytoplankton in euphotic layer, which exhibited different use patterns and intensities of 12C. In rainy season, the average δ13CDIC values of Xinzheng Reservoir increased from surface water (−9.96‰) to hypolimnetic water (−7.62‰, Fig. 7). This may be attributed to frequently vertical exchange of profiled water in a shallow reservoir and upwelling of hypolimnetic water with more negative δ13CDIC values (Fig. 7). The average δ13CDIC values of Dongxiguan Reservoir were slightly decreasing along with increasing water depth (Fig. 7). The δ13CDIC values of reservoir water in front of the Caojie dam (site 16) and that in the center of Caojie Reservoir (site 15) trend to become slightly negative along with increasing water depth, and ranged from −12.59‰ to −13.47‰ and from −12.88‰ to −13.18‰ (Fig. 7), respectively.
4.5 Natural and anthropogenic effects on DIC concentrations and δ13CDIC values
The DIC concentrations and δ13CDIC values had significant correlations with most of the ions and physiochemical parameters (p < 0.05 or 0.01), indicating that these factors had close relations with the DIC concentrations and its isotopic compositions and representativeness of these indexes can be confirmed (Table 3). In summer, the DIC concentrations combined with δ13CDIC values had significant correlations with Ca2++Mg2+, Si (calculated in SiO2), \({\text{SO}}_{4}^{2 - }\) and Cl– (p < 0.01), indicating the momentous influence of carbonate weathering on water chemistry, while anthropogenic inputs such as \({\text{SO}}_{4}^{2 - }\) and Cl– have important effects on the DIC concentrations/isotopic compositions (Table 3). Meanwhile, the DIC concentrations were significantly affected by thermal stratification in summer (Table 3, Figs. 4 and 6). The study area is located in the Chengdu Plain, which has a large population and is developed industrial and agricultural. The Sichuan Basin area had undergone rapid development since the end of 1970s, and is now seriously affected by acid rain, which resulted from high sulfur-content coal combustion. Thus, sulfuric acid might play a relatively important role in carbonate weathering (Li et al. 2011). This process can lead to additions of both DIC concentrations and δ13CDIC values (Table 3). Chen et al. (2002) reported that \({\text{SO}}_{4}^{2 - }\) concentration had significantly positive correlation with the amount of coal-burning. The Cl– ion of waters generally has three sources: atmospheric source, evaporate dissolution and anthropogenic source (mine, industry, city and agriculture). The study area is far away from the sea, thus very few Cl– were derived from sea salt circulation (Bao et al. 2010), and there was basically no distribution of evaporate in the study area (Fig. 1). Therefore, Cl– mainly was derived from anthropogenic inputs such as table and industrial salts, etc. Generally, additions of Cl– and \({\text{NO}}_{3}^{ - }\) concentrations in waters body would significantly lower the DIC concentrations in the bodies of water (Table 3).
In winter, the DIC concentrations and δ13CDIC values had significant correlations with \({\text{SO}}_{4}^{2 - }\) + \({\text{NO}}_{3}^{ - }\) + Cl–, Si (calculated in SiO2), \({\text{SO}}_{4}^{2 - }\) and Cl– (p < 0.01). All of these anthropogenic inputs would lower the δ13CDIC values (Table 3). Si (calculated in SiO2) concentrations had a significantly negative correlation with δ13CDIC values, and there were a number of silicate rocks distributed in study area (Figs. 1 and 2). Thus, this phenomenon may be ascribed to decomposition and mineralization of biogenic silica in sediments (nutrient release) because of low solubility of silicate minerals, while DIC produced by this process had lower δ13CDIC values. Even very limited Si comes from chemical weathering of silicate and aluminosilicate rocks can lead to silicon accumulation in sediments due to physical sedimentation and bio-absorption (Liu et al. 2009).
Depending on the above interpretation, atmospheric inputs and weathering of evaporites had limited impacts on \({\text{NO}}_{3}^{ - }\) and Cl–, while anthropogenic sources had important influences on both of them. Thus, correlation analyses were conducted with reference of \({\text{NO}}_{3}^{ - }\) and Cl– to estimate the extent of C dynamic influenced by anthropogenic inputs (Table 4). Results showed that Na+, K+, \({\text{SO}}_{4}^{2 - }\) were closely related to Cl– and \({\text{NO}}_{3}^{ - }\), especially in dry season (p < 0.01), indicating that all of these ions (Na+, K+, \({\text{SO}}_{4}^{2 - }\), Cl– and \({\text{NO}}_{3}^{ - }\)) were mainly derived from anthropogenic sources, and confirmed that the DIC concentrations and its isotopic compositions in the study area were affected by anthropogenic inputs significantly (Tables 3 and 4).
5 Conclusions
The solute compositions are dominated by carbonate weathering in the study area, while sulfuric acid may play a relatively important role in carbonate weathering. Besides, the water chemistry type can be mainly characterized as \({\text{HCO}}_{3}^{ - }\) − Ca2+·Mg2+ type in the study area. The dissolved inorganic carbon cycle in the Jialing River had been remolded after dam building, and the studied cascade reservoirs in the Jialing River can be divided into three types (lacustrine type, transitional type and fluvial type) based on spatiotemporal variations of physiochemical stratification, DIC concentrations and carbon isotopic compositions. Tingzikou Reservoir exhibited lacustrine features because of obvious physiochemical stratification (e.g. T, pH and DO), DIC concentrations increasing and δ13CDIC values decreasing as the water depth increased in summer. Xinzheng Reservoir and Dongxiguan Reservoir had transitional features and Caojie Reservoir had “fluvial” features. The DIC concentrations and isotopic compositions were affected by anthropogenic inputs such as Na+, K+, Cl–, \({\text{SO}}_{4}^{2 - }\) and \({\text{NO}}_{3}^{ - }\) which were most likely from rainwater, fertilizers, domestic wastewater or sulfide minerals oxidation. This study demonstrated that carbon biogeochemical cycling had been influenced by dam building in the Jialing River, and DIC concentrations combined with carbon isotopic compositions can be useful in tracing the anthropogenic pollutants and carbon biogeochemistry of damming rivers.
References
Atekwana EA, Krishnamurthy RV (1998) Seasonal variations of dissolved inorganic carbon and δ13C of surface water: application of a modified gas evolution technique. J Hydrol (Amst) 205:265–278. doi:10.1016/S0022-1694(98)00080-8
Bao LR, Li XD, Liu XL (2010) Space-time variation of the chemical composition of major ions in Jialing River. Adv Sci Technol Water Resour 30:35–40 (in Chinese)
Barros N, Cole JJ, Tranvik LJ (2011) Carbon emission from hydroelectric reservoirs linked to reservoir age and latitude. Nat Geosci 4:593–596. doi:10.1038/ngeo1211
Barth JAC, Cronin AA, Dunlop J et al (2003) Influence of carbonates on the riverine carbon cycle in an anthropogenically dominated catchment basin: evidence from major elements and stable carbon carbon isotopes in the Lagan River (N. Ireland). Chem Geol 200:203–216. doi:10.1016/S0009-2541(03)00193-1
Bayley PB (1991) The flood-pulse advantage and the restoration of river-floodplain system. Regul Rivers Res Manag 6:75–86. doi:10.1002/rrr.3450060203
Becker V, Huszar VLM, Naselli-Flores L et al (2008) Phytoplankton equilibrium phases during thermal stratification in a deep subtropical reservoir. Freshw Biol 53:952–963. doi:10.1111/j.1365-2427.2008.01957.x
Bonnet MP, Poulin M, Devaux J (2000) Numerical modeling of thermal stratification in a lake reservoir. Methodology and case study. Aquat Sci 62:105–124. doi:10.1007/s000270050001
Buhl D, Neuser RD, Richter DK et al (1991) Nature and nurture: environment isotope story of the river Rhine. Naturewissenschaften 78:337–346. doi:10.1007/BF01131605
Casamithjana X, Serra T, Colomer J et al (2003) Effects of the water withdrawal in the stratification pattern of a reservoir. Hydrobiologia 504:21–28. doi:10.1023/B:HYDR.0000008504.61773.77
Chen JS, Wang FY, Xia XH et al (2002) Major element chemistry of the Changjiang (Yangtze River). Chem Geol 187:231–255. doi:10.1016/S0009-2541(02)00032-3
Chetelat B, Liu CQ, Zhao ZQ et al (2008) Geochemistry of the dissolved load of the Changjiang Basin rivers: anthropogenic impacts and chemical weathering. Geochim Cosmochim Acta 72:4254–4277. doi:10.1016/j.gca.2008.06.013
Ducan A (1990) A review: limnological, management and biomanipulation in the London reservoir. Hydrobiogia 200:541–548. doi:10.1007/BF02530371
Giles J (2006) Methane quashes green credential of hydropower. Nature 444:524–525. doi:10.1038/444524a
Han GL, Liu CQ (2004) Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou province, China. Chem Geol 204:1–21. doi:10.1016/j.chemgeo.2003.09.009
Hélie JF, Hillaire-Marcel C, Rondeau B (2002) Seasonal changes in the sources and fluxes of dissolved inorganic carbon through the St. Lawrence River-isotopic and chemical constraint. Chem Geol 186:117–138. doi:10.1016/S0009-2541(01)00417-X
Herczeg AL (1987) A stable carbon isotope study of dissolved inorganic carbon cycling in a softwater lake. Biogeochemistry 4:231–263. doi:10.1007/BF02187369
Hu MH, Stallard RF, Edmond JM (1982) Major ion chemistry of some large Chinese rivers. Nature 298:550–553. doi:10.1038/298550a0
Humborg C, Ittekkot V, Cociasu A et al (1997) Effect of Danube river dam on black sea biogeochemistry and ecosystem structure. Nature 386:385–388. doi:10.1038/386385a0
Humborg C, Conley DJ, Rahm L et al (2000) Silicon retention in river basins: far-reaching effects on biogeochemistry and aquatic food webs in coastal marine environment. Ambio 29:45–50. doi:10.1579/0044-7447-29.1.45
Ittekkot V (1988) Global trends in the nature of organic matter in the river suspensions. Nature 332:436–438. doi:10.1038/332436a0
Jiao SL, Tao Z, Gao QZ et al (2008) Spatio-temporal variation of the stable isotopic composition of riverine dissolved inorganic carbon of the Xijiang inner estuary. Acta Geogr Sin 63:553–560. doi:10.3321/j.issn:0375-5444.2008.05.011 (in Chinese)
Jossette G, Leporcq B, Sanchez N et al (1999) Biogeochemical mass-balance (C, N, P, Si) in three large reservoirs of the Seine basin (France). Biogeochemistry 47:119–146. doi:10.1007/BF00994919
Klaver G, Van OB, Negrel P et al (2007) Influence of hydropower dams on the composition of the suspended and riverbank sediments in the Danube. Environ Pollut 148:718–728. doi:10.1016/j.envpol.2007.01.037
Knoll LB, Vanni MJ, Renwick WH et al (2013) Temperate reservoirs are large carbon sinks and small CO2 source: results from high-resolution carbon budgets. Glob Biogeochem Cycle 27:52–64. doi:10.1002/gbc.20020
Li SL, Calmels D, Han GL et al (2008a) Sulfuric acid as an agent of carbonate weathering constrained by δ13CDIC: examples from Southwest China. Earth Planet Sci Lett 270:189–199. doi:10.1007/BF02840269
Li SL, Liu CQ, Lang YC et al (2008b) Stable carbon isotope biochemistry and anthropogenic impacts on karst groundwater, Zunyi, Southwest China. Acta Geochim 14:211–221. doi:10.1007/s10498-008-9033-4
Li GR, Liu CQ, Chen C et al (2009) Dissolved inorganic carbon and its carbon isotope composition in cascade reservoir of the Maotiao River during summer and autumn. Chin J Environ Sci 30:2891–2897. doi:10.3321/j.issn:0250-3301.2009.10.013 (in Chinese)
Li XD, Liu CQ, Liu XL et al (2011) Identification of dissolved sulfate sources and the role of sulfuric acid in carbonate weathering using dual-isotopic data from the Jialing River, Southwest China. J Asian Earth Sci 42:370–380
Li XD, Liu XL, Yang Z et al (2015) Spatial and seasonal variation of dissolved inorganic carbon isotope compositions in the cascade reservoirs of the Jialing River. J Shanghai Univ (Nat Sci) 21(3):286–292 (in Chinese)
Liu CQ (2007) Biogeochemical processes and matter cycle of the earth’s surface—the basin weathering of south-west karst area and nutrients elements cycle. Science Press, Beijing (in Chinese)
Liu CQ, Wang FS, Wang YC et al (2009) Responses of aquatic environment to river damming-from the geochemical view. Resour Environ Yangtze Basin’ 18(4):384–395. doi:10.3969/j.issn.1004-8227.2009.04.015 (in Chinese)
Liu W, Pu JB, Yu S et al (2014) Preliminary research on the feature of the dissolved inorganic carbon in Wulixia reservoir in summer, Guangxi, China. Environ Sci 35(8):2959–2966. doi:10.13227/j.hjkx.2014.08.017 (in Chinese)
Louchouarn P, Lucotte MM, Mucci A et al (1993) Geochemistry of mercury in two hydroelectric reservoirs in Quebec, Canada. Can J Fish Aquat Sci 50:269–281. doi:10.1139/f93-031
Ludwig W, Probst JL, Kempe S (1996) Predicting the oceanic input of organic carbon by continental erosion. Glob Biogeochem Cycle 10:23–41. doi:10.1029/95GB02925
Ma Y, Guo QL, Huang TL et al (2013) Response characteristics of water quality to the seasonal thermal stratification in Jin-pen reservoir along the Heihe river, Xi’an city in China. J Hydraul Eng 44(4):406–415 (in Chinese)
Meybeck M (1981) Pathways of major elements from the land to ocean through rivers. United Nations Press, New York, pp 18–30
Meybeck M (1982) Carbon, nitrogen, and phosphorus transport by world rivers. Am J Sci 282:401–405. doi:10.2475/ajs.282.4.401
Milliman JD (1997) Blessed dams or damned dams? Nature 386:325–326. doi:10.1038/386325a0
Myrbo A, Shapley MD (2006) Seasonal water-column dynamics of dissolved inorganic carbon stable isotopic compositions (δ13CDIC) in small hardwater lakes in Minnesota and Montana. Geochim Cosmochim Acta 70:2699–2714. doi:10.1016/j.gca.2006.02.010
Orion-Jędrysek M, Kurasiewicz M, Trojanowska A et al (2006) Diurnal variations in carbon isotope composition of dissolved inorganic carbon (DIC) in a freshwater dam reservoir. Ecohydrol Hydrobiol 6:53–59. doi:10.1016/S1642-3593(06)70126-4
Pawellek F, Veizer J (1994) Carbon cycle in the upper Danube and its tributaries: δ13CDIC constrains. Isr J Earth Sci 43:187–194
Quay PD, Emerson SR, Quay BM et al (1986) The carbon cycle for lake Washington—a stable isotope study. Limnol Oceanogr 31:596–611. doi:10.4319/lo.1986.31.3.0596
Ran XB, Yu ZG, Yao QZ et al (2009) Advances in nutrient retention of dams on river. J Lake Sci 21(5):614–622. doi:10.3321/j.issn:1003-5427.2009.05.002 (in Chinese)
Tufford DL, Mckellar HN (1999) Spatial and temporal hydrodynamics and water quality modeling analysis of a large reservoir on the South Carolina (USA). Ecol Model 114:137–173. doi:10.1016/S0304-3800(98)00122-7
Wachniew P, Rόżński K (1996) Carbon budget of a mid-latitude, groundwater-controlled lake: isotopic evidence for the importance of dissolved inorganic carbon recycling. Geochim Cosmochim Acta 61:2453–2465. doi:10.1016/S0016-7037(97)00089-6
Wang YC, Wan GJ, Wang SL et al (2000) Forms of phosphorous in sediments of lake Baihua and lake Hongfeng, Guizhou. Acta Mineral Sin 20(3):273–277. doi:10.3321/j.issn:1000-4734.2000.03.010 (in Chinese)
Wang YC, Zhu J, Ma M et al (2005) Thermal stratification and paroxysmal deterioration of water quality in a canyon reservoir, Southwestern China. J Lake Sci 17(1):54–60. doi:10.3321/j.issn:1003-5427.2005.01.009
Wang BL, Liu CQ, Wang FS et al (2008) The distributions of autumn picoplankton in relation to environmental factors in the reservoirs along the Wujiang River in Guizhou Province, SW China. Hydrobiologia 598:35–45. doi:10.1007/s10750-007-9138-6
Wang FS, Liu CQ, Wang BL et al (2011) Disrupting the riverine DIC cycling by series hydropower exploitation in Karstic area. Appl Geochem 26:375–378. doi:10.1016/j.apgeochem.2011.03.065
Wang S, Qian X, Han BP et al (2012) Effects of local climate and hydrological conditions on the thermal regime of a reservoir at Tropic of Cancer, in southern China. Water Res 46:2591–2604. doi:10.1016/j.watres.2012.02.014
Wu QX, Han GL, Tang Y (2012) Temporal and spatial variation of water chemistry and dissolved inorganic carbon isotope characterization in Three Gorges Reservoir. Acta Scientiae Circumstantiae 32(3):654–661 (in Chinese)
Xie P (2003) Three-Gorges Dam: risk to ancient fish. Science 302:1149. doi:10.1126/science.302.5648.1149b
Yang C, Telmer K, Veizer J (1996a) Chemical dynamics of the “St. Lawrence River” riverine system: δ18OH2O, δ13CDIC, δ34SSulfate and dissolved 13C/86Sr. Geochim Cosmochim Acta 60:851–866
Yang C, Telmer K, Veizer J (1996b) Chemical dynamic of the “St. Lawrence” riverine system: δ18OH2O, δ13CDIC, δ34Ssulfate and dissolved 13C/86Sr. Geochim Cosmochim Acta 60:851–866
Yu YX, Liu CQ, Wang FS et al (2008) Dissolved inorganic carbon and its isotopic differentiation in cascade reservoirs in the Wujiang drainage basin. Chin Sci Bull 53:3371–3378. doi:10.1007/s11434-008-0348-8
Yu YX, Wang FS, Wang BL et al (2009) Response of dissolved inorganic carbon and its isotopic spatial and temporal characteristics to the earlier reservoir process. Acta Mineral Sin 29:268–273. doi:10.3321/j.issn:1000-4734.2009.02.021 (in Chinese)
Yuan DX (1997) Modern karstology and global change study. Earth Sci Front 4:17–25 (in Chinese)
Zhang LJ, Xue M, Wang M et al (2014) The spatiotemporal distribution of dissolved inorganic and organic carbon in the main stem of the Changjiang (Yangtze) River and the effect of the three gorges reservoir. J Geophys Res Biogeosci 119:741–757. doi:10.1002/2012JG002230
Acknowledgements
The authors thank Zhou Yang, Guodong Jia, Xiaolong Qiu, Zhongjun Wang and Xuetao Zhu for their help in the field. The technical laboratory assistance from Mr. Ning An also is appreciated. This study was financially supported by the National Key Research and Development Program of China (2016YFA0601000) and the National Natural Science Foundation of China (Grant No. 41373136).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, G., Li, X., Li, Q. et al. Damming effects on dissolved inorganic carbon in different kinds of reservoirs in Jialing River, Southwest China. Acta Geochim 36, 581–597 (2017). https://doi.org/10.1007/s11631-017-0155-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-017-0155-5