Abstract
The soil pollution by heavy metals was characterized in the mine tailings and surrounding soils of an old Pb–Zn mine in Huize County. Three hundred and ninety-six samples of agricultural and non-agricultural soil were analyzed for the total metal concentration by acid digestion and sixty-eight selected soil samples were used to determine the chemical fractionation of heavy metals with the modified BCR sequential extraction method. The pollution index of the heavy metals indices for As, Cd, Cr, Cu, Hg, Pb and Zn of non-agricultural soil and agricultural soils in the study area indicated the spreading of heavy metal pollution. Sequential extraction showed that most of Cd existed in an exchangeable form (31.2 %). The available content of Pb, Cu and Zn was mainly distributed in acid extractable fractions and Fe/Mn oxide fractions (27.9, 30 and 27.2 %), and Hg, As and Cr were mainly associated with residual fractions (90.4, 72.9 and 76.8 %). The risks of heavy metals were also evaluated by the risk assessment code (RAC) and potential ecological risk index, respectively. The results of RAC showed a medium and high risk of Cd (45.6 and 54.4 %), medium risk of Zn (100 %), low and medium risk of Cu (41.2 and 58.8 %), largely no risk of Hg (97.1 %), and mainly low risk of As and Pb (92.6 and 91.8 %). The range of the potential ecological risk of soil was 58.2–1839.3, revealing a considerably high ecological risk in the study area, most likely related to acid mine drainage and the mining complexes located in the area. The results can be used during the ecological risk screening stage, in conjunction with the total concentrations and metal fractionation values, to better estimate ecological risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metals polluting soil constitutes a major environmental problem, which has been the subject of detailed risk assessment and management studies (Peters et al. 1986; Schuhmacher et al. 1997; Prasad and Nazareth 2000; Zayed 2001; Granero and Domingo 2002). Metal-polluted soils are a major environmental problem. Consequently, much focus has been placed on the risk assessment and management studies of heavy metals in soil.
The risk assessment of heavy metals would provide theory support for risk management. The potential ecological risk index (RI) was a methodology developed by Hakanson (1980) to evaluate the ecological risks of heavy metals in sediments. Although RI considers both the toxicities and total contents of heavy metals, the chemical speciation is neglected. Significant differences of noxious properties between different chemical speciation of heavy metals existed (Li et al. 2007). Most of the recently reported studies dealing with the evaluation of heavy metal contamination in sediments only use the total content of heavy metal as a criterion for determining their potential effect on the environments. However, the total concentration of heavy metals provided inadequate information to assess their bioavailability or toxicity (Sundaray et al. 2006). The biological toxicity of heavy metals was generally related more closely to the concentrations of particular physicochemical forms. Sequential extractions could be useful by giving an operational classification of heavy metals in different geochemical fractions. Heavy metals in soil can be bound to various compartments; adsorbed on clay surfaces or iron and manganese oxyhydroxides; present in lattice of secondary minerals like carbonates, sulphates or oxides; occluded in amorphous materials such as iron and manganese oxyhydroxide; complexed with organic matter or present in lattice of primary minerals such as silicates (Chao 1972; Madrid et al. 2002; Hursthouse et al. 2004; Nagajyoti et al. 2010). Furthermore, studies on toxic metal fractionation in soils discriminated natural and anthropogenic sources (Tessier et al. 1979; Schramel et al. 2000; Gismera et al. 2004). The distribution of metals in the different phases of the modified BCR procedure offers an indication of their availability, which in turn enables a risk assessment study for the metals present in the soil (Chen et al. 2010). The risk assessment code (RAC) was a method used for the risk assessment of heavy metals. It classified the risk levels based on the chemical speciation of heavy metals (Singh et al. 2005).
With the rapid industrialization and economic development in the watershed region, the pollution of soil had become very widespread in China (Niu et al. 2009; Wang et al. 2010), which jeopardizes the ecology, food safety, people’s health and the sustainable development of agriculture. The Yunnan Province in the west of China is famous for its mining and smelting activities. A number of researchers have worked on the role of different urban and industrial effluents upon the soil quality (Wei et al. 1991; Teng et al. 2004; Xuan 2007; Teng et al. 2009, 2010). The sequential extraction methods have also become the most effective tools for assessing the risks generated by metal contamination in soils and sediments. For these reasons, the objectives of this study were to assess the degree of the most harmful heavy metals (As, Cd, Cr, Cu, Hg, Pb, and Zn) in soils at Huize Country, Yunnan Province, determine the mobility of heavy metals by a modified sequential extraction method, and investigate their potential ecological risk based on the examined data. The assessment’s conclusions would be beneficial for the management and control of heavy metal pollution in sediments of Huize country, which could allow us to establish monitoring strategies to support future action/remediation plans in the study area.
2 Materials and methods
2.1 Study area
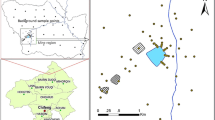
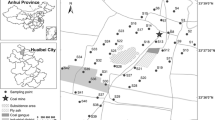
The study area (Huize Country) is located between 25°48′–27°04′ north latitude and 103°03′–103°55′ east longitude, in the northeast corner of the Yunan Province. The elevation is about 695–4017 m at Huize Country. The climate of the study area is in general described as middle subtropics. The mean annual temperature is 12.6 °C, and the mean annual rainfall is 1500 mm.
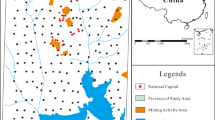
Yunnan Province is the famous foundation of nonferrous mineral resources in China. The main Pb–Zn mineral resources are deposited in Huize Country, in the study area. In Huize Country, there are some vast Pb–Zn mineral deposits with proven reserves of 153 million tons. In Huize Country, the ChiHongXinzhu is one of the greatest local Pb–Zn.
Paddy soil, yellow soil and red soil are the main soil types in the study area. Paddy soil is found in the plains, whereas yellow soil and red soil are distributed in the hilly areas.
2.2 Sample collection and pre-treatment
A total of 396 topsoil samples (including 195 agricultural topsoil samples) were collected in April 2011. In the same specific sample site, agricultural topsoil (0–20 cm) in the farmland and non-agricultural topsoil (0–20 cm) in the neighboring abandoned farmland were collected, and each sample was controlled at 1–1.5 kg. Sampling sites were carried out with a nominal density of one sample per 16 km2 and one sample per 4 km2, especially around some of the Pb–Zn mine areas.
The collected samples were air-dried at 35–40 °C for a few days. The soil was pretreated: it was sifted through a plastic net (the mesh was 6 mm) and mixed thoroughly; materials >6 mm were discarded. The soil was milled with a carnelian mortar pass to 0.015 mm sieve for chemical analysis (Guevara-Riba et al. 2004; Morillo et al. 2004; Yuan et al. 2004; Guillén et al. 2011). The samples were also selected to evaluate the mobility and availability of heavy metals in the soil by a BCR-sequential extraction combined with statistical analyses.
2.3 Reagents and apparatus
All chemicals in this study were of analytical grade and had to contain very low concentrations of trace metals. Normal precautions for trace metals analysis were observed throughout. Deionized water from a MILLI-Q system was used for preparing the solutions and dilutions. All the glassware and the Teflon vessels used in this study were previously soaked overnight with 20 % HNO3 and then rinsed thoroughly with deionized water.
Before the determination total contents of As, Cd, Cr, Cu, Hg, Pb and Zn, the samples were digested with 60 % perchloric acid, 40 % hydrofluoric acid, concentrated nitric acid and concentrated hydrochloric acid (Page et al. 1982). The total concentrations of Cu, Pb, Zn and Cr were analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES, Perkin-Elmer 3300 DV). Cd was analyzed by atomic absorption spectroscopy (AAS, Hitachi 508), As was analyzed by atomic fluorescence spectroscopy (AFS, AFS-1201 produced by KCHAIGUANG, China) and Hg was analyzed by atomic fluorescence spectroscopy (AFS, XGY-1011A produced by Langfang, China).
2.4 Sequential extraction procedure (SEP)
The SEP, based on an improved version of the initial three-step BCR scheme (Quevauviller et al. 1989), was applied to evaluate the metal fractionation in the soil. The procedure is summarized below and full details were reported elsewhere (Rauret et al. 1999; Sahuquillo, et al. 1999).
Step 1 (acid-soluble fraction—exchangeable and bound to carbonates) For each sample, 0.5 g of dry sediment was placed into a 50 mL polypropylene centrifuge tube. Twenty milliliters of 0.11 mol L−1 acetic acid was added to the tube, which was then shaken for 16 h at 22 ± 5 °C (overnight). The extract was separated from the solid phase via centrifugation at 4000 rpm for 10 min. The supernatant liquid was then decanted into a 250 mL flask. The residue was washed twice with de-ionized water and the supernatant liquid was decanted into the same flask, then diluted with 3 % HNO3 and stored at 4 °C prior to analysis. The supernatant was decanted and removed carefully to avoid loss of the solid residue.
Step 2 (reducible fraction-bound to Fe/Mn oxides) Twenty milliliters of 0.5 mol L−1 hydroxylamine hydrochloride (adjusted to a pH of around 1.5 by adding 2 mol L−1 HNO3) was added to the residue in the centrifuge tube from step 1. The extraction was performed as described in step 1. The solid residue was washed, as in step 1, before we continued to step 3.
Step 3 (oxidizable fraction-bound to organic matter and sulfides) Five milliliters of 8.8 mol L−1 H2O2 (pH of 2–3) was added carefully to the residue in the centrifuge tube from step 2. The tube was covered loosely and digested at room temperature for about 1 h, occasionally being shaken. The tube was then continuously digested at 85 ± 2 °C for about 1 h in a water bath, being occasionally shaken for the first 30 min, and the volume was then reduced to around 2–3 mL with further heating of the uncovered tube. Another 5 mL of 8.8 mol L−1 H2O2 (pH of 2–3) was added. Again, the covered tube was heated to 85 ± 2 °C and digested for 1 h before the volume in the uncovered tube was almost dry. After cooling, 25 mL of 1.0 mol L−1 ammonium acetate (adjusted to a pH of 2 with the addition of concentrated HNO3) was added to the residue, which was separated and rinsed as described in step 1. The extract was separated from the solid phase via centrifugation and decantation, as described above, and stored at 4 °C.
Step 4 (Residual fraction): The residue from step 3 was treated, using the procedure described above, to determine the pseudo-total heavy metals content by aqua regia digestion (10 mL of a mixture of 12 M HCL and 15.8 M HNO3 in a 3:1 ratio) in Teflon reactors. Reactors were maintained for 20 h in a fume hood and then simmered on a hot plate for 1 h at 100 °C.
2.5 Accuracy control
According to Regional Geochemical Exploration Regulation (1:200,000) (China Ministry of Geology and Mineral Resources 1995), accuracy, precision, eligibility and spot-check were used for quality control in laboratory analysis. The standard reference materials (GSS-1, GSS-2, GSS-3, GSS-4 soil purchased from the National Research Center for Geoanalysis of China) were incorporated to control the analysis accuracy. The results showed no sign of contamination, which revealed that the precision and bias of the analysis were generally below 5 %. The recovery rates for the elements in the standard reference material ranged from 93 to 115 %. The geochemical sample percent of mass for heavy metals was: Hg was 93 %; Zn was 98 %; Cd and Pb were 95 %; and Cu was 96 %. The spot-check analysis results showed that the eligibility was: As was 85 %; Hg was 90 %; Cd and Cr were 93 %; the others were 100 %.
An internal check on the results of the sequential extraction procedure was also performed by comparing the sum of the four steps from the sequential extraction procedure (acid-soluble + reducible + oxidizable + residual) with the total metal content from the microwave-assisted acid digestion procedure. The recovery of the sequential extraction procedure was calculated as: Recovery (%) = (Fraction 1 + Fraction 2 + Fraction 3 + Residual fraction)/total digestion × 100. Comparing the sum of the metal contents in the sequential extraction steps with the total digestion metal contents showed a good agreement for all elements; the recoveries for all metals ranged from 81 to 108 %, indicating satisfactory accuracy and the validation of the sequential extraction procedure.
2.6 Risk assessment code
Sequential extraction investigations can be also used to estimate the potential risk of waste-soil sediment based on a relative comparison between extracted fractions. The distribution of metals in the different phases of the modified BCR procedure offered an indication of their availability, which in turn enabled a risk assessment study on the pollutants present in an aquatic environment (Chen et al. 2010). Different extents of risk corresponding to different fractions of heavy metal could be expressed and regulated by RAC (Sundaray et al. 2011).
The RAC measures risk by using the percentage of metal associated with the soil/sediment in the exchangeable and carbonate fractions: there is no risk when the F1 BCR fraction is lower than 1 %; low risk for a range of 1–10 %; medium risk for a range of 11–30 %; high risk from 31 to 50 %; and very high risk for F1 percentages over 50 %.
2.7 Potential ecological risk index
RI was introduced to assess the contamination degree of heavy metals. The equations for calculating the RI were proposed by Hakanson (1980), depending on the toxicity of heavy metals and response of the environment:
where \(C_{f}^{i}\) is the single-element pollution factor, \(C^{i}\) is the concentration of the element in samples, and \(C_{n}^{i}\) is a reference value for the element. In this study, \(C^{i}\) is taken to be the sum of the metal contents from the sequential extraction, and \(C_{n}^{i}\) is taken to be the background values of pristine coastal soils in the Yunnan Province (Wei et al. 1991). The sum of \(C_{f}^{i}\) for all metals examined represents the contamination degree (C d ) of the environment. \(E_{r}^{i}\) is the potential ecological RI of an individual element. \(T_{r}^{i}\) is the toxic factor of an individual element. Because the toxic factors of heavy metals developed by Hakanson are restricted to limnic ecosystems but unsuitable for soil ecosystems, we use the standardized toxic factors of heavy metals, Zn = 1<Cr = 2<Cu = Ni = Pb = 5<As = 10 < Cd = 30 < Hg = 40, proposed by Xu et al. (2008) in this study. RI is the comprehensive potential ecological RI, which is the sum of \(E_{r}^{i}\). RI represents the sensitivity of the biological community to the toxic substance and illustrates the potential ecological risk caused by the overall contamination. The Grade standards for \(C_{f}^{i}\), C d , \(E_{r}^{i}\), and RI are listed at Table 1.
2.8 Statistical analysis
Univariate and bivariate statistical analysis, principal components (PCA) and cluster analysis (CA) were performed by the SPSS 13.0 for Windows. The cluster was performed with the method of weighted average linkage between the groups. The 1-pearson correlation coefficient for the cluster intervals and the elements, which showed a close correlation, were identified and grouped for further analysis.
3 Results and discussion
3.1 Heavy metal concentration in soil
The results of statistical analysis (range, median, mean, standard deviation and coefficient of variation) for Cd, Hg, As, Pb, Cr, Cu and Zn in the 396 soil samples from the research area after acid digestion has been summarized in Table 2. The highest average values correspond to Cd, Hg, As, Pb, Cr, Cu and Zn (8.3 mg kg−1, 9.5 mg kg−1, 3.3 mg kg−1, 132 mg kg−1, 2186 mg kg−1, 279 mg kg−1, 52 mg kg−1 and 697 mg kg−1, respectively). 88.9 % of Cd, 27.3 % of Hg, 13.2 % of As, 63.5 % of Pb, 5.1 % of Cr, 1.5 % of Cu and 77.5 % of Zn exceeded the local background value. The distribution of mean values and the extreme ranges of variation suggest an abnormal distribution of the chemical data. This was confirmed by the standard deviation and also corroborated by the values of kurtosis and asymmetry.
The pollution degree of agricultural topsoil was higher than that of non-agricultural topsoil. In non-agricultural topsoil, there were about 57 % sites with its pollution index (PI) below 1.0; about 16 % from 1.0 to 2.0; about 15 % from 2.0 to 3.0; about 10 % from 3.0 to 5.0; and about 2 % above 5.0. However, in agricultural topsoil, there were about 10 % sites with its PI below 1.0; about 16 % from 1.0 to 2.0; about 19 % from 2.0 to 3.0; about 20 % from 3.0 to 5.0; and about 35 % above 5.0.
The higher PI was distributed around the Pb–Zn mining area, either in non-agricultural topsoil or in agricultural topsoil. In the Au, Cu, Mo, Pb, and Zn mining area, acid mine drainage (AMD), which had high concentration of trace elements, was the most important pollution source (Rodríguez et al. 2009). In some areas located in and around the Cu, Pb, and Zn extraction plant and smelting mill, alkaline effluent, which had a high concentration of trace elements, was another important pollution source. Therefore, the AMD and wind transport of dust were proposed to be the main causes of the dispersion of pollution.
In order to assess the impact of cultivation on agricultural topsoil pollution, we compiled the PI to cultivation pollution index (CPI). The CPI was calculated with the following formula:
where n is a number of the measured elements.
There were a total of 163 samples with CPIs higher than 1, which indicated that the pollution was more serious in non-agricultural topsoil than in agricultural topsoil. The CPI patterns also revealed that the topsoil pollution distribution was located in the neighboring mining area. The quality of agricultural topsoil might be pejorative due to irragation with polluted water.
3.2 Bivariate analysis, correlation coefficients
The element association of heavy metals in the soil was mainly restricted to the local environmental features, geological processes and characteristics of heavy metals. This study area was important to mineralized regions of base metals (Cd, Pb and Zn), so the geochemical association of trace elements was controlled firstly by the geogenic process. In addition, trace element assemblage was influenced by mining and processing activities. Here, the principal component factor analytical method was applied, and the correlation matrix of tracer elements was obtained. The analysis results revealed that factor 1 included As, Cd, Pb and Zn; factor 2 included Cu, Hg and Zn; and factor 3 included As, Cr and Mn, therefore these trace elements could be from the same sources (Xuan 2007; Teng et al. 2010).
The principal component analysis (PCA) and cluster analysis (CA) were also applied to understand the relationships between the heavy metals responsible for pollution in the research area.
In the agricultural topsoil, As, Hg, Cu, Cd, Zn, Pb and Cr were classified by three groups. Group one included Cu, Cd and Zn; group two included As and Hg; and group three included Cr and Pb. For non-agricultural topsoil, the classification was gently different from that for agricultural topsoil. The first group included As, Cd, Pb, and Zn; the second group included Cr and Hg; the third group included Cu. According to Goldschmidt’s geochemical classification (Goldschmidt 1954), Cr, Mg and Fe were the siderophile group; As, Mo, Cu, Hg, Cd, Pb, and Zn were cthe halcophile group; and, K, Na, and Al were the lithophile group. In the investigation of the Yunan Province soil background (He et al. 2001), the 7 elements were divided into the chalcophile group (Cu, Pb, Cd, Hg and As) and siderophile group (Cr and Zn).
The possible reason for the phenomenon was inferred that, in the processing of metal mining and extracting, some sulfides (i.e. sphalerite, galenite, sphalerite, pyrite, chalcopyrite, molybdenite, bornite, chalcocite, tennatite) would be oxidized to release As, Cd, Cu, Pb, Zn, Hg into the environment.
3.3 Metal speciation
In order to determine the reactivity of the mineral phases susceptible to incorporating metals and metalloids, 68 of sampling soils were selected and the leachates of each step from the SEP of the soils were analyzed.
The potential mobile fraction is considered to be the sum of the first three steps of the SEP-BCR (F1 + F2 + F3) of the soils, i.e. the fraction soluble in water or weakly acidic conditions and carbonates (F1), the reducible fraction linked to the Fe–Mn oxyhydroxides and the oxidisable fraction related to sulphur and organic matter. The extractable contents of Cd, Hg, As, Pb, Cr, Cu and Zn were shown in Table 3. The metal contents in the fractions were evaluated in percentages compared to the sum of the metal contents in the sequential extraction steps, which represents 100 %.
The fractionation profile for Cd was totally different from the other metals, which indicates that it is mostly bound to first three fractions i.e. exchangeable (20.3–49.8 %), reducible (5.9–19.6 %) and oxidisable (11.5–21.8 %) fractions in the non-residual phase, indicating that its source was mostly anthropogenic and hazardous to the environment in specfic areas. These results were similar to those of other works carried out previously. The major fraction of Cd in its carbonate form indicated that at a slightly lower pH, an appreciable percentage of cadmium would have been remobilized and become readily available (Jain 2004; Huang et al. 2004). Further, the similarity of the ionic radius of Ca and Cd should favor the co-precipitation of Cd carbonates and its incorporation into the calcite lattice, giving solid solutions of CdαCa1−αCO3. The high percentage of Cd in the non-residual phase indicated the bioavailability of this element to the organisms in the studied area. There were several sources of Cd, which included runoff containing phosphate fertilizer from agricultural areas near the river, except Pb–Zn and non-ferrous metal smelting. This phosphate fertilizer, which was most likely applied to the agricultural farms, contains Cd (Huang et al. 2004). Presence of Cd could also be as a result of the transport of dust, which has been described as an important source of Cd emission (Ianni, et al. 2000). Cadmium could easily enter the food chain and pose a serious threat to the ecosystem due to its higher toxicity and availability.
On the contrary, Hg, As and Cr were found the most (86.2–98.1 %, 58.4–90.5 % and 58.9–86.1 %, respectively) in the residual fraction bound in mineral lattice, from which the release of heavy metals was unlikely under the environmental conditions (Carral et al. 1995), indicating relatively less mobility and bioavailability and therefore less harm to the environment.
Large amounts of Pb, Cu, Zn and Ni were mainly associated with the reducible fraction (11.4–29.0 %, 7.8–23.2 %, 10.3–38.8 % and 8.2–23.9 %, respectively), indicating their strong association with Fe/Mn oxides, from which the release of heavy metals into the water column can be expected to occur more readily under prevailing environmental conditions (e.g., lower pH, redox potential, etc.). The order of the other fractions of Pb and Cu decreased as follows: the residual (18.3–35.6 %, 23.2–34.5 %, respectively) > the acid-soluble (8.7–12.6 %, 15.2–29.7 %, respectively) > the oxidizable (3.7–9.8 %, 7.2–13.8 %, respectively).
The mobility and bioavailability of the metals, based on the mean values of the relative abundance of the sums of the first three fractions, were in the following order: Cd (57.0 %) > Zn (43.1 %) > Cu (42.2 %) > Ni (35.7 %) > Pb (33.3 %) > As (25.6 %) > Cr (23.2 %) > Hg (6.2 %). When each of the three mobile phases was examined separately, the results showed that the distributions of metals were somewhat different for each fraction, i.e., Cd (31.2 %) > Zn (13.8 %) > Ni (13.0 %) > Cu (12.0 %) > Pb (5.4 %) > Cr (4.1 %) > As (2.8 %) > Hg (0.6 %) for acid-soluble fraction, while Zn (22.2 %) > Pb (19.1 %) > Cu (15.0 %) > Cr (13.8 %) > Ni (13.7 %) > Cd (10.9 %) > As (6.1 %) > Hg (0.9 %) for the reducible fraction, and As (16.7 %) > Cu (15.1 %) > Cd (14.9 %) > Ni (9.0 %) > Pb (8.8 %) > Zn (7.0 %) > Cr (5.4 %) > Hg (4.7 %) for the oxidizable fraction. And the order of the residual fraction for the studied elements was as follows: Hg (90.4 %) > Cr (76.8 %) > As (73.0 %) > Pb (66.5 %) > Ni (63.2 %) > Cu (59.1 %) > Zn (56.2 %) > Cd (41.9 %).
3.4 Results of RAC
The soil samples were classified according to the RAC used by several authors for the heavy metal pollution of sediments (Perin et al. 1985; Jain 2004; Singh et al. 2005). This classification is based on the strength of the bond between metals and the different geochemical fractions in sediments or soils and the ability of metals to be released and enter the food chain. The RAC is assigned by taking into account the percentage of metal in the exchangeable and carbonate fractions (F1), although the RAC does not take into account the total concentration (Keller and Hammer 2004). RAC may be useful to assess the environmental risk using sequential extraction as a characterization method (Rodríguez et al. 2009). The distributions of heavy metals in various geochemical fractions are depicted in Table 3.
The results revealed that Cd at 45.6 % of the sites posed a medium risk and at 54.4 % of the sites posed a high risk to the environment, due to its higher ratios of F1. For Cu, 58.8 % of sites of posed a medium risk and 41.2 % of sites posed a low risk. For Zn, all sites may be classified as medium risk, due to its ratios of F1. 97.1 % of the RAC values of Hg were below 10 %, suggesting a relatively low risk. The RAC values of Cr were all below 10 %, suggesting a relatively low risk. For As and Pb, 92.6 and 91.8 % of sites were classified as low risk, and 7.4 and 8.2 % posed a medium risk, respectively.
Moreover, the obtained RAC values (Fig. 1) had allowed for the classification of elements as a function of their potential hazard as Cd > Zn > Cu > As > Pb > Hg > Cr.
The first fraction contained those metals that were weakly bound, which were readily soluble in water or a slightly acidic medium. The ease with which metals were freed from this fraction provides an idea of the high potential risk associated to it. According to the RAC, the risks of Cd and Zn were very high. That is, the priority pollutants in the soil of research area were Cd and Zn. As we know, the second and third fraction, associated with oxides and hydroxides of Fe and Mn and sulfides and organic matter, respectively, were susceptible to releasing those metals present in the structure, depending on variations that occured with changes potential or pH. It should also be noted that the potential ecological risk of Cu, As, Cr and Pb cannot be ignored due to their high ratios of oxyhydroxides or oxidisable fraction.
3.5 Assessment of ecological risk of heavy metals in soil
Based on the interpolated map of the C d values calculated for the seven elements (Cd, Hg, As, Pb, Cr, Cu and Zn) (Table 4), non-agricultural topsoil generally presented contamination varying from a moderate to a very high degree with values between 8.1 and 94.5; agricultural topsoil generally presented contamination varying from a low to a very high degree with values between 1.6 and 24.1.
The elements of Cd, Hg, As, Pb, Cr, Cu and Zn in agricultural topsoil showed low and moderate potential ecological risk in all sites, combining the potential ecological RI of individual metals \((E_{r}^{i} )\) (Data not shown) with its grade classifications (Table 1). The consequence of the average \(E_{r}^{i}\) for heavy metals was Cd > Hg > Zn > As > Pb > Cu > Ni > Cr. On the contrary, the elements of Cd, Hg, As, Pb, Cr, Cu and Zn in non-agricultural topsoil showed very high, high or medium potential ecological risk in all samples. The consequence also was Cd > Hg > Zn > As > Pb > Cu > Ni > Cr.
The range for the potential ecological risk of agricultural and none-agricultural soil was 29.3–262.5 and 113.3–959.7, respectively, which indicates that some sites presented high degree potential ecological risk. The extremely high potential ecological RI values (959.7, 932.5 and 930.8) were seen in sites 3, 9 and 10 in non-agricultural soil, respectively. These sites coincided with the area where mining activities were historically concentrated.
The comparison results of risk values of heavy metals based on different methods showed that there were several disagreements. The main reason may be that RAC determined the risk level of heavy metals by only focusing on chemical speciation and the toxic-response factors for different heavy metals were ignored. RI considered both the total concentration and toxic-response factors of heavy metals. But the toxic indexes corresponding to the chemical speciation of heavy metals were neglected. The chemical speciation and toxicities of heavy metals were significant factors in the risk assessment process. The improvement of ecological risk assessment need further research in the future.
4 Conclusions
The RAC and RI method applied to the analysis of the ecological risk of heavy metals in soil in study areas was successful and the results yielded the information below:
The high content of Cd, Hg, As, Pb, Cr, Cu and Zn in the study area revealed that the highest level of pollution was distributed in the research area where mining activities were historically concentrated. The agricultural soil was also affected by the mining activities. PCA and CA had been also applied. In the agricultural topsoil, As, Hg, Cu, Cd, Zn, Pb and Cr were classified by three groups. Group one included Cu, Cd and Zn; group two included As and Hg; and group three included Cr and Pb. For the non-agricultural topsoil, the classification was slightly different from that for the agricultural topsoil. The first group included As, Cd, Pb and Zn; the second group included Cr and Hg; the third group included Cu. Furthermore, this study suggested that major portion of Cr, Pb, Zn, Ni and Cd were due to anthropogenic factors.
The chemical speciation of the heavy metals (Cd, Hg, As, Pb, Cr, Cu and Zn) was determined using a BCR sequential extraction procedure in order to evaluate the mobility potential. The metals like Cd, Cu, Zn, Ni and Pb represented an appreciable portion in the carbonate phase, as these metals have a special affinity toward carbonate and may co-precipitate with its minerals. The present study suggested that colloids of Fe–Mn oxides act as efficient scavengers for metals like Zn, Pb, Cu, Cr and Ni, while organic matter and CaCO3 was found to be more a effective scavenger for As, Cu and Cd, respectively. Cadmium was mostly bound to the first three fractions in the non-residual phase, which indicated the bioavailability of this metal to the aquatic organisms in the studied area. This suggested that Cd was highly mobile and under high environmental concern.
According to RAC, Cd posed a very high risk to the ecosystem due to its higher toxicity and percentage in the exchangeable and carbonate fractions. Hg and Zn also posed a high risk. The overall risks levels of heavy metals were, in declining order, Cd > Hg > Zn > As > Pb > Cu > Ni > Cr. The results of the RI showed that Cd was the only metal posing a very high risk to the environment. The risks of heavy metals under RI were, in declining order, Cd > Hg > Zn > As > Pb > Cu > Ni > Cr. The overall RIs in agricultural and non-agricultural soils caused by the toxic heavy metals ranged from 29.3–262.5 to 113.3–959.7, respectively, indicating some of the sites where mining activities were concentrated presented a high degree of potential ecological risk.
Overall, the chemical speciation and toxicities of heavy metals were two significant factors in the risk assessment process. The comparison results of risk values of heavy metals based on different methods showed that there were several disagreements, because the total concentration, chemical speciation and toxic response factor of individual heavy metals were not considered with one method. Therefore, improving the ecological risk assessment of heavy metals is of great importance.
References
Carral E, Villares R, Puente X (1995) Influence of watershed lithology on heavy-metal levels in estuarine sediments and organisms in Galicia (north-west Spain). Mar Pollut Bull 30:604–608
Chao LL (1972) Selective dissolution of manganese oxides from soils and sediments with acidified hydroxylamine hydrochloride. Soil Sci Soc Am J 36:764–768
Chen C, Lu Y, Hong J, Ye M, Wang Y, Lu H (2010) Metal and metalloid contaminant availability in Yundang Lagoon sediments, Xiamen Bay, China, after 20 years continuous rehabilitation. J Hazard Mater 175:1048–1055
China Ministry of Geology and Mineral Resources (1995) Regulation of regional geochemical exploration (1:200000)
Gismera MJ, Lacal J, Silva P, Garcia R, Sevilla MT, Procopio JR (2004) Study of metal fractionation in river sediments. A comparison between kinetic and sequential extraction procedures. Environ Pollut 127:175–182
Goldschmidt VM (1954) Geochemistry. In: Muir A (ed.). Oxford University Press, Oxford
Granero S, Domingo JL (2002) Levels of metals of Alcala de Henares, Spain: human health risks. Environ Int 28:159–164
Guevara-Riba A, Sahuquillo A, Rubio R (2004) Assessment of metal mobility in dredged harbor sediments from Barcelona, Spain. Sci Total Environ 321:241–255
Guillén MT, Delgado J, Albanese S, Nieto JM, Lima A, De Vivo B (2011) Environmental geochemical mapping of Huelva municipality soils (SW Spain) as a tool to determine background and baseline values. J Geochem Explor 109:59–69
Hakanson L (1980) An ecological risk index for aquatic pollution control—A sediment logical approach. Water Res 14:975–1001
He J, Xu G, Zhu H, Peng G (2001) Soil background values of Hunan Province. Chinese Environmental Science Press, Beijing
Huang B, Kuo S, Bembenek R (2004) Availability of cadmium in some phosphorus fertilizers to field grown lattuce. Water Air Soil Pollut 158:35–51
Hursthouse A, Tognarelli D, Tucker P, Ajmone-Marsan F, Martini C, Madrid L, Madrid F, Díaz-Barrientos E (2004) Metal content of surface soils in parks and allotments from three European cities: initial pilot study results. Land Contam Reclam 12:189–196
Ianni C, Magi E, Rivaro PE, Ruggieri N (2000) Trace metals in Adriatic coastal sediments: distribution and speciation patterns. Environ Toxicol Chem 78:73–92
Jain CK (2004) Metal fractionation study on bed sediments of River Yamuna, India. Water Res 38:569–578
Keller C, Hammer D (2004) Metal availability and soil toxicity after repeated croppings of Thlaspi caerulescens in metal contaminated soils. Environ Pollut 131:243–254
Li RY, Yang H, Zhou Zh-G, Lu JJ, Shao XH, Jin F (2007) Fractionation of heavy metals in sediments from Dianchi lake, China. Pedosphere. 17:265–272
Madrid L, Díaz-Barrientos E, Madrid F (2002) Distribution of heavy metal contents of urban soils in parks of Seville. Chemosphere 49:1301–1308
Morillo J, Usero J, Gracia I (2004) Heavy metal distribution in marine sediments from the southwest coast of Spain. Chemosphere 55:431–442
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Niu HY, Deng WJ, Wu QH, Chen XG (2009) Potential toxic risk of heavy metals from sediment of the pearl river in south China. J Environ Sci 21:1053–1058
Page AL, Miller RH, Keeney DR (1982) Methods of soil analysis. Part 2. Chemical and microbiological properties, 2nd edn. ASA, SSSA, CSSA No. 9, Madison, Wisconsin
Perin G, Craboledda L, Lucchese M et al (1985) Heavy metal speciation in the sediments of northern Adriatic Sea. A new approach for environmental toxicity determination. In: Lakkas TD (ed) Heavy metals in the environment, vol 2. CEP Consultants, Edinburgh
Peters JM, Thomas D, Falk H, Oberdorster G, Smith TJ (1986) Contribution of metals to respiratory cancer. Environ Health Perspect 70:71–83
Prasad LR, Nazareth B (2000) Contamination of allotment soil with lead: managing potential risks to health. J Public Health Med 22:525–530
Quevauviller P, Lavigne R, Cortez L (1989) Impact of industrial and mine drainage wastes on the heavy metal distribution in the drainage basin and estuary of the Sado River (Portugal). Environ Pollut 59:267–286
Rauret G, López-Sánchez JF, Sahuquillo A, Rubio R, Davidson C, Ure AM, Quevauviller PH (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1:57–61
Rodríguez L, Ruiz E, Alonso-Azcárate J, Rincón J (2009) Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J Environ Manag 90:1106–1116
Sahuquillo A, Lopez-Sanchez JF, Rubio R, Rauret G (1999) Use of a certified reference material for extractable trace metals to assess sources of uncertainty in the BCR three-stage sequential extraction procedure. Anal Chim Acta 382:317–327
Schramel O, Michalke B, Kettrup A (2000) Study of the copper distribution in contaminated soils of hop fields by single and sequential extraction procedures. Sci Total Environ 263:11–22
Schuhmacher M, Meneses M, Granero S, Llobet JM, Domingo JL (1997) Trace element pollution of soils collected near a municipal solid waste incinerator: human health risks. Bull Environ Contam Toxicol 59:861–867
Singh KP, Mohan D, Singh VK, Malik A (2005) Studies on distribution and fractionation of heavy metals in Gomti river sediments–A tributary of the Ganges, India. J Hydrol 312:14–27
Sundaray SK, Panda UC, Nayak BB et al (2006) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of the Mahanadi river-estuarine system (India): a case study. Environ Geochem Health 28(4):317–330
Sundaray SK, Nayak BB, Lin S, Bhatt D (2011) Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—A case study: Mahanadi basin, India. J Hazard Mater 186:1837–1846
Teng Y, Ni S, Jiao P, Deng J, Zhang C, Wang J (2004) Eco-environmental geochemistry of heavy metal pollution in Dexing mining area. Chin J Geochem 23:349–358
Teng Y, Ni S, Wang J, Niu L (2009) Geochemical baseline of trace elements in the sediment in Dexing area, South China. Environ Geol 57:1646–1660
Teng Y, Ni S, Wang J, Zuo R, Yang J (2010) A geochemical survey of trace elements in agricultural and non-agricultural topsoil in Dexing area, China. J Geochem Explor 104:118–127
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Bioanal Chem 51:844–851
Wang SF, Jia YF, Wang SY, Wang X, Wang H, Zhao ZhX, Liu BZ (2010) Fractionation of heavy metals in shallow marine sediments from Jinzhou Bay China. J Environ Sci 22:23–31
Wei F, Zheng C, Chen J, Wu Y (1991) Study on the background contents on 61 elements of soils in China. Chin J Environ Sci 12:12–19
Xu Z, Ni S, Tuo X, Zhang C (2008) Calculation of heavy metals’ toxicity coefficient in the evaluation of potential ecological risk index. Environ Sci Technol (China) 31:112–115
Xuan H (2007) Geochemical baseline of trace elements in soil in Dexing area. Dissertation of MSc, Beijing Normal University
Yuan C, Shi J, He B, Liu J, Liang L (2004) Speciation of heavy metals in marine sediments from the East China Sea by ICP-MS with sequential extraction. Environ Int 30:769–783
Zayed J (2001) Use of MMT in Canadian gasoline: health and environment issue. Am J Ind Med 39:426–433
Acknowledgments
This work is funded by National Natural Science Foundation of China (No. 41303069).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, S., Teng, Y., Wang, Y. et al. Research on the ecological risk of heavy metals in the soil around a Pb–Zn mine in the Huize County, China. Chin. J. Geochem. 34, 540–549 (2015). https://doi.org/10.1007/s11631-015-0062-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-015-0062-6