Abstract
A highly reproducible plant regeneration protocol through somatic embryogenesis and shoot organogenesis has been developed for Cenchrus ciliaris. Three explants (seeds, shoot apices, and immature inflorescences) of four genotypes (IG-3108, IG-718, IG-74, and DBC15-8/32/10) were used for callus induction and plant regeneration. The highest rate of callus formation was found using Murashige and Skoog (MS) medium supplemented with 0.5 mg L−1 benzylaminopurine (BA) and 3.0 mg L−1 2,4-dichlorophenoxyacetic acid (2,4-D). The largest number of somatic embryos was generated with the addition of 400 mg L−1 L-proline, 400 mg L−1 L-glutamine, and 300 mg L−1 casein hydrolysate. Somatic embryos were successfully germinated on MS medium with 3.0 mg L−1 BA and 0.25 mg L−1 2,4-D. In vitro plant regeneration was accomplished through somatic embryogenesis using all three explants. Ultra-structural features of somatic embryos confirmed proper formation and ontogeny.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apomictic Cenchrus ciliaris L. (Buffel grass; syn. Pennisetum ciliare) is a warm-season, perennial forage grass for livestock and with high value as a bioenergy feedstock (Whyte et al. 1959; Bray 1978). The genus Cenchrus includes more than 25 species which are widely distributed throughout the world (Sherwood et al. 1980; Sanderson et al. 1999). Some of these species are resilient to extreme environmental conditions (Griffa et al. 2006) including strong winds, low rainfall, soil erosion, and poor soil conditions (Ayerza 1981). Cenchrus is a member of the tribe Paniceae, family Poaceae, and a component of Dichanthium-Cenchrus-Lasiurus grasslands of India. The majority of plants in C. ciliaris are polyploid (2n = 4x = 36) (Fisher et al. 1954; Burson et al. 2002). It reproduces through apospory combined with pseudogamy and hence is considered a model taxa to study apospory. Due to apomixis, breeding Cenchrus genotypes for improved agronomic traits has been limited and is restricted to selection methodologies only. Hence, in vitro plant regeneration through somatic embryogenesis (SE) can provide an important alternate method to genetically improve this grass. Induction and progression of SE can be enhanced using different explants, media compositions, plant growth regulators, culture conditions, and conditioned media. Empirical information for key physiological cues associated with increased embryogenic potential are highly useful for specific taxa. Although there are several reports of in vitro plant regeneration in C. ciliaris, an efficient protocol for in vitro plant regeneration using diverse explants suitable for genetic transformation studies is not yet available.
A fundamental pre-requisite for many biotechnological applications is the ability to regenerate whole plants from cultured cells, tissues, or organs. Earlier studies on in vitro plant regeneration in Cenchrus used immature and mature embryos (Murty et al. 1992; Ross et al. 1995; Colomba et al. 2006) and immature inflorescences (Sankhla and Sankhla 1989; Kackar and Shekhawat 1991; Yadav et al. 2009; Kumar et al. 2015). Initial success on callus induction was observed with young immature inflorescences, although quantitative details of frequency of regenerable callus and the rate of plant regeneration were not ascertained (Sankhla and Sankhla 1989). Callus could be induced in Cenchrus species at different concentrations of 2,4-D (1.0 to 20.0 mg L−1) with a combination of the phytohormones indole-3-acetic acid (IAA) and kinetin (Sankhla and Sankhla 1989) or by including supplements such as ascorbic acid (Kackar and Shekhawat 1991) or coconut milk water (Ross et al. 1995) in MS medium. Recent studies suggested addition of 2,4-D at a range of 2.5 to 6.0 mg L−1 for C. ciliaris and at 4.0 to 14.0 mg L−1 for C. setigerus as optimal concentrations for callus induction and maintenance. However, Sankhla and Sankhla (1989) reported callus production even with 1.0 mg L−1 2,4-D, 5.0 mg L−1 IAA, and 0.5 mg L−1 kinetin. Kackar and Shekhawat (1991) suggested including ascorbic acid as an antioxidant source for callus cultures of C. ciliaris. Similarly, adding 5% coconut water was also found beneficial for inducing callus (Ross et al. 1995). Rogers et al. (1993) were the first report on the initiation of suspension cultures from shoot apices–derived callus using two distinct media formulations, MS basal salts (Murashige and Skoog 1962), and B5 vitamins (Gamborg et al. 1968). These callus lines, which were morphologically distinct to each other, were identified and separated from the original explant material. Developing a highly proficient and reproducible in vitro plant regeneration protocol across diverse genotypes for multiple explants is challenging but a pre-requisite for any genetic manipulation study in this grass. Nevertheless, owing to its mode of reproduction, its genetic improvement mostly relies on development of an efficient tissue culture protocol (Bhat et al. 2001; Batra and Kumar 2002, 2003).
In this study, seeds, shoot apices, and immature inflorescences were tested for callus-mediated SE and in vitro plant regeneration in four genotypes of C. ciliaris. Towards accelerating the rate of SE and plant regeneration, several amino acids and growth supplements including L-proline, L-glutamine, and casein hydrolysate were investigated along with exogenously supplied plant growth regulators.
Materials and Methods
Plant materials and explant preparation
Mature seeds of four apomictic buffel grass genotypes IG-3108, IG-718, IG-74, and DBC15-8/32/10 were obtained from botanical garden under natural conditions at the Department of Botany, University of Delhi, Delhi, India.
The explants investigated for callus induction and SE were 1-yr-old mature seeds, shoot apices dissected from 2 to 3-d-old seedlings, and immature inflorescences (1.5 to 3.0 cm) about to emerge out of the boot leaf. Mature seeds and immature inflorescences were surface sterilized by rinsing initially in 70% (v/v) ethanol (MB106; Himedia®, Mumbai, India) for 1 min, then in 0.1% (w/v) aqueous HgCl2 (GRM1067; Himedia®) for 5 min with occasional stirring (Yadav et al. 2009). For culturing, inflorescences were cut into small portions of 3.0 to 5.0 mm length using a sterile scalpel blade (Cynamed, Lortan, VA). For shoot apex explants, surface-sterilized mature seeds were cultured on Murashige and Skoog (MS basal salts, Himedia®; Murashige and Skoog 1962) medium in 90 × 15 mm Petri plates (Himedia®) under fluorescent light with light intensity of 40 μmol m−2 s−1 at 25 ± 2°C and 16/8 h (light/dark). After 2 to 3 d, a portion of the shoot containing the shoot apical meristem and mesocotyl was dissected out and formed the shoot apex explants for SE.
Callus induction
Sterilized explants were placed on MS callus induction medium containing 2,4-D (PCT0825; Himedia®) (2.0, 3.0, 5.0, 6.0, or 7.0 mg L−1), 0.5 mg L−1 BA (PCT0802; Himedia®), 30.0 g L−1 sucrose (GRM601; Himedia®), and 8.0 g L−1 agar-agar type I (GRM666; Himedia®). The pH of all media was set at 5.8 before adding agar. The media were sterilized by autoclaving at 121°C for 17 min and 25 mL of media was distributed into each 100-mL autoclaved conical flask (Borosil, Mumbai, India) and closed with sterile non-absorbent cotton plugs. Eight to ten explants were aseptically placed on the MS medium in each flask. The cultured explants were kept in darkness at 25 ± 2°C for callus induction and subcultured every 20 d for 2 mo. The percentage of induced callus was calculated as the number of explants that produced callus divided by the total number of explants on callus induction medium, multiplied by 100. The data was recorded after 20 d of culturing of primary explants.
Induction of embryogenic callus and its maintenance
The induced callus from primary explants was cut into small pieces (approximately 100 mg) and inoculated onto MS medium containing 2.0, 3.0, 5.0, 6.0, or 7.0 mg L−1 2,4-D and 0.5 mg L−1 BA to obtain embryogenic callus. All cultures were maintained at 25 ± 2°C under dark conditions. The cultures were examined periodically and visual observations were made to record any morphological changes, quality of callus, and growth rate. The percentage of embryogenic callus was calculated as the number of callus bearing regions of embryogenic portion divided by the total number of callus subculture multiplied by 100. Data on embryogenic callus frequency was recorded at different time intervals for different explants: after one to two subcultures in the case of immature inflorescence; after second to third subculture for shoot apices explants and third to fourth subculture for seed explants.
Effect of growth supplements on somatic embryo maturation
Callus induction medium was augmented with L-proline (Pro; PCT0317; Himedia®), L-glutamine (Glu; PCT0308; Himedia®), and casein hydrolysate (CH; PCT0403; Himedia®) either alone or in combination at various concentrations ranging from 100 to 500 mg L−1. The embryogenic callus induction medium used was MS with 3.0 mg L−1 2,4-D and 0.5 mg L−1 BA. The cultures were incubated at 28 ± 2°C in the dark for 20 d and were observed regularly until the appearance of somatic embryos. The percentage of somatic embryos was calculated as the number of calluses containing somatic embryos per 100 mg calluses cultured, multiplied by 100. The number of somatic embryos developed in 100 mg callus cultures was recorded after 2-wk incubation.
Scanning electron microscopy and histological observations on somatic embryos
Intact embryogenic callus was fixed in 2% (w/v) glutaraldehyde (RM5927; Himedia®) prepared in phosphate buffer (pH 6.8) for 24 h at 4°C, and dehydrated through a graded ethanol series and stored in 70% (v/v) acetone (MB179; Himedia®). The calluses were critical point dried, coated with gold, examined, and photographed using a LEO 435 VP Scanning Electron Microscope (Cambridge U.K. model).
For histological observations, fresh embryogenic callus was fixed in a mixture of acetic acid:alcohol (1:3 ratio) for 24 h and subsequently transferred to 70% (v/v) ethanol. The samples were treated with a tertiary butyl alcohol (AS083; Himedia®) dehydration-infiltration series and embedded in paraffin wax (GRM10301; Himedia®; Johnsen 1940). The sequential sections (10 μm thick) were obtained using a microtome (Thermo Shandon, Thermo Scientific, Waltham, MA) and spread onto microscope slides (Blue Star©, Mumbai, India). The paraffin sections containing tissues were affixed onto the slides using Mayer’s adhesive, dewaxed with xylene (AS078; Himedia®) and dehydrated and stained in a graded alcohol series with Safranin (GRM129; Himedia®) and Fast Green FCF (MB187; Himedia®). The sequential sections were again cleared using xylene and fixed with Distyrene Plasticizer Xylene (DPX; GRM655; Himedia®) mountant. Images were captured using an upright compound microscope (Nikon, Tokyo, Japan).
Plant regeneration from somatic embryos
Proliferating embryogenic calluses (approximately 100 mg each) containing somatic embryos were further subcultured onto MS regeneration medium containing 1.0 to 4.0 mg L−1 BA and 0.25 mg L−1 2,4-D. Regeneration from somatic embryos was carried out in glass jars with 75 mL of culture medium to allow space for shoot elongation and maintained at 25°C under 16/8 (light/dark) photoperiod with light intensity 40 μmol m−2 s−1. After 2 wk, the percentage of plant regeneration was determined as the number of calluses producing shoots divided by the total number of embryogenic calluses inoculated onto regeneration medium multiplied by 100. The mean number of shoots per callus was recorded 30 d after transferring to regeneration medium.
Root induction
Regenerated shoots (10 to 15 cm height) were subcultured onto rooting media containing various combinations of hormones for optimizing root induction. Semi-solid MS or ½MS medium with or without 0.8% (w/v) charcoal (PCT1001; Himedia®) or MS medium containing 2.0 mg L−1 indole-3-butyric acid (IBA; PCT0804; Himedia®) was tested for rooting. The percent root induction was determined by counting the number of shoots producing any roots divided by the total number of shoots, multiplied by 100. To estimate the number of roots formed by single shoot, an average of roots produced from several single shoots in a flask was calculated. After 3 wk, the rooted plantlets were washed with sterile distilled water to remove adhering media, and then maintained in liquid MS medium containing 0.4% sucrose for 1 wk to allow root growth. Healthy plants were then moved to test tubes (Borosil) containing sterile tap water for 1 d, and later transferred to pots as described below.
Acclimatization of regenerated plants
Hardened plantlets were transferred to Soilrite Mix-TC (Keltech Energies Ltd., Bangalore, India) in plastic pots and nutrients were provided through Hoagland’s solution (Hoagland and Arnon 1950). Initially, these pots were kept inside the culture room and covered with plastic bags (Hidispo bag; Himedia®) with numerous small holes to retain humidity. After 15 d, the bags were removed in order to reduce humidity. Finally, the plants were transferred to autoclaved garden soil in pots, further acclimatized by removal of plastic bag after 15 d. These plants were subsequently grown in a greenhouse and later transferred to field conditions until maturity.
Statistical analysis
The SPSS ver. 16 software was used to analyze the data collected on various factors such as explants, genotypes, and media which influenced callus, shoot, and root induction. Analysis of variance (ANOVA) was used to determine the significant variation for all the data sets. Four genotypes and media composition were the factors in the analysis. Each test consisted of 10 explants per flask (100 mL) in three replicates (three flasks per treatment) and the experiment was repeated two times. Two-way ANOVA and least significant difference at p ≤ 0.05 formed the basis on which significant differences among means were compared.
Results and Discussion
Callus induction
Callus cultures were induced from the three explants incubated on MS medium containing different levels of 2,4-D (2.0 to 7.0 mg L−1) along with 0.5 mg L−1 BA. For seed and shoot apex explants, callus initiation was first observed after 3 to 5 d of culture, compared to 10 to 12 d for immature inflorescence. After 20 d, all explants displayed morphological changes resulting in the induction of callus at all 2,4-D concentrations tested. All three explants failed to induce callus on hormone-free MS medium. Maximum callus induction frequency, 87 ± 2.2%, was obtained from genotype IG-3108 seed explants subcultured on MS medium containing 3.0 mg L−1 2,4-D, whereas the minimum, 15 ± 2.1%, was observed from DBC15-8/32/10 immature inflorescence explants cultured on MS medium containing 2.0 mg L−1 2,4-D (Fig. 1). Among the four genotypes, IG-3108 showed the best callus induction frequency, followed by IG-718 (83.3 ± 2.1%), IG-74 (75 ± 4.3%), and DBC15-8/32/10 (66.6 ± 2.0%). The inference that genotype and explants had significant influence on callus induction frequency from our study is consistent with the earlier results in C. ciliaris (Colomba et al. 2006; Yadav et al. 2009) and Dichanthium annulatum (Kumar et al. 2005) (Table 1).
Frequency of callus induction from three Cenchrus ciliaris L. explants (Seed, shoot apex (SA), immature inflorescence (Im In)) from four genotypes cultured on Murashige and Skoog medium containing 0.5 mg L−1 benzylaminopurine (BA) and varying levels of 2,4-dichlorophenoxyacetic acid (2,4-D) (2.0 to 7.0 mg L−1). Mean ± standard error, letters on the standard error bars represent mean separation by LSD at p ≤ 0.05.
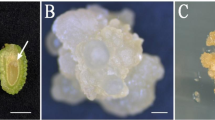
Once callus has been induced, the rate of cell proliferation depends on several factors including genotype, phytohormones (auxin and cytokinin), and media composition (Gupta et al. 2002). When a high level of 2,4-D (6.0 to 7.0 mg L−1) was used, callus induction during the first 20 d was at a relatively low frequency and growth rate, ultimately resulting in brown-colored callus (Fig. 2). Among the different explants used, mature seed was the best source for callus induction with more than a four-fold difference among all explants tested. Maximum callus growth was recorded from seed and immature inflorescences in all genotypes on MS medium containing 3.0 mg L−1 2,4-D and 0.5 mg L−1 BA (Fig. 2). The primary callus induced was watery, loose, and pale yellow in the case of seed and shoot apex explants whereas calluses of immature inflorescence were mostly soft, compact, white, and non-regenerative in nature (Fig. 2).
Comparison of the responses of three different explants on MS medium with varying levels of 2,4-dichlorophenoxyacetic acid (2,4-D) and 0.5 mg L−1 benzylaminopurine (BA) on callus growth rate and quality. Callus induced from three explants such as seeds, shoot apices, and immature inflorescences cultured on MS medium with different levels (2.0 to 7.0 mg L−1) of 2,4-D and 0.5 mg L−1 BA was tested. Data were recorded after 20 d of first subculture. Based on visual appearance, maximum callus growth was recorded from seed-derived callus on MS medium containing 3.0 mg L−1 2,4-D and 0.5 mg L−1 BA. For embryogenic calluses induced from shoot apices and immature inflorescences, MS medium with varying levels (2.0 to 5.0 mg L−1) of 2,4-D + 0.5 mg L−1 BA + 400 mg L−1 proline + 400 mg L−1 glutamine + 300 mg L−1 casein hydrolysate and for seeds, MS medium with varying levels of 2,4-D (5.0 to 7.0 mg L−1) + 0.5 mg L−1 BA + 400 mg L−1 proline + 400 mg L−1 glutamine, 300 mg L−1 casein hydrolysate were used. Based on visual appearance, the callus type and growth rate were assessed in 2-mo-old cultures. Callus induced from shoot apices and immature inflorescences turned brown at higher levels of 2,4-D (6.0 to 7.0 mg L−1) and for seeds at low levels of 2,4-D (2.0 to 3.0 mg L−1). The quality/texture of callus induced from three explants showed in images with arrows: (a) dry, granular, yellowish; (b) hard, granular, compact, white; (c) watery, gelatinous, yellow-brown; (d) hard, nodular, white; (e) soft, nodular, creamish white; (f) hard, nodular, fragile, milky white.
Callus maintenance and embryogenic callus formation
After 20 d, the primary callus was separated into smaller portions of approximately 100 mg and transferred onto fresh MS medium with 2.0 to 7.0 mg L−1 2,4-D and 0.5 mg L−1 BA. The seed-derived calluses required high levels of 2,4-D (5.0 to 7.0 mg L−1) to become embryogenic callus as they turned brown at low levels of 2,4-D (2.0 to 3.0 mg L−1) in all genotypes. However, shoot apices and immature inflorescences failed to develop into embryogenic callus at higher levels of 2,4-D (6.0 to 7.0 mg L−1) and 0.5 mg L−1 BA (Figs. 2 and 3). The embryogenic callus that developed from immature inflorescence and shoot apices could be maintained at lower levels of 2,4-D (2.0 to 5.0 mg L−1) and 0.5 mg L−1 BA. All explant-derived calluses after 2 to 4 subcultures showed morphological changes; they became white, hard, granular, and embryogenic in nature. Two characteristic types of callus were observed, one fast-growing and non-regenerable and the other slow-growing and regenerable. These same types were also reported in Cenchrus (Kackar and Shekhawat 1991; Colomba et al. 2006; Yadav et al. 2009), Paspalum (Vikrant and Rashid 2003), and Dichanthium (Kumar et al. 2005). Thus, our results are consistent with earlier observations on Cenchrus and other Graminaceous genera.
Frequency of Cenchrus ciliaris L. embryogenic calli from three explants (seed, shoot apex (SA), immature inflorescence (Im In)) in four genotypes cultured on Murashige and Skoog medium containing 0.5 mg L−1 benzylaminopurine (BA) and varying levels of 2,4-dichlorophenoxyacetic acid (2,4-D) (2.0 to 7.0 mg L−1). Embryogenic callus could not be produced at some levels of 2,4-D from seed (2.0 and 3.0 mg L−1) and SA and Im In (6.0 and 7.0 mg L−1). Mean ± standard error, letters on the standard error bars represent mean separation by LSD at p ≤ 0.05.
The maximum percent embryogenic callus formation (89.6 ± 4.0%) was from immature inflorescence explants of IG-3108 cultured on MS medium containing 3.0 mg L−1 2,4-D and 0.5 mg L−1 BA, whereas the least amount of embryogenic callus was obtained from shoot apex explants of DBC15-8/32/10 (30 ± 3.6%) cultured on MS medium containing 5.0 mg L−1 2,4-D and 0.5 mg L−1 BA (Fig. 3). There was a significant influence of genotype, 2,4-D level, and explant type on embryogenic callus formation (Table 1). Embryogenic callus from seed explants of all four genotypes could be maintained on MS medium containing 6.0 mg L−1 2,4-D and 0.5 mg L−1 BA. These combinations of phytohormones and subculture periods resulted in significant enhancement in the rate of embryogenic callus production compared to previous efforts (Yadav et al. 2009).
Impact of amino acids and growth supplements on somatic embryogenesis
Development of somatic embryos from all three explants has been successfully demonstrated in Cenchrus ciliaris. In order to further enhance the quality and quantity of somatic embryos produced, we tested the effects of Pro, Glu, and CH. CH and Pro were used for inducing a large number of somatic embryos and regeneration in rice (Ozawa and Komamine 1989). CH is an organic nitrogen source which stimulates somatic embryo development as well as multiple shoot regeneration (Ramakrishnan et al. 2013; Satish et al. 2015). All these organic nitrogenous additives had a significant effect on increased somatic embryo formation when used in embryogenic callus maintenance medium (Elkonin and Pakhomova 2000; Dal Vesco and Guerra 2001).
MS medium with 400 mg L−1 L-glutamine gave a moderate response (88%) and 300 mg L−1 casein hydrolysate produced the least response (81%) compared to control (50%, without supplement) on increasing the frequency of SE (Table 2). L-proline at 400 mg L−1 alone induced 90% somatic embryogenesis, which was superior compared to those obtained from the media containing L-glutamine or casein hydrolysate. The addition of L-glutamine to the medium with casein hydrolysate showed further increase in SE formation. The combination of 400 mg L−1 L-proline, 400 mg L−1 L-glutamine, and 300 mg L−1 casein hydrolysate could induced higher rate (92%) of the somatic embryogenesis within 6 d (Table 2). Earlier reports also had mentioned Pro and Glu as key components in the induction of SE in sugarcane (Sinha et al. 2000; Desai et al. 2004). An increased number of somatic embryos was also observed in the presence of amino acids in maize (Carvalho et al. 1997) and wheat (Yadava and Chawla 2002) in vitro cultures. In our study, MS medium containing 400 mg L−1 Pro, 400 mg L−1 Glu, and 300 mg L−1 CH gave better response for somatic embryo development than control medium (MS medium containing 2,4-D and BA). Similarly, callus induction from immature embryos was enhanced by using CH and Pro in turf-type tall fescue (Bai and Qu 2001) and in maize (Gleddie et al. 1983; Armstrong and Green 1985; Shohael et al. 2003; Sharma et al. 2012; Dhillon and Gosal 2013). All three amino acids significantly enhanced rate of SE in the present study.
Histological, stereomicroscopic, and scanning electron microscopic studies of developmental stages of somatic embryo
Histological studies have been performed in many Gramineae species reporting that somatic embryos develop de novo from proliferating parenchyma cells present in cultured mature embryos or from leaf mesophyll cells (McDaniel et al. 1982; Conger et al. 1983). In previous studies, it was shown that SEs originated from single cells in embryogenic callus induced either from cultured mature embryos or inflorescence of Pennisetum americanum (Vasil and Vasil 1982; Ho and Vasil 1983; Botti and Vasil 1984). In Sorghum bicolor, SEs originated from young leaves or by simple folding of the embryo scutellum and through the de novo formation of an embryogenic axis (Dunstan et al. 1978; Vasil and Vasil 1985). Recently, globular somatic embryos were induced by Wus2 and Bbm gene expression from single scutellar epithelial cells. Anatomical observations confirmed single cell origin of the embryo connecting it to the scutellum of the original zygotic embryo (Lowe et al. 2018).
Stereomicroscopic studies (Fig. 4a–c) showed embryogenic callus cultures consisting of compact cell masses hard and white in color. In the presence of high cytokinin and light, calluses turned green and somatic embryo started to germinate when later stages of embryo development were visible. SEM images provided further evidence for somatic embryo development in genotype IG-3108 (Fig. 4d–f).
Development of somatic embryos from embryogenic callus culture in Cenchrus ciliaris L. Stereomicroscopic photographs of somatic embryo development from callus: (a) Proliferating white nodular embryogenic callus containing somatic embryo formed after subculture on Murashige and Skoog (MS) medium containing 3.0 mg L−1 2,4-D + 0.5 mg L−1 BA + 400 mg L−1 Pro + 400 mg L−1 Glu + 300 mg L−1 CH. (b) Yellow globular-shaped somatic embryo (SE). (c) Germination of somatic embryo on MS medium containing 3.0 mg L−1 BA and 0.25 mg L−1 2,4-D after 7 d. Scanning electron microphotographs showing (d) Development of many proembryos of globular stage (GE) on the surface of callus (EC) mass. (e) Differentiation of scutellum (SC), somatic embryo (GE), organized in form of disc-shaped scutellum. (f) Formation of fused somatic embryo with lateral scutellar notch (SN, which partially separates the future embryonic axis from scutellum) and differentiates into scutellum. Histological sections of callus showing (g) Young somatic embryo (SE) of epidermal origin, small projections on the surface of callus (marked by arrows). Well-developed upper epidermis (UE) and parenchyama cell layers (PC) visible. (h) An oval-shaped somatic proembyo (PE) with well-defined protodermis (PT) formed from meristematic cell (MC). (i) An enlarged view of globular staged somatic embryo with procambial strands (PS). (j) Scutellar notch (SN) at the terminal region of embryo and a globular somatic embryo (GE) without connection to mother tissue and suspensor like. (k) Somatic embryo with developing shoot meristem (SM). (l) Fully developed somatic embryo with dome-shaped shoot apical meristem (SAM), coleorhiza (CR), coleoptile (CO), and vascular bundles (VB). Somatic embryos proliferation, growth, and acclimatization: (m) Regenerated shoots from embryogenic callus. (n) Shoot elongation and multiplication. (o) In vitro flowering in genotype IG-3108. (p) Rooting on MS with 2.0 mg L−1 IBA media. (q) Rooted shoots on liquid MS medium with 0.4% sucrose. (r) Regenerated plants transferred to Soilrite in pots and well-established plants hardened in soil.
Histological observations of embryogenic cultures revealed small cytoplasmically dense meristematic cells that were starch enriched. Cross sections showed the initiation and development of somatic embryos. Around the peripheral region of embryogenic calluses, small, compact, and cytoplasmically dense meristematic cells were observed (Fig. 4g–i). Initiation of embryogenesis with continuous divisions of single cells resulted in six- and eight-celled embryos, which after 1 wk formed globular embryos (Fig. 4i, j). Initially the somatic embryo was connected to the embryogenic callus through a prominent procambial strand and exhibited signs of polarization with apical and radical meristems at opposite poles. The shoot apex appeared very distinct and the coleoptile was visible as a circular primordium (Fig. 4k, l). The shoot apex was lateral in position, while the root apex could be observed towards the opposite side of shoot apex. Clear distinguishing parts of somatic embryos such as scutellum, shoot apex, coleorhiza, and coleoptile were visible which had a separate vascular system that was not connected to the maternal tissue (Fig. 4l).
Plant regeneration through somatic embryogenesis
When the auxin level was gradually reduced, embryogenic cells developed into small filamentous globular-shaped embryos from nodular compact embryogenic calluses (Fig. 4c). Cytokinin induces shoot regeneration from competent cells which appears to be mediated by molecular components associated with cytokinin perception and signaling (Ikeuchi et al. 2019). In the present study, shoots with leaves from all genotypes were successfully formed from germination of somatic embryos as well as through shoot organogenesis. Culturing on MS medium containing 3.0 mg L−1 BA and 0.25 mg L−1 2,4-D, these somatic embryos turned green resulting in protruding green, tiny shoot primordia in the presence of light (Fig. 4m, n). It was observed that plant regeneration from somatic embryo and also number of shoots formed was faster from immature inflorescence-derived callus in comparison to seed as well as shoot apex–derived cultures. Shoot regeneration percentage varied from 26.6 to 85%, across all the different levels of BA (Table 3). Maximum frequency of shoot regeneration (85% ± 2.2) and the highest number of shoots per callus (12 ± 1.0) was observed on MS medium containing 3.0 mg L−1 BA and 0.25 mg L−1 2,4-D in IG-3108 (Table 3). Our results corroborate the earlier findings, which reported shoot regeneration after 3 to 4 wk on regeneration medium containing 1-naphthaleneacetic acid (NAA) (Colomba et al. 2006) and BA (Yadav et al. 2009). As indicated in our study, there were significant genotypic differences in shoot differentiation and prolonged plant regeneration in Sorghum bicolor (Cai and Butler 1990), Hordeum vulgare (Hanzel et al. 1985), Triticum aestivum (Rajyalakshmi et al. 1991), and Avena sativa (Cummings et al. 1976). Genotypes, explants, and BA levels as well as significant interaction between the latter two factors had significantly influenced the rate of shoot induction (Table 3).
Rhizogenesis, hardening, and acclimatization
Well-formed shoots were transferred to various media to test the optimum rooting conditions. Earlier reports showed roots formation on MS (Sankhla and Sankhla 1989), ½MS (Kackar and Shekhawat 1991), ½MS containing charcoal (Yadav et al. 2009), and MS media containing NAA (Batra and Kumar 2002) in C. ciliaris. Optimal root induction frequency of 90.0 ± 2.2 and roots per shoot of 5.3 ± 0.4 were from shoots incubated for 3 wk on MS medium containing 2.0 mg L−1 IBA (similar as reported by Kumar et al. 2015) (Fig. 4p, Table 4). In vitro flowering was found in 1% of the total plants subcultured on MS medium after a duration of 2 to 3 mo (Fig. 4o), which was also reported by Yadav et al. (2009). After rooting, plantlets were placed direct into MS liquid medium with 0.4% sucrose for 1 wk for rapid growth and preventing root damage (Fig. 4q). Rooted plants were successfully hardened, acclimatized by transferring to pots filled with Soilrite and soil (1:3), and growing in a greenhouse (Fig. 4r).
Conclusion
A reproducible protocol for in vitro plant regeneration has been developed through SE using three explants and 4 genotypes of C. ciliaris. In vitro plant regeneration using mature seed and shoot apex derived callus in C. ciliaris is reported for the first time in this study. Somatic embryogenesis efficiency could be increased by adding growth supplements in MS medium containing growth regulators. The explant type, genotype, and growth media factors optimized for plant regeneration in the present study could be utilized for developing Agrobacterium-mediated transformation protocol in C. ciliaris.
References
Armstrong CL, Green CE (1985) Establishment and maintenance of friable, embryogenic maize callus and the involvement of L.-proline. Planta 164:207–214
Ayerza R (1981) El’ Buffel grass: utilidad y manejo de una promisoria gramínea. Hemisferio Sur, Buenos Aires
Bai Y, Qu R (2001) Factors influencing tissue culture responses of mature seeds and immature embryos in turf-type tall fescue. Plant Breed 120:239–242
Batra S, Kumar S (2002) In-vitro high frequency plant regeneration in buffel grass (Cenchrus ciliaris L.). J Plant Biol 29:191–194
Batra S, Kumar S (2003) Agrobacterium-mediated transient GUS gene expression in buffel grass (Cenchrus ciliaris L.). J Appl Genet 44:449–458
Bhat V, Dalton SJ, Kumar S, Bhat BV, Gupta MG, Morris P (2001) Particle- inflow-gun-mediated genetic transformation of buffel grass (Cenchrus ciliaris L.): optimizing biological and physical parameters. J Appl Genet 42:405–412
Botti C, Vasil IK (1984) Ontogeny of somatic embryos of Pennisetum americanum. II. In cultured immature inflorescences. Can J Bot 62:1629–1635
Bray RA (1978) Evidence for facultative apomixis in Cenchrus ciliaris. Euphytica 27:801–804
Burson BL, Hussy MA, Actkinson JM, Shafer GS (2002) Effect of pollination time on the frequency of 2n+n fertilization in apomictic buffel grass. Crop Sci 42:1075–1080
Cai T, Butler L (1990) Plant regeneration from embryogenic callus initiated from immature inflorescences of several high-tannin Sorghum. Plant Cell Tiss Org Cult 20:101–110
Carvalho CHS, Bahorova N, Bordallo PN, Abreu LL, Valicente FH, Bressan W, Paiva E (1997) Type II callus production and plant regeneration in tropical maize genotypes. Plant Cell Rep 17:73–76
Colomba EL, Grunberg K, Griffa S, Ribotta A, Mroginski L, Biderbost E (2006) The effect of genotype and culture medium on somatic embryogenesis and plant regeneration from mature embryos of fourteen apomictic cultivars of buffel grass (Cenchrus ciliaris L.). Grass Forage Sci 6:2–8
Conger BV, Hanning GE, Gray DJ, McDaniel JK (1983) Direct embryogenesis from mesophyll cells of orchard grass. Science 221:850–851
Cummings DP, Green CE, Stuthman DD (1976) Callus induction and plant regeneration in oats. Crop Sci 16:465–470
Dal Vesco LL, Guerra MP (2001) The effectiveness of nitrogen sources in Feijoa somatic embryogenesis. Plant Cell Tiss Org Cult 64:19–25
Desai NS, Suprasanna P, Bapat VA (2004) Simple and reproducible protocol for direct somatic embryogenesis from cultured immature inflorescence segments of sugarcane. Curr Sci 87:764–768
Dhillon NK, Gosal SS (2013) Effect of growth adjuvants of somatic embryogenesis in maize (Zeamays L.). J Cell Tiss Res 13:3557–3563
Dunstan DI, Short KC, Thomas E (1978) The anatomy of secondary morphogenesis in cultured scutellum tissues of Sorghum bicolor. Protoplasma 97:251–260
Elkonin LA, Pakhomova NV (2000) Influence of nitrogen and phosphorus on induction embryogenic callus of Sorghum. Plant Cell Tiss Org Cult 61:115–123
Fisher WD, Bashaw EC, Holt EC (1954) Evidence for apomixis in Pennisetum ciliare and Cenchrus setigerus. Agron J 46:401–404
Gamborg OL, Miller RA, Ohima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gleddie S, Keller W, Setterfield G (1983) Somatic embryogenesis and plant regeneration from leaf explants and cell suspensions of Solanum melongena (eggplant). Can J Bot 61:656–666
Griffa SDD, Ribotta A, Castelli SL, Munoz N, Colomba EL, Luna C, Grunberg K, Biderbost E (2006) Molecular genetic discrimination of Buffel grass genotypes and F1 hybrids for breeding purposes using amplified fragment length polymorphism analyses. Grass Forage Sci 61:454–458
Gupta S, Khanna VK, Singh R, Garg GK (2002) Effect of media and explant on callus formation and plant regeneration in sorghum. J Plant Biol 29:39–44
Hanzel JJ, Miller JP, Brinkmann MA, Fendos E (1985) Genotype and media effects on callus formation and regeneration in barley. Crop Sci 25:27–31
Ho W, Vasil IK (1983) Somatic embryogenesis in sugarcane (Saccharum officinarum L.) I. The morphology and physiology of callus formation and the ontogeny of somatic embryos. Protoplasma 118:169–180
Hoagland M, Arnon DI (1950) The water culture method to grow plants without soil. Calif Agric Expt Station Cir 347. Berkeley California
Ikeuchi M, Favero DS, Sakamoto Y, Iwase A, Coleman D, Rymen B, Sugimoto K (2019) Molecular mechanisms of plant regeneration. Annu Rev Plant Biol 70:377–406
Johnsen DA (1940) Plant micro-techniques. McGraw-Hill, NewYork
Kackar A, Shekhawat NS (1991) Plant regeneration from cultured immature inflorescences of Cenchrus setigerus and C. ciliaris. Indian J Exp Biol 29:62–64
Kumar J, Shukla SM, Bhat V, Gupta S, Gupta MG (2005) In vitro plant regeneration and genetic transformation of Dichantium annulatum. DNA Cell Biol 24:670–679
Kumar S, Sahu N, Singh A (2015) High-frequency in vitro plant regeneration via callus induction in a rare sexual plant of Cenchrus ciliaris L. In Vitro Cell Dev Biol - Plant 51:28–34
Lowe K, Rota ML, Hoerster G, Hastings C, Wang N, Chamberlin M, Wu E, Jones T, Gordon-Kamm W (2018) Rapid genotype “independent” Zea mays L. (maize) transformation via direct somatic embryogenesis. In Vitro Cell Dev Biol – Plant 54:240–252
McDaniel JK, Conger BV, Graham ET (1982) A histological study of tissue proliferation, embryogenesis and organogenesis from tissue cultures of Dactylis glomerata L. Protoplasma 110:121–128
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murty UR, Bharathi M, Visadara M, Annapurna A (1992) Embryogenic callus formation and plant regeneration in Cenchrus ciliaris (L). Cereal Res Commun 20:7–12
Ozawa K, Komamine A (1989) Establishment of a system of high frequency embryogenesis from long-term cell suspension cultures of rice (Oryza sativa L.). Theor Appl Genet 77:205–211
Rajyalakshmi K, Grover A, Maheshwari N, Tyagi AK, Maheshwari SC (1991) High frequency regeneration of plantlets from the leaf bases via somatic embryogenesis and comparison of polypeptide profiles from morphogenic and non-morphogenic calli in wheat (Triticum aestivum). Plant Physiol 82:617–623
Ramakrishnan M, Ceasar SA, Duraipandiyan V, Daniel MA, Ignacimuthu S (2013) Efficacious somatic embryogenesis and fertile plant recovery from shoot apex explants of onion (Allium cepa L.). In Vitro Cell Dev Biol - Plant 49:285–293
Rogers SMD, Dahmer ML, Stair DW (1993) Characterization of buffel grass (Cenchrus ciliaris L.) cell suspension cultures. In Vitro Cell Dev Biol - Plant 29:51–54
Ross AH, Manners JM, Birch RG (1995) Embryogenic callus production, plant regeneration and transient gene expression following particle bombardment in the pasture grass, C. ciliaris (Graminae). Aust J Bot 43:193–199
Sanderson MA, Voigt P, Jones RM (1999) Yield and quality of warm season grasses in central Texas. Rangel Ecol Manag 52:45–50
Sankhla A, Sankhla N (1989) Tissue culture studies on desert plants: Cenchrus ciliaris cv. 75. Curr Sci 58:872–874
Satish L, Ceasar SA, Shilpha J, Rency AS, Rathinapriya P, Ramesh M (2015) Direct plant regeneration from in vitro-derived shoot apical meristems of finger millet (Eleusine coracana (L.) Gaertn). In Vitro Cell Dev Biol - Plant 51:192–200
Sharma S, Sandhu MK, Kaur P, Kaur A, Gosal SS (2012) Factors affecting somatic embryogenesis in maize (Zea mays L.). J Cell Tiss Res 12:3103–3108
Sherwood RT, Young BA, Bashaw EC (1980) Facultative apomixis in buffel grass. Crop Sci 20:375–379
Shohael AM, Akanda MAL, Parvez S, Mahfuja S, Alam MF, Islam R, Joarder N (2003) Somatic embryogenesis and plant regeneration from immature embryo derived callus of inbred maize (Zea mays L.). Biotechnology 2:154–161
Sinha H, Gill M, Gosal S (2000) Regulation of somatic embryogenesis and plant regeneration in sugarcane (Saccharum officinarum L.). Indian J Agric Sci 70:181–183
Vasil V, Vasil IK (1982) The ontogeny of somatic embryos of Pennisetum americanum (L.) K. Schum. I. In cultured immature embryos. Bot Gaz 143:454–465
Vasil V, Vasil IK (1985) Initiation and maintenance of cell suspension cultures of Gramineae. In: Vasil IK (ed) Cell culture and somatic cell genetics of plants, vol 1. Laboratory procedures and their applications. Academic Press, New York, pp 152–157
Vikrant, Rashid A (2003) Somatic embryogenesis or shoot formation following high 2,4-D pulse-treatment of mature embryos of Paspalum scrobiculatum. Biol Plant 46:297–300
Whyte RO, Moir TRG, Cooper JP (1959) Grasses in agriculture. FAO agricultural study no. 42. Rome, FAO
Yadav CB, Jha P, Mahalakshmi C, Anjaiah V, Bhat V (2009) Somatic embryogenesis and regeneration of Cenchrus ciliaris genotypes from immature inflorescence explants. Biol Plant 53:603–609
Yadava R, Chawla HS (2002) Role of genotypes, growth regulators and amino acids on callus induction and plant regeneration from different developmental stages of inflorescence in wheat. Indian J Genet 62:55–60
Funding
This research was supported by the R & D grant, University of Delhi. This paper is presented by Shashi in partial fulfillment of requisites for a thesis of Doctor of Philosophy in Botany at the University of Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Editor: Charles Armstrong
Rights and permissions
About this article
Cite this article
Shashi, Bhat, V. Enhanced somatic embryogenesis and plantlet regeneration in Cenchrus ciliaris L.. In Vitro Cell.Dev.Biol.-Plant 57, 499–509 (2021). https://doi.org/10.1007/s11627-020-10148-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-020-10148-y