Abstract
To optimize a bioreactor callus culture system for Rhodiola sachalinensis, several physicochemical factors affecting callus growth and bioactive compound accumulation were studied using a 3-L, balloon-type airlift bioreactor system. A bioreactor with a sparger size of 3.5-cm diameter was optimal for the callus culture of R. sachalinensis. Suitable light intensities for R. sachalinensis callus growth and biosynthesis of various kinds of bioactive compounds (salidroside, polysaccharides, phenolics, and flavonoids) were different. Maximum productivity of all bioactive compounds was found at a light intensity of 30 μmol m−2 s−1. Callus growth and production of salidroside, polysaccharides, and flavonoids were stimulated with sucrose at a concentration of 30 g L−1, but 50 g L−1 was favorable for the production of phenolics. Salidroside accumulation was promoted when MS medium salt strength increased to 1.5 × MS, 1 × MS was best for the production of polysaccharides and flavonoids, and 0.5 × MS was proper for phenolic production. A kinetics study indicated that a culture duration of 20–25 d was appropriate for the mass production of bioactive compounds from R. sachalinensis callus in a bioreactor system. Callus culture in a bioreactor can be an alternative method for producing materials from R. sachalinensis for commercial drug production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, plant cell, tissue, and organ cultures have provided alternatives to whole-plant cultivation for producing valuable metabolites. Tissue culture can offer higher yields and more consistent quality of bioactive compounds (Hahn et al. 2003). Bioreactors have been configured and used for large-scale production of effective metabolites. Cells (Ahmad et al. 2008), adventitious roots (Cui et al. 2013), shoots (Varsha et al. 2011), and embryos (Shohael et al. 2005) have been successfully cultured in bioreactors. During bioreactor culture, bioactive compound accumulation is affected by various factors such as the efficiency of oxygen transfer, shear forces, hydrodynamic forces, and control of the physicochemical environment (Paek et al. 2005). Consequently, studies have focused on controlling these parameters to enhance the yields of biomass and bioactive compounds during bioreactor culture (Ahmad et al. 2008; Varsha et al. 2009).
Rhodiola sachalinensis A. Bor. (Crassulaceae) is a precious perennial, herbaceous plant that is widely used in traditional Chinese medicine. Its roots and stems contain various bioactive compounds, including salidroside, polysaccharides, phenolics, flavonoids, glycosides, and coumarin. Salidroside is the most important active compound of R. sachalinensis and has proven effective in treating patients suffering from anoxia, microwave radiation, and fatigue (Li et al. 2014). Polysaccharides from R. sachalinensis can regulate immunity, blood glucose and lipids, and improve viral resistance (Cheng et al. 1993). Flavonoids in R. sachalinensis also have antioxidant, anticancer, and anti-inflammatory functions (Song et al. 2003; Choe et al. 2012). However, natural sources of R. sachalinensis are endangered because of over collection. Field cultivation is limited because of the temperature-sensitive nature of the plant and frequent occurrence of root-rotting diseases (Meng et al. 1994). Investigations of R. sachalinensis indicated that bioactive compound production by cell suspension cultures is a possible alternative (Xu et al. 1999; Wu et al. 2003). However, most of these studies have used small-scale flask culture. Only two studies on callus culture of R. sachalinensis in bioreactors are known to exist: compact callus aggregates of R. sachalinensis were successfully cultured in a 5-L airlift bioreactor by Xu et al. (1998), and the effects of air volume, inoculation density, medium pH, and methyl jasmonate on callus growth and accumulation of salidroside and polysaccharides in 3-L airlift bioreactors were investigated by Li et al.(2014). However, there still are many factors that have not been studied and that can affect callus bioreactor culture for the high-efficient production of R. sachalinensis bioactive compounds.
During bioreactor culture, the yield of secondary metabolites in the cultures can be increased by controlling the culture media and environmental conditions, such as plant growth regulators, carbon sources, mineral or organic nutrition, temperature, and light. At present, though several culture conditions have been investigated during bioreactor callus culture of R. sachalinensis, many other factors that may affect bioactive compound accumulation remain to be studied.
To optimize the bioreactor callus culture system of R. sachalinensis, the present study used 3-L, balloon-type airlift bioreactors to investigate physicochemical factors (sparger diameter, light intensity, sucrose concentration, and medium salt strength) affecting callus biomass and accumulation of bioactive compounds (salidroside, polysaccharides, phenolics, and flavonoids). Furthermore, the kinetics of callus growth and bioactive compound accumulation were studied.
Materials and Methods
Plant material and maintenance of callus culture
Grainy, fragile calluses of R. sachalinensis were induced according to Li et al. (2014) and used as the experimental material.
Effect of sparger diameter on biomass and bioactive compound accumulation
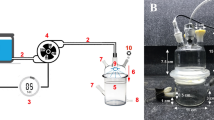
Three-liter, balloon-type airlift bioreactor immersion system with 2-L working volume was used for all experiments. Sparger diameters were 2.0, 2.5, or 3.5 cm, and had 30-μm pores. Each bioreactor contained Murashige and Skoog (Murashige and Skoog 1962) (MS) medium (Jinan Plant Bio-Tech Co., Ltd., Jinan, China), supplemented with 13.32 μM benzylaminopurine (BA) (Jinan Plant Bio-Tech Co., Ltd.), 1.61 μM α-naphthalene acetic acid (NAA) (Jinan Plant Bio-Tech Co., Ltd.), and 30 g L−1 sucrose (Jinan Plant Bio-Tech Co., Ltd.). The pH of the medium was adjusted to 5.8 before autoclaving, and the medium was autoclaved for 20 min at 121°C and 130 kPa. Each bioreactor was inoculated with 10 g L−1 of calluses (fresh weight, FW), aerated with filtered air (0.2 μm) at 100 mL min−1, and maintained at 25 ± 2°C with a 16-h photoperiod under white fluorescent light (Opple Lighting Electronic (Zhongshan) Co., Ltd., Zhongshan, China) of 15 μmol m−2 s−1 intensity. After 25 d of culture, callus FW and dry weight (DW) were recorded, and the amounts of salidroside, polysaccharides, phenolics, and flavonoids are determined. In addition, the initial volumetric oxygen transfer coefficient (kLa) was measured before callus culture in the experiment of sparger diameter.
Effect of light intensity on biomass and bioactive compound accumulation
Based on the results of the sparger diameter study, bioreactors with sparger diameters of 3.5-cm diameter were used to determine the effect of light on biomass and bioactive compound accumulation. Except for varying light intensities (15, 30, or 45 μmol m−2 s−1), experimental conditions were as described above.
Optimization of the sucrose concentration and salt strength of MS medium
To examine the effect of sucrose and salt strength on bioactive compound accumulation of R. sachalinensis culture tissue, the experimental conditions were as described above except as follows. Medium was supplemented with various concentrations of sucrose (10, 20, 30, 40, or 50 g L−1) and various concentrations of MS salts (0.5×, 1.0×, 1.5×, or 2.0×). The light intensity was 30 μmol m−2 s−1.
Kinetics of callus growth and bioactive compound accumulation
To assess callus growth and bioactive compound accumulation, experimental conditions were as described above, with sucrose at 30 g L−1 and light intensity at 30 μmol m−2 s−1. Samples of callus and culture medium were obtained from the bioreactors at 5-d intervals over a 30-d culture period. Callus biomass, specific growth rate (μ), and doubling time (Td) were recorded. The specific oxygen uptake rate (SOUR), bioactive compound content (salidroside, polysaccharides, phenolics, and flavonoids), pH, and electrical conductivity (EC) in the culture medium were determined.
Measurement of the initial volumetric oxygen transfer coefficient ( k L a ) value
The kLa value was determined by the conventional dynamic gassing-out method (Taguchi and Humphery 1966) before inoculation of calluses. Nitrogen was injected at a certain time (T0) to minimize the dissolved oxygen (DO) value in the medium. The bioreactor was then resupplied with constant air to increase DO value. The DO value was determined with a DO probe (SG6, Mettler − Toledo Instrument Co., Ltd., Shanghai, China). kLa was calculated by using the following formula:
where C* is the value of saturated DO (mol O2 L−1), and C is the DO value (mol O2 L−1).
Determination of biomass, specific growth rate (μ), and biomass of calluses
FW and DW were determined by passing calluses through a stainless steel sieve and washing them twice or thrice with tap water to remove the medium. FW biomass was measured after blotting away the surface water. DW was recorded after drying at 50°C for 48 h.
μ value of the calluses is defined as:
where X is the callus FW (g L−1), t is the time (d), and μ is the specific growth rate (per d). The doubling time (Td) of the calluses is defined as Td = In2/μ = 0.693/μ.
Determination of the specific oxygen utilization rate (SOUR)
SOUR was measured according to the method described by Jeong et al. (2006). Calluses (10 g FW) was added to a 340-mL chamber with air-saturated water. A DO probe was quickly inserted (SG6, Mettler − Toledo Instrument Co., Ltd.) and sealed with a rubber cap. The calluses were kept in suspension by mixing with a magnetic stirring bar. The decrease in the DO level was then recorded and the oxygen uptake rate (OUR) was estimated from the slope of DO against time, whereas SOUR was calculated from OUR and DW.
Determination of pH and EC in culture medium
Media samples (10 mL) were collected and filtered through a 0.2-μm membrane filter before measurement of pH (HI8424N, Hanna Instruments, Padova, Italy) and EC (Leici DDS-30, Shanghai Precision & Scientific Instrument Co., Ltd., Shanghai, China).
Determination of salidroside content
Salidroside was extracted and measured according to Li et al. (2014) with slight modification. Dry callus (0.5 g) was soaked with 10 mL of methanol. The suspension was ultrasonically (THC, Jining Tianhua Ultrasonic Electronic Instrument Co., Ltd., Jining, China) treated at 125 W for 30 min and then placed in a 75°C water bath for 5 h. The suspension was then filtered through a 0.22-μm membrane filter, and the filtrate (10 μL) was injected into a high-performance liquid chromatography (HPLC) system. The HPLC analysis used a C18 reverse phase column (4.6 × 250 mm, 5 μm, Thermo Scientific, Waltham, Massachusetts, USA) and a UV detector (SPD-15C, Shimadzu Corporation, Kyoto, Japan) at 275 nm. A mobile phase mixture of water (85%, v/v) and methanol (15%, v/v) was used at a flow rate of 1.0 mL min−1. The retention time of salidroside was 11.8 min. Salidroside content was determined by using an external standard method with the peak height as the quantitative parameter. Salidroside standard was provided by Chengdu Must Bio-Technology Co., Ltd., Chengdu, China.
Determination of total polysaccharide content
Total polysaccharides were extracted according to Li et al. (1990). Dried calluses were powdered, and 0.1 g of the powder was soaked in 90% (v/v) ethanol for 6 h at 25°C. The precipitate was collected and mixed with 90% (v/v) ethanol to discard the interfering compounds (monosaccharides, oligosaccharides, and glycosides). Twenty milliliters of distilled water was added to the extract and after 30 min of ultrasonic treatment, the extract was filtered, and the filtrate was collected. The remaining residue was soaked in water and ultrasonically treated at 125 W for 30 min thrice. All filtrates from a single sample were pooled. The polysaccharides were quantitatively determined spectrophotometrically (UV-2600, Shimadzu Corporation) at an absorbance of 490 nm according to the phenol-sulfuric acid assay (Dubois et al. 1956) with glucose (Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China) as a reference standard.
Determination of total phenolic content
Powdered calluses (0.1 g) were incubated at 80°C for 2 h with 10 mL of 80% (v/v) methanol in a thermostatic water bath (HH − S, Jintan Hengfeng Instrument Manfacturing Co., Ltd., Jintan, China). After filtration and centrifugation (Himac CR22G, Hitachi Instruments Co., Ltd., Shanghai, China) at 4000×g for 10 min, the supernatant was collected and adjusted to 25 mL with 80% (v/v) methanol. The total phenolic content in the extract was determined by using the Folin − Ciocalteu method (Singleton et al. 1999). Two milliliters of each extract was added to test tubes containing 0.2 mL of 10% (v/v) Folin − Ciocalteu reagent and 1 mL of sodium carbonate (2%, w/v). The tubes were thoroughly shaken and allowed to stand in the dark for 1 h. Total phenolic content was measured spectrophotometrically (UV-2600, Shimadzu Corporation) at an absorbance of 760 nm. Gallic acid (Tianjin Kemiou Chemical Reagent Co., Ltd.) was used as standard to obtain a calibration curve.
Determination of total flavonoid content
Powdered calluses (0.1 g) were digested in 10 mL of 70% (v/v) ethanol at 60°C for 3 h and filtered through filter paper. The filtrate was centrifuged at 4000×g for 10 min. Subsequently, the supernatant was adjusted to 25 mL with 70% (v/v) ethanol. Total flavonoid content was determined by using the aluminum chloride colorimetric method (Wu et al. 2011). One milliliters of extract was placed in a test tube, after which 0.3 mL of 5% (w/v) sodium nitrite, 0.3 mL of 10% (w/v) aluminum nitrate, and 2 mL of 4% (w/v) sodium hydroxide were added at intervals of 6 min. The reagents were mixed and incubated at a room temperature (∼25°C) for 15 min. Rutin (Tianjin Kemiou Chemical Reagent Co., Ltd.) was used as the standard to prepare the calibration curve. The absorbance was measured at 510 nm spectrophotometrically (UV-2600, Shimadzu Corporation).
Experimental design and data analysis
Data were collected from three experimental replicates. In the sparger diameter, light intensity, sucrose concentration, and MS medium strength experiments, the mean values were subjected to Duncan’s multiple-range test by using the SAS program (SAS Institute, Inc., Cary, North Carolina, USA) using P < 0.05. In the kinetics study experiment, the results are presented as mean ± standard deviation (SD).
Results and Discussion
Effect of sparger diameter on biomass and bioactive compound accumulation
Sparger type is an important consideration because the sparger controls oxygen transfer efficiency (Kim et al. 2005). In general, initial kLa is affected by several factors, including bioreactor type, cell density, medium strength, and air volume (Kato et al. 1975), which is particularly important in air supply conditions (Pan et al. 2000). Table 1 shows that the kLa value increased as sparger diameter increased, with a maximum of 7.5 h−1 when a 3.5-cm sparger diameter was used.
Callus biomass and bioactive compound accumulation were affected by initial kLa. The maximum callus biomass was found at an initial kLa of 7.5 h−1, where callus FW and DW reached 248.0 and 8.3 g L−1, respectively. These values were significantly higher than in bioreactors with sparger diameters of 2.0 and 2.5 cm (Table 1).
Larger sparger diameter also increased the content and productivity of salidroside or phenolics Fig. 1. Total polysaccharide content was significantly enhanced with increasing sparger diameter, but both 2.5 and 3.5-cm diameter spargers supported the highest levels. Flavonoid content was not affected by sparger diameter, but the maximum productivity was observed at 3.5 cm.
Very few reports on the effect of sparger size on culture growth and metabolite accumulation during bioreactor culture have been published. Kim et al. (2005) investigated the effect of sparger diameter on the adventitious root growth of Panax ginseng during bioreactor culture and reported results that were similar to those of the present study. The present study indicated that the sparger diameter controlled initial kLa value, which subsequently affected callus biomass and bioactive compounds during bioreactor culture of R. sachalinensis. These results suggested that selecting a bioreactor with a suitable sparger is critical for cell and organ cultures and must be considered when designing an airlift bioreactor.
Effect of light intensity on biomass and bioactive compound accumulation
Light is important for the production of metabolites in plant cell culture. In the present study, callus biomass reached the highest FW and DW values of 263.4 and 9.2 g L−1, respectively, under a light intensity of 30 μmol m−2 s−1. This was significantly higher than what was observed under light intensities of 15 and 45 μmol m−2 s−1 Fig. 2. The highest values for salidroside content (0.85 mg g−1 DW) and productivity (7.84 mg L−1) were also found under 30 μmol m−2 s−1 of light intensity. Polysaccharide content was not affected by light intensity, but maximum productivity was found at 30 μmol m−2 s−1. The highest contents of phenolics and flavonoids were obtained from calluses grown under the highest light intensity (45 μmol m−2 s−1), but their productivities did not differ from those obtained from calluses grown under 30 μmol m−2 s−1 of light intensity.
In a number of plant species, light intensity strongly affected callus growth and metabolite biosynthesis during cell and tissue culture. Wen et al. (2007) found the callus growth and resveratrol content of Polygonum cuspidatum was optimal under low light intensity (nearly 30 μmol m−2 s−1). Ouyang et al. (2003) reported that callus biomass and production of phenylethanoid glycosides in Cistanche deserticola was highest under 24 μmol m−2 s−1 light intensity. The present study found that a light intensity of 30 μmol m−2 s−1 was suitable for obtaining the maximum productivity of all bioactive compounds in R. sachalinensis.
Optimization of the sucrose concentration and salt strength of MS medium
Most cultured plant cells, tissues, and organs require an exogenous source of carbohydrates because they do not support photosynthesis. Callus FW and DW increased with increasing sucrose concentration from 10 to 30 g L−1, but decreased at higher sucrose concentrations Fig. 3. Salidroside and flavonoid accumulation patterns were similar. The highest contents of salidroside and flavonoids were obtained with sucrose at 30 g L−1, whereas the highest contents of polysaccharides and phenolics were obtained with sucrose at 40 and 50 g L−1, respectively. The highest productivities of salidroside, polysaccharides, and flavonoids were found at a sucrose concentration of 30 g L−1. However, the maximum productivity of phenolics was found at the highest sucrose concentration of 50 g L−1. The importance of sucrose concentration as a critical regulating chemical factor on metabolite production has been reported in several plant species. In cell suspension cultures, optimal values for cell biomass and accumulation of withanolide A in Withania somnifera (Nagella and Murthy 2010) and of ginsenoside in P. ginseng (Lian et al. 2002) were found at 30 g L−1 of sucrose. Cell growth rate of Perilla frutescen increased when the initial sucrose level in the culture medium was increased up to 60 g L−1 (Zhong and Yoshida 1995). In callus cultures of Calendula officinalis, a sucrose concentration of 60–70 g L−1 resulted in maximum carotenoid content (Legha et al. 2012). For R. sachalinensis, Xu et al. (1999) demonstrated that 40 g L−1 of sucrose was favorable for increasing salidroside production, which differed from the results of the present study. During plant cell and tissue culture, culture growth and metabolite accumulation are affected by many factors and their interactions. The varied results of sucrose experiments between Xu et al. (1999) and the present study for R. sachalinensis may be due to an interaction effect among the factors. Xu et al. (1999) used compact callus aggregates to conduct the suspension culture in Erlenmeyer flasks, whereas in the present study, grainy, fragile calluses were cultured in bioreactors. Thus, the effect of initial sucrose concentration on plant cell growth and metabolite accumulation depended on several factors, such as plant species, cell lines, culture methods, and metabolite types.
Optimum nutrient concentration is an important determinant in controlling culture growth and metabolite accumulation (Rao and Ravishankar 2002). In the present study, the highest FW and DW of the calluses were found at 1 × MS Fig. 4. This finding can be explained by the lack of nutrients at the 0.5 × MS and high osmotic stress in the 1.5 × MS (Yin et al. 2013). MS salt strengths affected the contents of bioactive compounds Fig. 4. The maximum content of salidroside was found in 1.5 × MS, of polysaccharides in 1 × MS, of phenolics in 0.5 × MS, and of flavonoids in 0.5 × MS or 1 × MS. The highest productivity of salidroside was found in 1.5 × MS, of polysaccharides and flavonoids in 1 × MS, and of phenolics in 0.5 × MS.
Previous studies reported inconsistent results with respect to the suitability of MS salt strength in promoting culture growth and metabolite production. Lian et al. (2002) reported that 0.5 × MS and 1 × MS were equally suitable for cell growth and ginsenoside productivity in cell suspension cultures of P. ginseng. Nagella and Murthy (2010) described that 1 × MS favored the accumulation of cell biomass and withanolide A production in W. somnifera. Shim et al. (2010) obtained the highest cell biomass in 1 × MS, whereas accumulation of anthraquinone, phenolics, and flavonoids reached maximum values in 2 × MS. MS medium has high levels of nitrogen, potassium, and some micronutrients (Cohen 1995) and is commonly used in plant tissue culture because of the favorable reaction of most plant cultures to this medium. However, this nutrient medium is not always optimal for all culture growth and metabolite accumulation. Therefore, the salt strength of the MS medium should be properly regulated to optimize the production of metabolites in medicinal plant cell, tissue, and organ culture.
Kinetics of callus growth and bioactive compound accumulation
FW and DW of R. sachalinensis calluses cultured in 3 L balloon − type airlift bioreactors increased similarly and gradually during culture to 220.9 and 9.1 g L−1, respectively, at 20 d Figs. 5 and 6a . The specific growth rate, μ, of calluses changed during culture, with the peak rate of 0.450 at 10 d and Td of 0.312 d (Table 2). The callus biomass remained stable from 20 to 25 d, whereas μ decreased. After 25 d, calluses in the middle of bioreactors turned brown, which resulted in the rapid decline of biomass and μ. This result was similar to the findings of Wu et al. (2003) that showed maximum biomass of compact callus aggregates in R. sachalinensis after 25 d in an Erlenmeyer-flask suspension culture.
SOUR is a measurement of oxygen mass transfer capability. In the present study, SOUR increased quickly from 5 to 10 d and reached the peak at 10 d. SOUR sharply dropped to almost zero after 20 d of culture Fig. 6b . A similar behavior was found in the bioreactor cell culture of Glycyrrhiza uralensis (Wang et al. 2013). SOUR is related to OUR, but the relationship between SOUR and biomass cannot be clearly explained (Wang et al. 2013). The medium EC decreased from 4.28 to 2.04 ms cm−1 during culture Fig. 6c . EC is inversely correlated with callus growth because of the uptake of inorganic ions by plant cells (Hahlbrock and Kuhlen 1972). A similar phenomenon has been reported in the cell bioreactor culture of P. ginseng (Thanh et al. 2006). Culture medium pH value increased with prolonged bioreactor culture from pH 5.1 to pH 6.1 Fig. 6c . The increase in culture medium pH value in the present study can be explained by McDonald and Jackman’s theory (McDonald and Jackman 1989), i.e., that nitrate uptake by cultures increases media pH.
Similar trends were observed for the accumulation of salidroside, polysaccharides, phenolics, and flavonoids. The contents of bioactive compounds decreased in the first 15 d, whereas productivity slightly increased. Content and productivity peaked at 20 d for salidroside and phenolics and at 25 d for polysaccharides and flavonoids. A decrease in bioactive compound accumulation was observed in the succeeding days Fig. 7. The decrease of bioactive compound contents within the first 15 d was possibly due to greater catabolism during the first 15 d than anabolism. Catabolism provides the carbon skeleton and energy needed to promote cell growth. After 15 d of culture, anabolism was enhanced, thereby dramatically increasing the contents of bioactive compounds. However, Wu et al. (2003) reported that salidroside content of R. sachalinensis peaked at 5 d and then decreased. These differing results may be a result of studying different cell lines.
Conclusion
Physicochemical factors can regulate callus biomass and bioactive compound accumulation in the culture of R. sachalinensis. Sparger size is suitable at 3.5 cm for the 3-L, balloon-type airlift bioreactor. Calluses need to be cultured in full-strength MS medium supplemented with 30 g L−1 sucrose under a light intensity of 30 μmol m−2 s−1. However, factors can be slightly adjusted according to the type of bioactive compound. In addition, the bioactive compound accumulation can be improved by studying the effects of specific treatments, including the use of elicitors, in the future.
References
Ahmad S, Hahn EJ, Y PK (2008) Aeration volume and photosynthetic photon flux affect cell growth and secondary metabolite contents in bioreactor cultures of Morinda citrifolia. J Plant Biol 51:209–212
Cheng XJ, Di L, Wu Y, Zhao QC, Du GZ, Liu YQ (1993) Studies on the hypoglycemic effect of Rhodiola sachalinensis A. Bor polysaccharides. Chin J Chin Mater Med 18:557–559, 575
Choe KI, Kwon JH, Park KH, Oh MH, Kim MH, Kim HH, Cho SH, Kyung CE, Ha SY, Won LM (2012) The antioxidant and anti-inflammatory effects of phenolic compounds isolated from the root of Rhodiola sachalinensis A. Bor Molecules 17:11484–11494
Cohen D (1995) The culture medium. Acta Hortic 393:15–24
Cui HY, Baque MA, Lee EJ, Paek KY (2013) Scale-up of adventitious root cultures of Echinacea angustifolia in a pilot-scale bioreactor for the production of biomass and caffeic acid derivatives. Plant Biotechnol Rep 7:297–308
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Hahlbrock K, Kuhlen E (1972) Relationship between growth of parsley and soybean cells in suspension cultures and changes in the conductivity of the culture medium. Planta 108:271–278
Hahn EJ, Kim YS, Yu KW, Jeong CS, Paek KY (2003) Adventitious root cultures of Panax ginseng C V Meyer and ginsenoside production through large-scale bioreactor system. J Plant Biotechnol 5:1–6
Jeong CS, Chakrabarty D, Hahn EJ, Lee HL, Paek KY (2006) Effects of oxygen, carbon dioxide and ethylene on growth and bioactive compound production in bioreactor culture of ginseng adventitious roots. Biochem Eng J 27:252–263
Kato A, Shimizu Y, Nagai S (1975) Effect of initial kLa on the growth of tobacco cells in batch culture. J Ferment Technol 53:744–751
Kim YS, Hahn EJ, Shin CG, Paek KY (2005) Effects of aeration rate and sparger type on growth and ginsenoside accumulation in bioreactor cultures of ginseng adventitious root (Panax Ginseng C.A. Meyer). Korean J Plant Biotechnol 32:111–116
Legha MR, Prasad KV, Singh SK, Kaur C, Arora A, Kumar S (2012) Induction of carotenoid pigments in callus cultures of Calendula officinalis L. in response to nitrogen and sucrose levels. In Vitro Cell Dev Biol–Plant 48:99–106
Li M, Hirata Y, Xu G, Niwa M (1990) Determination of polysaccharide contents in the drugs of Dendrobium. Chin Tradit Herbal Drugs 21:10–12
Li Y, Shao CH, Park SY, Chun PX, Lian ML (2014) Production of salidroside and polysaccharides in Rhodiola sachalinensis using airlift bioreactor systems. Acta Physiol Plant 36:2975–2983
Lian ML, Chakrabarty D, Paek KY (2002) Effect of plant growth regulators and medium composition on cell growth and saponin production during cell suspension culture of mountain ginseng (Panax ginseng C.A. Mayer). J Plant Biol 45:201–206
McDonald KA, Jackman AP (1989) Bioreactor studies of growth and nutrient utilization in alfalfa suspension cultures. Plant Cell Rep 8:455–458
Meng QY, Jian ML, Zhong WT (1994) Controlling the root-rot disease of Rhodiola sachalinensis A. Bor with pesticide. J Shenyang Agric Univ 25:264–267
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nagella P, Murthy HN (2010) Establishment of cell suspension cultures of Withania somnifera for the production of withanolide A. Bioresour Technol 101:6735–6739
Ouyang J, Wang XD, Zhao B, Wang YC (2003) Light intensity and spectral quality influencing the callus growth of Cistanche deserticola and biosynthesis of phenylethanoid glycosides. Plant Sci 165:657–661
Paek KY, Chakrabarty D, Hahn EJ (2005) Application of bioreactor systems for large scale production of horticultural and medicinal plants. Plant Cell Tiss Org Cult 81:287–300
Pan ZW, Wang HQ, Zhong JJ (2000) Scale-up study on suspension cultures of Taxus chinensis cells for production of taxane diterpene. Enzyme Microb Technol 27:714–723
Rao RS, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Shim KM, Hahn EJ, Jeon WK, Peak KY (2010) Accumulation of cell biomass anthraquinones, phenolics, and flavonoids as affected by auxin, cytokinin, and medium salt strength in cell suspension culture of Morinda citrifolia. Korean J Hortic Sci Technol 28:288–294
Shohael AM, Chakrabarty D, Yu KW, Hahn EJ, Paek KY (2005) Application of bioreactor system for large-scale production of Eleutherococcus sessiliflorus somatic embryos in an air-lift bioreactor and production of eleutherosides. J Biotechnol 120:228–236
Singleton V, Orthofer R, Lamuela − Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin − ciocalteu reagent. Method Enzymol 299:152–158
Song EK, Kim JH, Kim JS, Cho H, Nan JX, Sohn DH (2003) Hepatoprotective phenolic constituents of Rhodiola sachalinensis on tacrine-induced cytotoxicity in Hep G2 cells. Phytother Res 17:563–565
Taguchi H, Humphery AE (1966) Dynamic measurement of the volumetric oxygen transfer coefficient in fermentation systems. J Ferm Technol 44:881–889
Thanh NT, Murthy HN, Yu KW, Jeong CS, Hahn EJ, Paek KY (2006) Effect of oxygen supply on cell growth and saponin production in bioreactor cultures of Panax ginseng. J Plant Physiol 163:1337–1341
Varsha S, Shaily G, Kishan GR (2009) Scale up production of isoflavonoids in cell suspension cultures of Pueraria tuberosa grown in shake flasks and bioreactor. Eng Life Sci 9:267–271
Varsha S, Shaily G, Kishan GR (2011) Increased puerarin biosynthesis during in vitro shoot formation in Pueraria tuberosa grown in bioreactor with aeration. Physiol Mol Biol Plants 17:87–92
Wang J, Zhang J, Gao WY, Wang Q, Yin SS, Liu H, Man SL (2013) Identification of triterpenoids and flavonoids, step-wise aeration treatment as well as antioxidant capacity of Glycyrrhiza uralensis Fisch. cell. Ind Crop Prod 49:675–681
Wen T, Liang L, Zeng Y, Yu X (2007) Effect of different light intensity on Polygonum cuspidatum callus. China J Chin Mat Med 32:1277–1280
Wu SQ, Lian ML, Park SY, Piao XC (2011) Bioreactor application on adventitious root culture of Astragalus membranaceus. In Vitro Cell Dev Biol–Plant 47:719–724
Wu SX, Zu YG, Wu M (2003) High yield production of salidroside in the suspension culture of Rhodiola sachalinensis. J Biotechnol 106:33–43
Xu JF, Su ZG, Feng PS (1998) Suspension culture of compact callus aggregate of Rhodiola sachalinensis for improved salidroside production. Enzym Microb Technol 23:20–27
Xu JF, Ying PQ, Han AM, Su ZG (1999) Enhanced salidroside production in liquid-cultivated compact callus aggregates of Rhodiola sachalinensis: manipulation of plant growth regulators and sucrose. Plant Cell Tiss Org Cult 55:53–58
Yin SS, Liang YY, Gao WY, Wang J, Jing SS, Zhang Y, Liu H (2013) Influence of medium salt strength and nitrogen source on biomass and metabolite accumulation in adventitious root cultures of Pseudostellaria heterophylla. Acta Physiol Plant 35:2623–2628
Zhong JJ, Yoshida T (1995) High-density cultivation of Perilla frutescens cell suspensions for anthocyanin production: effects of sucrose concentration and inoculum size. Enzyme Microb Technol 17:1073–1079
Acknowledgment
Project 81160497 is supported by the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Editor: Zeng-Yu Wang
Rights and permissions
About this article
Cite this article
Li, H., Piao, X.C., Gao, R. et al. Effect of several physicochemical factors on callus biomass and bioactive compound accumulation of R. sachalinensis bioreactor culture. In Vitro Cell.Dev.Biol.-Plant 52, 241–250 (2016). https://doi.org/10.1007/s11627-016-9758-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-016-9758-5