Abstract
Castilleja tenuiflora, a species highly valued for its medicinal properties, is threatened in the wild. We evaluated the effects of six different immersion cycles in a temporary immersion bioreactor on C. tenuiflora shoot growth, proliferation rate, phenolics content, flavonoid content, and antioxidant activity. We also evaluated the regeneration capacity of the shoots. The highest proliferation rate (nine shoots per explant) was obtained using an immersion cycle of 5 min every 12 h, and the longest shoots (38.8 ± 1.9 mm) were obtained using an immersion cycle of 5 min every 24 h. Shoots obtained from immersion cycles of 30 min every 24 h or 5 min every 24 h showed 100% rooting efficiency. Shoots obtained from immersion cycles of 30 min every 3 h or 30 min every 12 h accumulated H2O2, developed abnormal stomata, and showed symptoms of hyperhydricity. These characteristics were associated with a low survival rate (16–80%) when the plants were transferred to potting mix. The shoots from an immersion cycle of 30 min every 24 h showed the highest total phenolics content, which coincided with the highest antioxidant activity in the 2,2′-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS) free-radical scavenging assay (161.74 ± 10.06 μmol Trolox/g dry weight (DW)). The shoots from an immersion cycle of 5 min every 24 h showed the highest activity in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free-radical scavenging assay, and those from an immersion cycle of 5 min every 3 h showed the strongest reducing power. These results show that temporary immersion culture represents a reliable and efficient method for in vitro micropropagation of C. tenuiflora.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Castilleja tenuiflora Benth. (Orobanchaceae), commonly known as “Indian paintbrush,” is a perennial hemiparasitic plant distributed in mountainous areas of the southern USA and Mexico (Holmgren 1976). It has long been used in folk medicine (Béjar et al. 2000), and it is still used in both rural and urban regions to treat the symptoms of various cancers (Alonso-Castro et al. 2011) and to treat coughs, inflammation, and gastrointestinal disorders (Biblioteca Digital de la Medicina Tradicional Mexicana 2011). The traditional uses of C. tenuiflora may be related to the biological activities of its secondary products, including iridoid glycosides, phenylethanoid glycosides (Gómez-Aguirre et al. 2012), and flavonoids (López-Laredo et al. 2012).
Wild populations of C. tenuiflora are vulnerable to the indiscriminate commercial harvesting of this species. Also, it is available only seasonally, and falling trees and fires often affect its habitat. To avoid possible loss of this important plant and to obtain a permanent source of its secondary metabolites, we have established efficient procedures to propagate C. tenuiflora in semisolid cultures in vitro (Salcedo-Morales et al. 2009; Martínez-Bonfil et al. 2011) and to acclimatize the plants propagated in culture (Martínez-Bonfil et al. 2011). However, despite having a successful micropropagation protocol, commercial-scale implementation of these procedures remains limited by the high production costs. A promising alternative is the use of temporary immersion culture (Etienne and Berthouly 2002; Ashraf et al. 2013). Temporary immersion culture combines the positive effects of aeration with liquid culture medium, thereby stimulating shoot proliferation and growth (Etienne and Berthouly 2002). This method avoids problems such as asphyxia and hyperhydricity, so the plant materials produced are often of better quality than those produced by conventional culture systems, i.e., on semisolid media (Sreedhar et al. 2009). This method is also useful for basic research on plant physiology (Michoux et al. 2013).
The main factors influencing the performance of a given plant species in temporary immersion cultures are the immersion time and frequency. These factors need to be optimized for different genotypes and applications (Albarran et al. 2005; Ivanov et al. 2011; Zhao et al. 2012; Ashraf et al. 2013). The aim of this study was to evaluate the effect of immersion cycles on the growth, proliferation rate, phenolics contents, and antioxidant properties of C. tenuiflora shoots cultured in temporary immersion bioreactors, as well as their effects on the ability of the shoots to regenerate into whole plants.
Materials and Methods
Plant material.
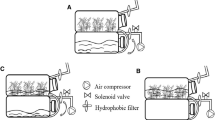
Shoot cultures of C. tenuiflora were initiated and propagated in vitro as described previously (Trejo-Tapia et al. 2012). Shoots were subcultured in 200-ml jars (Fig. 1A ), with a 6-cm-diameter mouth that was sealed with a polypropylene closure. Each jar contained 30 ml of B5 culture medium (Gamborg et al. 1968) containing 3% (w/v) sucrose and no plant growth regulators (PGRs). Cultures were maintained under continuous agitation at 110 rpm on an orbital shaker in a growth room at 25 ± 2 °C under a 16-h light/8-h dark photoperiod with illumination of 103 μmol/m2/s provided by 75-W cool-white fluorescent lamps (SL5938, Osram, Tultitlan, México).
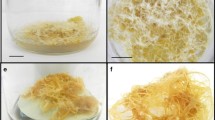
Stages of C. tenuiflora plants regenerated in temporary immersion bioreactors (RITA®). (A) Shoots cultured in B5 liquid culture medium (Gamborg et al. 1968) containing 3% (w/v) sucrose and no plant growth regulators. (B) Shoot explant. (C) Multiple shoot regeneration in RITA® after 3 wk of culture (from treatment IC-5). (D) Uniform shoots/young plants transferred to pots filled with a sterilized mixture of peat moss, agrolite, and vermiculite. (E) Regenerated plantlets after 3 wk of acclimatization. Third plant from left is from treatment IC-6, which gave the longest shoots. (F) Regenerated plant from treatment IC-6, which gave the longest roots.
Temporary immersion culture.
The 3-wk-old in vitro shoots of C. tenuiflora were cultured in RITA® (Sigma-Aldrich, St. Louis, MO) temporary immersion bioreactors with 200 ml of B5 culture medium (Gamborg et al. 1968) supplemented with 3% sucrose (w/v) without PGRs. In each bioreactor, the flow rate of the inlet air was 1 l/min. We evaluated the effects of immersion time (30 or 5 min) and immersion frequency (every 3, 12, or 24 h) in six different immersion cycle (IC) treatments as follows: IC-1, 30 min every 3 h; IC-2, 30 min every 12 h; IC-3, 30 min every 24 h; IC-4, 5 min every 3 h; IC-5, 5 min every 12 h; and IC-6, 5 min every 24 h. Each IC treatment consisted of five replicates of 40 shoots (2.5 cm in length; Fig. 1B ), giving a total of 200 initial shoots per IC treatment. Cultures were maintained in a growth chamber under the conditions described above. At the end of the 3-wk in vitro culture period, all of the shoots were counted and the proliferation rate was calculated as follows: (number of shoots and buds at the end of culture period)/(number of shoots inoculated). We also evaluated the following morphological characteristics: shoot length (mm), hyperhydricity (%), fresh mass (g), and rooting rate (%). The experiments were performed three times.
Anatomical analysis and subcellular localization of H2O2.
To visualize the subcellular location of H2O2, we used a histochemical method based on the generation of an insoluble brown precipitate after the reaction of 3,3-diaminobenzidine (DAB) with H2O2 (Thordal-Christensen et al. 1997). Leaves from normal and hyperhydric shoots were cut and immersed in DAB solution (1 mg/ml DAB dissolved in deionized water, pH 3.8). Samples were incubated for 8 h at 25 °C, cleared in boiling ethanol (96%) for 10 min, and mounted on slides. The presence of H2O2 is indicated by a reddish-brown color. Samples were observed (×40) and images were acquired under a light microscope (Eclipse 80I, Nikon, Tokyo, Japan) equipped with a digital camera (DC330, Dage-MTI, Tokyo, Japan).
Rooting and plant acclimatization.
Uniform shoots/young plants with 6–8 expanded leaves (3.0 cm long) were selected from each IC tested and transferred to pots (10 × 7.5 cm; Fig. 1D ) filled with a sterilized mixture of peat moss, agrolite (agroLITA, State of Mexico, Mexico), and vermiculite (60:20:20 v/v/v) adjusted to pH 5.8 ± 0.3 and moistened with water (40 ml). The pots were covered individually with plastic foil (12.5 × 20 cm) for 3 wk to maintain a relative humidity of ≥95% and kept under the conditions described by Martínez-Bonfil et al. (2011). In all cases, the following data were recorded after 35 d: survival (%), plant height, number of roots per shoot, and length of the longest root. These experiments were performed three times, and 50 shoots from each IC were cultured each time.
Quantification of total phenolics and flavonoids.
Plant material was freeze-dried and ground into a fine powder (particle size <250 μm) using a mortar and pestle. Phenolics and flavonoids were extracted from 100-mg lyophilized tissue in 100-ml methanol, as described previously (López-Laredo et al. 2012). The total phenolics content was estimated by the Folin–Ciocalteu method, and the flavonoid content was determined by a colorimetric assay as described previously (López-Laredo et al. 2009). Standard curves for total phenolics and flavonoids were prepared with gallic acid and catechin, respectively. Total phenolics content is expressed as milligram gallic acid equivalents per gram dry weight (GAE/g DW). Total flavonoid content is expressed as microgram catechin equivalents per gram dry weight (CE/g DW). Three independent samples were analyzed each time.
DPPH and ABTS free-radical scavenging assays.
Free-radical scavenging activity was quantified spectrophotometrically using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay and the 2,2′-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS) radical scavenging assay as described previously (López-Laredo et al. 2009). The results are expressed as micromoles of Trolox per gram DW based on a calibration curve. Three independent samples were analyzed for each assay.
Reducing power assay.
Reducing power was quantified by the phosphomolybdenum (PPM) assay as described elsewhere (Prieto et al. 1999). The results are expressed as micromoles Trolox per gram DW (Diouf et al. 2009) based on a calibration curve. Three independent samples were analyzed.
Statistical analysis.
The differences among ICs were tested by two-way ANOVA, and Tukey’s all-pairwise multiple comparison procedure was used to determine statistically different values at a significance level of p < 0.05. Percentage values were arcsine-transformed to obtain normally distributed data. The software SigmaPlot for Windows version 11.0 (Systat Software Inc., San Jose, CA) was used to perform statistical analyses.
Results
Shoot proliferation and morphological characteristics.
After 3 wk, the C. tenuiflora shoot proliferation rate ranged from six to nine shoots per explant, depending on the IC treatment (p < 0.05; Table 1). The highest proliferation rate (nine shoots per explant) was in IC-5 (5 min every 12 h; Fig. 1C ) and the lowest (six shoots per explant) was in IC-3 and IC-6 (30 or 5 min every 24 h, respectively). The longest shoots (38.8 ± 1.9 mm) were from IC-6, followed by IC-4 and IC-3.
The shoots cultured in immersion bioreactors developed roots from their bases after 2 wk and did not form callus. The highest rooting efficiency (100%) was observed for shoots from IC-3 and IC-6. Shoots from IC-1 and IC-2, which had the longest total daily immersion times (240 and 60 min per day, respectively), showed hyperhydricity at levels ranging from 16 to 40% (p < 0.05). In hyperhydric shoots, the water content (WC) was 93–96% (Table 1; p < 0.05). The symptoms of hyperhydricity in shoots were observed after 1 wk of culture, when shoots began to show brittle and translucent leaves with a paler green color than that of normal shoots. Hyperhydric leaves had larger stomata (Fig. 2B ) than those of normal leaves (Fig. 2A ). In the histochemical analysis of C. tenuiflora leaves, we observed red-brownish polymerization products from DAB reacting with H2O2 in hyperhydric leaves (Fig. 2D ) but not in control leaves (Fig. 2C ).
Histochemical analysis of control and hyperhydric leaves of C. tenuiflora. (A, B) Semi-thin sections of a control leaf (A) and a hyperhydric leaf showing an altered anatomy (B). (C, D) H2O2 accumulation in control leaf (C) and hyperhydric leaf (D) detected by DAB staining. H2O2 is indicated by the reddish-brown color. st stoma.
Secondary metabolites and antioxidant properties of shoots.
The highest contents of total phenolics and flavonoids were in shoots from IC-3 (30-min immersion every 24 h) followed by IC-4 (5-min immersion every 3 h). These shoots also showed among the highest free-radical scavenging and reducing activities (Table 1). Shoots that showed less vigorous growth and symptoms of hyperhydricity (from IC-1 to IC-2) showed the lowest contents of total phenolics and flavonoids.
Plant characteristics and survival rate.
Table 2 shows the characteristics of plants grown in potting mix after different IC treatments. The tallest plants (10.4 ± 0.5 cm; Fig. 1E ) were those regenerated from shoots from IC-6 (5-min immersion every 24 h). These plants also showed the longest roots (6.0 ± 0.3 cm; Fig. 1F ) and were among those with the highest survival rate (100%). Plants regenerated from shoots from ICs 3, 4, and 5 were significantly shorter than those regenerated from shoots from IC-6 (p < 0.05), ranging from 4.9 to 6.7 cm in height, but they showed high survival rates (100%). As expected, the hyperhydric plants (from IC-1 to IC-2) showed lower survival rates (16–80%).
Discussion
Our results showed that C. tenuiflora shoots grown in a temporary immersion bioreactor successfully regenerated into whole plants. A 5-min immersion every 24 h resulted in the production of many vigorous C. tenuiflora shoots without symptoms of hyperhydricity. All of the shoots developed under these conditions developed roots, and the regenerated plants showed a 100% survival rate. The temporary immersion bioreactor system improved the morphological characteristics and shoot proliferation rate (nine shoots per explant) of in vitro C. tenuiflora shoots, compared with that obtained using the conventional method of propagation on semisolid medium (four shoots per explant; Martínez-Bonfil et al. 2011). In the present study, a short immersion time (5 min) every 24 h gave better results than more frequent immersions (every 3 and 12 h) or longer immersions (30 min). The same trend of shorter immersions producing better results has been observed for shoot proliferation of Saccharum spp. (Lorenzo et al. 1998) and Ananas comosus (Escalona et al. 1999), while in other cases, longer and more frequent immersions produced better results. For instance, for microtuberization of Chlorophytum borivilianum, a 15-min immersion every hour produced better results than did more or less frequent immersions (every 45 or 75 min) (Ashraf et al. 2013). For biomass accumulation in Leucojum aestivum shoots, the best conditions were a 15-min immersion every 8 h (Ivanov et al. 2011).
The shoots of C. tenuiflora grown under different immersion cycle treatments showed different rooting efficiencies. Shoots with the least frequent immersion (once every 24 h) showed the highest rooting efficiency (100%). There are other reported cases where roots developed from shoots during immersion culture. For instance, temporary immersion culture stimulated root development from somatic embryos of Hevea brasiliensis (Etienne et al. 1997) and from shoots of A. comosus (Ayenew et al. 2013). The conditions used here represent a promising alternative for bypassing the rooting phase, which lasts for 21 d in C. tenuiflora (Martínez-Bonfil et al. 2011). This enhanced rooting response may have occurred because plantlets growing in immersion culture assimilate more nutrients such as sugars, nitrate, and ammonium than do those growing in conventional conditions (semisolid culture) (Escalona et al. 2003). In addition, shoots growing in temporary immersion culture tend to accumulate higher levels of polyamines (Scherer et al. 2013) than those in permanent immersion culture. Polyamines have been reported to promote adventitious root formation in Curcuma longa (Viu et al. 2009) and Vitis vinifera (Neves et al. 2002) and to reverse hyperhydricity in Thymus daenensis (Hassannejad et al. 2012).
Among the six immersion cycles tested here, those with the longest total daily immersion times (240 and 60 min per d) resulted in shoots with hyperhydric symptoms. This phenomenon was characterized by an increase in shoot WC, a decreased tendency to form roots, abnormal stomata, and H2O2 accumulation. These results are consistent with those reported for Dianthus caryophyllus, in which hyperhydric shoots produced more H2O2 than did control shoots (Saher et al. 2004). Also, in T. daenensis, hyperhydricity was characterized by higher WC and lower rates of differentiation (Hassannejad et al. 2012). As well as showing morphological changes, hyperhydric C. tenuiflora shoots showed the lowest contents of phenolic compounds and flavonoids, and low free-radical scavenging and reducing activities. In D. caryophyllus, phenylalanine ammonia-lyase (PAL) activity was significantly lower in hyperhydric tissues than in control tissues (Saher et al. 2004). PAL is a key enzyme for the biosynthesis of phenolic compounds, so it is possible that lower PAL activity was responsible for the lower levels of phenolic compounds and flavonoids in hyperhydric C. tenuiflora tissues. Together, these results show that longer immersion times resulted in hyperhydricity and oxidative stress in C. tenuiflora shoots, leading to lower contents of secondary metabolites and lower survival rates.
Conclusions
The results reported here show that temporary immersion culture is a reliable and efficient methodology for in vitro micropropagation of C. tenuiflora. The plants produced using this method showed good survival rates.
References
Albarran J, Bertrand B, Lartaud M, Etienne H (2005) Cycle characteristics in a temporary immersion bioreactor affect regeneration, morphology, water and mineral status of coffee (Coffea arabica) somatic embryos. Plant Cell Tissue Organ Cult 81:27–36
Alonso-Castro AJ, Villarreal ML, Salazar-Olivo LA, Gomez-Sanchez M, Dominguez F, Garcia-Carranca A (2011) Mexican medicinal plants used for cancer treatment: pharmacological, phytochemical and ethnobotanical studies. J Ethnopharmacol 133:945–972
Ashraf MF, Abd Aziz M, Stanslas J, Kadir MA (2013) Optimization of immersion frequency and medium substitution on microtuberization of Chlorophytum borivilianum in RITA system on production of saponins. Process Biochem 48:73–77
Ayenew B, Tadesse T, Gebremariam E, Mengesha A, Tefera W (2013) Efficient use of temporary immersion bioreactor (TIB) on pineapple (Ananas comosus L.) multiplication and rooting ability. J Microbiol Biotechnol Food Sci 2:2456–2465
Béjar E, Reyes-Chilpa R, Jiménez-Estrada M (2000) Bioactive compounds from selected plants used in the XVI century Mexican traditional medicine. In: Atta-ur-Rahman (ed) Studies in natural products chemistry, vol. 24. Elsevier, Amsterdam, pp 799–844
Biblioteca Digital de la Medicina Tradicional Mexicana (2011) UNAM, México City, México. http://www.medicinatradicionalmexicana.unam.mx/index.php. Cited 5 Feb 2014
Diouf PN, Stevanovic T, Cloutier A (2009) Antioxidant properties and polyphenol contents of trembling aspen bark extracts. Wood Sci Technol 43:457–470
Escalona M, Lorenzo JC, González B, Daquinta M, González JL, Desjardins Y, Borroto CG (1999) Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep 18:743–748
Escalona M, Samson G, Borroto C, Desjardins Y (2003) Physiology of effects of temporary immersion bioreactors on micropropagated pineapple plantlets. In Vitro Cell Dev Biol Plant 39:651–656
Etienne H, Berthouly M (2002) Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult 69:215–231
Etienne H, Lartaud M, Michaux-Ferriére N, Carron MP, Berthouly M, Teisson C (1997) Improvement of somatic embryogenesis in Hevea brasiliensis (Müll. Arg.) using the temporary immersion technique. In Vitro Cell Dev Biol Plant 33:81–87
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:150–158
Gómez-Aguirre YA, Zamilpa A, González M, Trejo-Tapia G (2012) Adventitious root cultures of Castilleja tenuiflora Benth. as a source of phenylethanoid glycosides. Ind Crop Prod 36:188–195
Hassannejad S, Bernard F, Mirzajani F, Gholami M (2012) SA improvement of hyperhydricity reversion in Thymus daenensis shoots culture may be associated with polyamines changes. Plant Physiol Biochem 51:40–46
Holmgren NH (1976) Four new species of Mexican Castilleja (subgenus Castilleja, Scrophulariaceae) and their relatives. Brittonia 28:195–208
Ivanov I, Georgiev V, Georgiev M, Ilieva M, Pavlov A (2011) Galanthamine and related alkaloids production by Leucojum aestivum L. shoot culture using a temporary immersion technology. Appl Biochem Biotechnol 163:268–277
López-Laredo A, Gómez-Aguirre Y, Medina-Pérez V, Salcedo-Morales G, Sepúlveda-Jiménez G, Trejo-Tapia G (2012) Variation in antioxidant properties and phenolics concentration in different organs of wild growing and greenhouse cultivated Castilleja tenuiflora Benth. Acta Physiol Plant 34:2435–2442
López-Laredo A, Ramírez-Flores F, Sepúlveda-Jiménez G, Trejo-Tapia G (2009) Comparison of metabolite levels in callus of Tecoma stans (L.) Juss. ex Kunth. cultured in photoperiod and darkness. In Vitro Cell Dev Biol Plant 45:550–558
Lorenzo J, González B, Escalona M, Teisson C, Borroto C (1998) Sugarcane shoot formation in an improved temporary immersion system. Plant Cell Tissue Organ Cult 54:197–200
Martínez-Bonfil B, Salcedo-Morales G, López-Laredo A, Ventura-Zapata E, Evangelista-Lozano S, Trejo-Tapia G (2011) Shoot regeneration and determination of iridoid levels in the medicinal plant Castilleja tenuiflora Benth. Plant Cell Tissue Organ Cult 107:195–203
Michoux F, Ahmad N, Hennig A, Nixon PJ, Warzecha H (2013) Production of leafy biomass using temporary immersion bioreactors: an alternative platform to express proteins in transplastomic plants with drastic phenotypes. Planta 237:903–908
Neves C, Santos H, Vilas-Boas L, Amâncio S (2002) Involvement of free and conjugated polyamines and free amino acids in the adventitious rooting of micropropagated cork oak and grapevine shoots. Plant Physiol Biochem 40:1071–1080
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–340
Saher S, Piqueras A, Hellin E, Olmos E (2004) Hyperhydricity in micropropagated carnation shoots: the role of oxidative stress. Physiol Plant 120:152–161
Salcedo-Morales G, Rosas-Romero G, Nabor-Correa N, Bermúdez-Torres K, López-Laredo AR, Trejo-Tapia G (2009) Propagation and conservation of Castilleja tenuiflora Benth. (“hierba del cáncer”) through in vitro culture. Polibotánica 28:119–137
Scherer RF, Garcia AC, Fraga HPF, Vesco LLD, Steinmacher DA, Guerra MP (2013) Nodule cluster cultures and temporary immersion bioreactors as a high performance micropropagation strategy in pineapple (Ananas comosus var. comosus). Sci Hortic 151:38–45
Sreedhar RV, Venkatachalam L, Neelwarne B (2009) Hyperhydricity-related morphologic and biochemical changes in vanilla (Vanilla planifolia). J Plant Growth Regul 28:46–57
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. Plant J 11:1187–1194
Trejo-Tapia G, Rosas-Romero G, López-Laredo AR, Bermúdez-Torres K, Zamilpa A (2012) In vitro organ cultures of the cancer herb Castilleja tenuiflora Benth. as potential sources of iridoids and antioxidant compounds. In: Orhan I (ed) Biotechnological production of plant secondary metabolites. Bentham Science, Sharjah, pp 87–106
Viu AFM, Viu MAO, Tavares AR, Vianello F, Lima GPP (2009) Endogenous and exogenous polyamines in the organogenesis in Curcuma longa L. Sci Hortic 121:501–504
Zhao Y, Sun W, Wang Y, Saxena PK, Liu CZ (2012) Improved mass multiplication of Rhodiola crenulata shoots using temporary immersion bioreactor with forced ventilation. Appl Biochem Biotechnol 166:1480–1490
Acknowledgments
This work was supported by the Secretaría de Investigación y Posgrado del IPN-México (SIP–IPN Grants 20131786) and by the Fondo Mixto de Fomento a la Investigación Científica y Tecnológica CONACYT–Gobierno del Estado de Morelos (Grant MOR-2007-C01-79409). The funding sources were not involved in the preparation of this paper or in the decision to submit it for publication. RVT is indebted to the CONACYT and PIFI-IPN for the Master in Sciences fellowship awarded. GTT, JLTE, and ARLL are grateful to the Sistema de Becas por Exclusividad (IPN) and Programa de Estímulos al Desempeño de los Investigadores (IPN).
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Praveen Saxena
Rights and permissions
About this article
Cite this article
Valdez-Tapia, R., Capataz-Tafur, J., López-Laredo, A.R. et al. Effect of immersion cycles on growth, phenolics content, and antioxidant properties of Castilleja tenuiflora shoots. In Vitro Cell.Dev.Biol.-Plant 50, 471–477 (2014). https://doi.org/10.1007/s11627-014-9621-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-014-9621-5