Abstract
Development of in vitro techniques has enabled rapid clonal propagation, regeneration, and multiplication of genetically manipulated superior clones, production of secondary metabolites, and ex situ conservation of valuable germplasm. This has been possible not only due to the refinements of culture methodologies and applications of cutting-edge areas of molecular biology but also due to the judicious inclusion of engineering principles and methods to improve and refine the system. In the present study, we used engineering principles and methods to transform basic in vitro techniques into commercially viable technologies. We investigated two types of temporary immersion systems (TIS), two types of lighting, and two different gas exchange systems during in vitro banana (Musa spp. cv. 'Grande naine') shoot cultures. After 7 wks, all banana shoots cultured in standard TIS (5-L glass vessels, type 1) showed superior vegetative growth for the evaluated parameters. We also found that illumination provided by light emission diodes (LEDs) was superior to the use of white fluorescent lamps at the same light intensity (40 μmol m−2 s−1). Shoots treated with compressed air for immersion and additional gas exchange during the culture period in the glass vessel of TIS systems resulted in higher propagation rates and a larger number of shoots harvested as well as a larger number of roots formed per shoot after the 7-wk culture period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bananas and plantains (Musa spp.) are important crops cultivated in tropical and subtropical regions of the world. World Musa production was estimated to be 106,541,709 metric tons in 2011, and 72.5% of this production was bananas (FAOSTAT 2013). The expansion of banana production is limited due to a shortage of available healthy plant material from the farmers (Haq and Dahot 2007a). The transmission of harmful insects, nematodes, and viral diseases by field-grown suckers has heightened interest in the use of in vitro culture techniques. However, high production costs generally limit the commercial use of in vitro micropropagation of banana due to inefficiencies of the system.
Recent development of in vitro techniques has enabled rapid clonal propagation, regeneration, and multiplication of genetically manipulated superior clones. The inclusion of engineering principles and methods has transformed fundamental in vitro techniques to become commercially viable technologies. The application of plant tissue culture for various biotechnological purposes will increasingly depend on the adoption of engineering principles and the interaction with biological systems (Gupta and Ibaraki 2006).
Commercial laboratories need to produce a large number of high-quality plants at a low and efficient cost level. Large-scale plant propagation by using tissue culture techniques is often criticized because of the intensive labor requirements. Thus, scaling-up and automation of production process are necessary to reduce production costs (Gupta and Ibaraki 2006). Therefore, using liquid nutrient medium (either with temporary and/or permanent immersion) during routine banana micropropagation is considered to be ideally suited for automation and cost efficiency (Alvard et al. 1993; Albany et al. 2005; Roels et al. 2006; Haq and Dahot 2007a, b; Aragon et al. 2010).
Tisserat and Vandercook (1985) first applied the idea of temporary immersion systems (TIS) in plant tissue culture by designing a system consisting of a large elevated culture chamber that was drained and then refilled with fresh medium at regular intervals. In 1993, Alvard et al. applied this method to grow banana meristems by using a standard autoclavable filtration unit with two compartments. A similar apparatus was commercialized, namely, Recipient for Automated Temporary Immersion (RITA®, Vitropic, Saint-Mathieu-de-Treviers, France). In all these cases, compressed air is used to overflood the plant material with liquid nutrient medium for only a few minutes, two to four times per day. After stopping the airflow, the liquid medium returned to the bottom of the vessel by gravity. Whereas Teisson and Alvard (1995), Escalona et al. (1999), Etienne and Berthouly (2002), and Jiménez González (2005) used compressed air in TIS with two flasks (twin flasks system: one flask for the plant material and the second flask for the liquid nutrient medium; both flasks are connected by tubes and also to the compressed air source; compressed air regulated the flow of the liquid medium inside the plant culture vessel and the return flow into the storage flask). Other authors, such as Aragon et al. (2010) demonstrated that compressed air was more effective in providing an appropriate environment (gas exchange rate) combined with nutrient supply than immersion systems using only gravity without active gas exchange. However, at present, a system where the exchange process of the culture medium is realized by gravity in both directions has not been tested. Applying gravity to flood the in vitro plant material in one flask as well as to ensure the return flow into the second flask for storage of the liquid medium requires much more care and can be realized only by elevation.
Furthermore, currently, the use of light emitting diodes (LEDs) optimized for plant growth (combining LEDs emitting in far red, red and blue colors) can result in better plant growth and morphology. Nhut and Nam (2010) successfully used a mixture of LEDs for tissue culture in banana micropropagation. However, they used a semisolid medium in small containers, not utilizing TIS. Therefore, we aimed to determine whether LED or white fluorescent light was optimal illumination for banana propagation and biomass production in a standard TIS.
To enable these experiments to be conducted, the project (Innovative Pflanzenerzeugung im Temporären Immersions System, Number: 01RI0614B) was developed between Fraunhofer IFF (Magdeburg, Germany), Institute for Factory Operation and Automation, BioPlanta GmbH (Leipzig, Germany; since November 2012 Vita 34 AG [Germany] is the legal successor of BioPlanta GmbH [Germany]) and Plant Biotechnology Institute, IBP (Santa Clara, Cuba) for development of a new design of TIS and biological validation of the first prototype. Therefore, this study compares two types of TIS, two types of lighting, and effects of different immersion systems during in vitro culture of banana shoots.
Materials and methods
Plant material.
Banana (Musa spp.) cv. 'Grande naine' (AAA) plants were selected from a stock field collection. Meristematic shoot tips were excised and used as explants. They were surface-sterilized by washing with detergent and running water, stirred in 3.0% (w/v) NaOCl for 20 min, and rinsed with sterile distilled water three times in a laminar air flow cabinet. Shoot tips (1–1.5 cm) were isolated aseptically and then cultured for organogenesis on Murashige and Skoog (MS) basal medium (Murashige and Skoog 1962), supplemented with 8.6 μM 6-benzylaminopurine (BAP), 0.98 μM indolebutyric acid (IBA), 1 mg L−1 thiamine, and 30 g L−1 sucrose. The pH of all media was adjusted to 5.7–5.8 before autoclaving media at 121°C for 15 min. After organogenesis (production of in vitro shoot in 3 to 4 wk), explants were subcultured every 21 d, on the same medium supplemented with only 16.8 μM BAP and 3.8 μM indole-3-acetic acid (IAA) for shoot multiplication. All shoots were subcultured four times on MS multiplication medium supplemented with 16.8 μM BAP, 3.8 μM IAA, 1 mg L−1 thiamine, 30 g L−1 sucrose, and 6 g L−1 agar. Cultures were maintained at 25 ± 2°C under a 16/8-h (light/dark) photoperiod with a photosynthetic photon flux of 40 μmol m−2 s−1.
For all experiments, individual well-formed shoots, 2–3 cm in height without roots were used as explants. Experiments comparing TIS were repeated three times.
TIS designs.
Two experimental systems were compared: TIS type 1 consisting of standard TIS, 5-L glass flasks with 3.0 L of medium and 60 explants per flask; and TIS type 2 consisting of a 40-L plastic box with 24 L of medium and 480 explants. TIS type 2 complies with the new type of TIS (project with Fraunhofer Institute for Factory Operation and Automation, Magdeburg, Germany). The first demonstrator (prototype) was built and installed at BioPlanta GmbH in 2009. This system used gravity for medium transfer in both directions and eight polycarbonate boxes for parallel application (Fig. 1A ).
Micropropagation of banana (Musa spp. cv. 'Grande naine, AAA) in temporary immersion systems. (A) New type of TIS (type 2) with lighting from the top and medium change by gravity. (B) Five-liter TIS (type 1) with lighting from the side (white fluorescent lamp). (C) Polycarbonate box (PC) from new type of TIS (type 2) illuminated by LEDs with banana shoots after 4-wk growth. (D) Five-liter TIS standard lightened LEDs with banana shoots after 4-wk growth. (E) Aspect of banana plants in plastic box from new type of TIS (type 2) after 7-wk culture. (F) Aspect of banana plants harvested from standard TIS with white fluorescent light from the side and additional gassing at 7-wk culture.
The immersion program for standard TIS (5 L) and TIS type 2 (40 L) was four immersions per day for 1-min duration, for both systems. The type of immersion for standard TIS (5 L) used compressed air, and for TIS type 2 (40 L), immersion occurred using gravity at atmospheric pressure. In both types of TIS, the internal atmosphere was not supplemented with additional gas exchange. The light source for the standard TIS (5 L) system used cool white fluorescent lamps placed at the side of containers, and TIS type 2 (40 L) utilized white super-bright LEDs (Conrad Electronic SE, Hirschau, Germany) that were placed over the containers. Three standard TIS containers and three boxes from TIS type 2 were used. The experiment was repeated twice.
Experiment with light quality.
Two treatments were tested using standard TIS: 5-L glass flasks with 3.0 L of medium. Lighting was either by fluorescent lamps (40 μmol m−2 s−1) or by LEDs (40 μmol m−2 s−1). The inoculum density was 60 explants (banana shoots) per flask. Immersion programs were four immersions per d for 1 min each, assisted by compressed air.
Experiment with type of immersion.
Three immersion treatments were tested: (1) immersion via compressed air without additional gassing, (2) immersion via compressed air with additional gas exchange, and (3) immersion at atmospheric pressure via gravity without additional gassing.
For all immersion system utilized TIS (5 L), an inoculum density of 60 explants (banana shoots) per flask and an illumination by fluorescent lamps with 40 μmol m−2 s−1 were chosen. The immersion programs for all TIS (5 L) were. For all treatments, four immersions per day for 1 min were applied. Additionally, the gassing rate in treatment 2 was six times per d for 1 min each. As described above, treatments 1 and 2 were realized by compressed air, and treatment 3 by gravity.
Culture conditions.
For all experiments, a two-phase culture program totaling 7 wk was applied: a 4-wk multiplication phase and 3-wk rooting/shoot elongation phase. The composition of multiplication medium for banana contained MS macro and micronutrients, 30 g L−1 sucrose, and 1.0 mg L−1 thiamine HCl, 16.8 μM 6-BAP, and 8.5 μM paclobutrazole. The rooting/shoot elongation medium used MS macro and micronutrients, 40 g L−1 sucrose, and 1.0 mg L−1 thiamine HCl. The pH of all media was adjusted to 5.8 before autoclaving (Albany et al. 2005). The total time of culture period was 7 wk. Four replicates were used for each treatment, and the experiment was repeated twice.
The parameters recorded after a 7-wk culture period were initial fresh weight (to calculate the average initial explant weight); number of shoots produced (multiplication rate); plant length (cm); number of roots per shoots; root length (average and maximum); total fresh weight (to calculate final shoot or explant average weight by dividing the total fresh weight with the total number of explants or shoots); and plant vitality during culture period, such as shoot color, necrosis, chlorosis, hyperhydricity, and/or callus formation.
Statistical analysis.
All statistical analyses were carried out using SPSS software (version 13.0). The results were analyzed using one-way ANOVA followed by Tukey’s test at the 5% level.
Growing conditions during hardening.
Plants from the TIS experiment were transplanted to a mixture of soil and sand (2:1) and then transferred to a greenhouse (16/8-h light/dark; Tmin 20°C and Tmax 26°C) for hardening. The plants were watered twice a wk. Survival rates were determined for all treatments 15 d after transplanting to greenhouse.
Results and Discussion
Two experimental TIS systems were compared: TIS type 1 consisting of standard TIS consisting of two 5-L glass flasks connected to each other and to a compressed air source (standard TIS) and TIS type 2 consisting of a 40-L polycarbonate boxes as culture vessels for the in vitro plant material connected to a second vessel for the nutrient supply. This TIS type 2 complied with a new type of TIS (project with Fraunhofer Institute for Factory Operation and Automation, Magdeburg, Germany). The first demonstrator (prototype) was built and installed at BioPlanta GmbH in 2009.
TIS design.
After 7 wk, a range of vegetative growth parameters was evaluated (Table 1). This showed that all banana shoots cultured in standard TIS (5-L glass vessels, type 1) performed better for the case biomass increase, fresh weight of shoot and plant height. The biomass production was three times larger than TIS type 2. In the case of the number of roots, both TIS designs had the comparable effect (average 4.7 and 4.9 roots per shoot for type 1 and type 2 TIS, respectively). Only the maximum root lengths of TIS type 2 (2.3 ± 1.7 cm) were longer than the maximum root lengths of TIS type 1 (1.2 ± 1.1 cm), but no statistical differences could be determined. These results (lower biomass production, lower multiplication rate, comparable root formation, and longer maximum root lengths) may be due to the TIS type 2 design. The internal atmosphere within the polycarbonate plastic box will not change 100%, because the immersion of culture medium was realized only by gravity without application of compressed air (Fig. 1B ). The applied system (immersion via gravity without compressed air) had a negative impact on both phases of the micropropagation of banana (multiplication and rooting). This confirms the advantages of traditional temporary immersion systems (TIS type 1) with compressed air which is essential for an adequate gas exchange and sufficient mixing (Gupta and Ibaraki 2006) which is probably not achieved in the new system (TIS type 2) using only gravity for the inlet/outlet of the liquid nutrient medium inside the polycarbonate box with in vitro banana shoots.
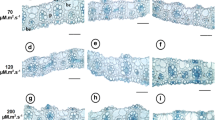
Furthermore, it is necessary to take into account the fact that the polycarbonate box exhibits a 10% lower transmittance in visual wavelength range compared to the glass bottle (Fig. 2). This also has a negative influence on the response of the banana shoots under the chosen culture conditions. In this regard, Adolfo et al. (2011) highlighted the importance of the characteristics of the culture flask influencing the growth and development of plant tissues in culture. Huang and Chen (2005) showed that polycarbonate filtered out the transmittance of wavelengths below 400 nm and excludes UV light from the internal space of the vessels, as confirmed in Fig. 2. Whereas, the glass vessels exhibited transmittance of light at wavelengths 300 to 700 nm. These physical properties could provide useful information to select vessels determining the suitable growth condition for plantlets. The irradiance and spectra of light reaching the top of in vitro plantlets are additionally affected by the transmittance of the caps and walls of vessels (Smith and Spomer 1995).
The position of the light source may have also affected the plant growth. In TIS type 1, fluorescent lamps were placed alongside the TIS vessel (Fig. 1B ) and the shoots were more exposed to the light. However, in TIS type 2, the LED light source was positioned above the container resulting in a larger distance between the light source and banana shoots (Fig. 1C ). Nguyen and Kozai (1998) reported that light intensity in vessels which were illuminated from the side were about five times higher than a downward lighting system. However, it is noteworthy that the results of the experiment with different TIS designs are a combination of the effects of the renewal of the internal atmosphere (100% change of the atmosphere with normal air the type of applied culture vessel) and the position of the light source.
The plant height of the banana shoots after a 7-wk culture was 12.5 ± 4.5 cm in TIS type 1 versus 6.2 ± 2.7 cm in TIS type 2. The shorter plantlet height seemed favorable for the plant management at the time of transplanting to ex vitro conditions. However, the survival rates following acclimatization of the plants in greenhouse conditions were similar (98.2 and 97.8% for TIS types 1 and 2, respectively). The comparable acclimatization rate might be influenced by the position of the light source for both designs. In the case of TIS type 2 (polycarbonate plastic boxes), shoots were exposed to LED light that were placed above the container.
Importantly, banana shoots and plants from all experiments did not show symptoms of necrosis, chlorosis, hyperhydricity, or callus formation.
Experiment with the type of light source.
In banana tissue culture, the goal is to produce a maximum number of shoots that are long enough for rooting under in vitro conditions (Haq and Dahot 2007b). Therefore, we designed an experiment to compare the effect of different light sources on the multiplication rate of banana tissue cultures. Two treatments were tested using standard TIS: 5-L glass flasks with 3.0 L of medium. Lighting was either by fluorescent lamps (40 μmol m−2 s−1) from the side or by LEDs (40 μmol m−2 s−1) placed above the culture vessels. The application of white LEDs in the standard TIS (type 1) resulted in more vigorous growth compared to white light fluorescent lamps (Table 2), as determined by the propagation coefficient of 7.1 verses 4.2 and the number of roots per plant of 7.7 ± 1.1 versus 4.7 ± 1.8 cm despite LEDs being placed above the culture vessel. Both parameters are important for commercial-scale development of micropropagation protocols. Good root development is also essential for survival and growth of plants during the acclimatization phase. However, the fresh weight of shoots was lower in the case of LED illumination compared to the fluorescent lighting (Table 2). Additionally, a reduced plant height was observed in the TIS system using the LED illumination (5.6 ± 3.2 cm) compared to white light fluorescent lamps (12.5 ± 4.5 cm). One possible explanation might be due to the position of the light sources. Fluorescent lamps were placed horizontally next to the glass vessel (Fig. 1B ), whereas LEDs were located above the culture flasks (Fig. 1D ), which means that the light intensity received towards the bottom of the culture vessel was lower. However, the more homogeneous distribution of overhead light induced a higher multiplication rate for all banana shoots, with the rate for white LEDs (Table 2). In the case of illumination by white fluorescent lamps, the multiplication rate was less, but the plant height was greater than for LED lighting. This may be due to a lower light intensity received by banana shoots cultured in 5-L glass flasks with fluorescent lamps as light source positioned horizontally, which caused the shoot elongation. Furthermore, the color of the harvested shoots changed slightly to green and yellow leaves when illuminated by fluorescent lamps compared to the green color of the leaves when illuminated with LEDs. Takayama and Akita (2006) reported that production of plants with well-developed and green leaves in the TIS is the main objective for successful establishment of the plants in soil. The production of such plants depends mainly on the illumination intensity provided by the culture light, but it can be difficult to efficiently introduce lighting into the TIS system. However, it seems that banana shoots need a reduced light intensity during the propagation phase for micropropagation compared to the rooting phase (Pérez et al. 1998). This effect could be shown with the propagation rate, in this study, which was higher in the experiments using LED located above the culture vessel (Table 2). Thereby, LEDs exhibit a lower light intensity compared to the white fluorescent lamps due to the relative positions.
On the other hand, the quality of the light spectrum may have also contributed to the different growth responses observed. Matsumoto and Caldas (2007) reported that blue light reduces plant growth, whereas yellow and red light induces growth in some species such as in the case of banana cultivar 'Nanicao' (Musa spp. AAA group). These results are also supported by Nhut and Nam (2010), who reported that the best combination of light was 80% red LED and 20% blue LED for propagation of banana. Although it should be noted that all studies were performed with banana shoots grown in culture flasks with semisolid medium, not with a TIS and liquid nutrient medium.
Another factor to take into account is the light intensity, i.e., the density of photosynthetic photon flux (PPFD). Using LEDs with a light intensity of 60 μmol m−2 s−1, the growth of banana explants was maximal compared to light intensities of 45 μmol m−2 s−1 and 75 μmol m−2 s−1 (Haq and Dahot 2007b). At this intensity, LEDs were also superior to the use of white fluorescent lamps. In the present study, the intensity of both types of light sources was 40 μmol m−2 s−1, which may have influenced the low fresh weight of shoots in the TIS system using LEDs. However, root formation and the propagation coefficients obtained for banana shoots (Musa spp. cv. 'Grande naine' AAA) using low LED lighting were superior to the use of fluorescent lamps.
Due to the low light intensity, the elongation of the in vitro shoots was greater in experiments using white fluorescent lamps compared to the LEDs. However, the opposite occurred when using the standardized white LEDs with the equal light intensity. Haq and Dahot (2007a) also achieved greater height of in vitro shoots (8.25 cm) using white fluorescent lamps in an experiment with TIS. Aragon et al. (2010) compared two light intensities using cold fluorescent lamps in a TIS system for banana (Musa spp. cv. 'CEMSA' ¾AAB) culture, and the best results for acclimatization of the plants were achieved when the light intensity (PPFD) was 80 μmol m−2 s−1, double of what is used in the present study. However, it is important to note that the banana plants were much shorter (2.5 cm height and at least two leaves) at this light intensity. An explanation could be due to the high light intensity applied among other factors, such as planting density and distance from light source. As well, the effect of blue light on banana plants is known to inhibit plant growth (Nhut and Nam 2010).
Previous experiments have been performed with LEDs where the transmission wavelength and range were unknown, but LEDs emit in white light range (sensitive to the human eye, but not effective for plant growth). Our differences observed between use of LEDs and fluorescent lamps may also be related to different light intensities rather than the specific light spectra (Nhut and Nam 2010).
Accordingly, Takayama and Akita (2006) reported that illumination of shoot in TIS system is not easy because of the logarithmic reduction of light intensity passing through the plant tissues and the distance from the light source. The relationship between the distance from the source of light and its intensity is an important factor to obtain good results. Light emitting diode (LED) is superior to other light sources because of its excellent focusing characteristics, i.e., the high-energy conversion rate and the reduced infrared heat radiation. Light transmittance was reduced drastically by the presence of shoot cultures in the TIS system, especially with a higher fresh weight. When shoot cultures of Spathiphyllum and Colocasia grown in a TIS system using glass vessels illuminated externally by fluorescent lamps, light was transmitted to the cultures only several centimeters from the vessel surface. The leaves of illuminated shoots became green and well-developed.
Experiment with type of immersion.
Three different immersion systems were compared using the type 1 TIS (5 L). Shoots treated with compressed air for immersion and additional gassing during the 7-wk culture period showed a better result for shoot multiplication, with a greater number of harvested shoots (337) compared to the same treatment without compressed air (253 shoots) or immersion using gravity without gassing (201 shoots). The plant height for the TIS with immersion via compressed air plus additional gassing (6.9 ± 3.6 cm) was less than TIS with immersion via compressed air without additional gassing (12.5 ± 4.5 cm), after the 7-wk culture (Table 3; Fig. 1E, F ). Roels et al. (2006) reported that frequent headspace renewal by surrounding air is responsible for the quality increase of plantain (Musa spp. AAB group) shoots cultured in a small TIS system. Haq and Dahot (2007a) indicated that when a plant is fixing CO2, the rate of shoot multiplication is comparable to the decrease of growth using proper in vitro conditions. However, in this study, using gravity for medium renewal without additional gassing resulted in the lowest values for shoot multiplication (3.4 for the propagation coefficient) and the final number of shoots harvested (201; Table 3). Furthermore, the renewal of the atmosphere inside the culture vessel is more frequent and without requiring the culture medium which reduces the risk of developing hyperhydric shoots due to the repeated immersions in TIS system (Ducos et al. 2008).
According to the present study, the application of LEDs in TIS system located above the culture vessels and employment of additional gassing without immersion is a recommended setup for the micropropagation of banana cv. 'Grande naine'. However, it is necessary to continue further studies regarding the position of the LEDs in relation to the TIS (light from above or from the side) and also to the position of the glass culture bottles (vertical or horizontal position).
References
Adolfo JJ, Alonso-Blázquez N, López-Vela D, Celestino C, Toribio M, Alegre J (2011) Influence of culture vessel characteristics and agitation rate on gaseous exchange, hydrodynamic stress, and growth of embryogenic cork oak (Quercus suber L.) cultures. In Vitro Cell Dev Biol Plant 47:578–588
Albany N, Jiménez E, Vilchez J, García L, de Feria, Pérez N (2005) Use of growth retardants for banana shoot multiplication in TIS. In: Hvoslef-Eide AK, Preil W (eds) Liquid systems for in vitro mass propagation of plants. Springer, Dordrecht, pp 213–224
Alvard D, Cote F, Teisson C (1993) Comparison of methods of liquid medium culture for banana micropropagation. Plant Cell Tissue Organ Cult 32:55–60
Aragon CE, Escalona M, Rodríguez R, Canal MJ, Capote I, Pina D, Olmedo JG (2010) Effect of sucrose, light, and carbon dioxide on plantain micropropagation in temporary immersion bioreactors. In Vitro Cell Dev Biol Plant 46:89–94
Ducos JP, Terrier B, Courtois D, Petiard V (2008) Improvement of plastic-based disposable bioreactors for plant science needs. Phytochem Rev 7:607–613
Escalona M, Lorenzo JC, Gonzalez B, Daquinta M, Gonzalez JL, Desjardins Y, Borroto CG (1999) Pineapple (Ananas comosa L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep 18:743–748
Etienne H, Berthouly M (2002) Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult 69:215–231
FAOSTAT (2013) http//www.faostat.fao.org/site/567/desktopdefault. Cited 28 June 2013
Gupta SD, Ibaraki Y (2006) Plant tissue culture engineering. Focus on biotechnology, vol 6. Springer, Netherlands, 477 pp
Haq I, Dahot MU (2007a) Effect of immersion systems on chlorophyll contents in micropropagating banana. Afr J Biotechnol 6:1095–1101
Haq I, Dahot MU (2007b) Micropropagation efficiency in banana (Musa spp.) under different immersion systems. Pak J Biol Sci 10:726–733
Huang C, Chen C (2005) Physical properties of culture vessels for plant tissue culture. Biosyst Eng 91:501–511
Jiménez González E (2005) Mass propagation of tropical crops in temporary immersion systems: present status and future prospects. In: Hvoslef-Eide AK, Preil W (eds) Liquid systems for in vitro mass propagation of plants. Springer, Dordrecht, pp 197–211
Matsumoto K, Caldas LS (2007) Stimulation of banana in vitro shoot growth by yellow-cellophane-film shading. Fruit 62:143–148
Murashige T, Skoog F (1962) A revised media for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nguyen QT, Kozai T (1998) Environmental effects on the growth of plantlets in micropropagation. Environ Control Biol 36:59–75
Nhut DT, Nam NB (2010) Light-emitting diodes (LEDs): An artificial lighting source for biological studies In: Proceedings of the Third International Conference on the Development of Medical and Biological Engineering (MBE), Vietnam pp 133–138
Pérez PJ, Jiménez González E, Agramonte PD (1998) Aumento de la eficiencia en la micropropagación. In: Pérez PJ (ed) Propagación y Mejora genética de plantas por Biotecnología. Instituto de Biotecnología de las plantas, Cuba, pp 179–191 (in Spanish)
Roels S, Noceda C, Escalona M, Sandoval J, Canal MJ, Rodríguez R (2006) The effect of headspace renewal in a temporary immersion bioreactor on plantain (Musa AAB) shoot proliferation and quality. Plant Cell Tissue Organ Cult 84:155–163
Smith MA, Spomer LA (1995) Vessels, gels, liquid media and support systems. In: Aitken-Christic J, Kozai T, Smith MAL (eds) Automation and environmental control in plant tissue culture. Kluwer Academic Publishers, Dordrecht, pp 371–404
Takayama S, Akita M (2006) Bioengineering aspect of bioreactor in plant propagation. In: Gupta SD, Ibaraki Y (eds) Plant tissue culture engineering. Springer, Dordrecht, pp 83–100
Teisson C, Alvard D (1995) A new concept of plant in vitro cultivation liquid medium: temporary immersion. In: Terzi M, Celia R, Falavigna A (eds) Current issues in plant molecular and cellular biology. Kluwer, Dordrecht, pp 105–110
Tisserat B, Vandercook CE (1985) Development of an automated plant culture system. Plant Cell Tissue Organ Cult 5:107–117
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: John W. Forster
Rights and permissions
About this article
Cite this article
Wilken, D., Jiménez Gonzalez, E., Gerth, A. et al. Effect of immersion systems, lighting, and TIS designs on biomass increase in micropropagating banana (Musa spp. cv. 'Grande naine' AAA). In Vitro Cell.Dev.Biol.-Plant 50, 582–589 (2014). https://doi.org/10.1007/s11627-014-9605-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-014-9605-5