Abstract
A protocol for somatic embryogenesis was developed for Thymus hyemalis, a wild species in the Mediterranean region. First, the effects of explant type, plant growth regulators [kinetin (KIN) and 2,4-dichlorophenoxyacetic acid (2,4-D)], and genotype on callus induction were tested. For callus induction, the node was the best explant; Murashige and Skoog (MS) medium supplemented with 1.8 μM 2,4-D and 0.5 μM KIN was the best medium, and the genotype had a highly significant effect. To induce production of somatic embryos, the effects of KIN, 6-benzylaminopurine (BAP), and naphthalene acetic acid (NAA) were evaluated. After 5 wk of culture in the dark, MS medium supplemented with 4.44 μM BAP, 0.54 μM NAA, and 4.65 μM KIN gave the highest percentage (85%) of embryogenic callus and the highest number of somatic embryos (27.00) per 45 mg of callus. For germination and plant recovery, somatic embryos were transferred to MS medium without plant growth regulators and plantlet conversion from developed somatic embryos was 90%. In vitro plants with adequate growth and sufficient root systems were subsequently transplanted into a mixture of peat and vermiculite (2:1 v/v) under greenhouse conditions. The survival rate of the plantlets under ex vitro conditions was 80%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The genus Thymus is one of the eight most important genera in the Lamiaceae family because of the large number of species in this genus (215) (Stahl-Biskup and Sáez 2002). The Mediterranean region, specifically the West Mediterranean region, is the center of origin of the genus (Stahl-Biskup and Sáez 2002). The most characteristic constituents of the herb, especially of the leaves, are essential oil, flavones, rosmarinic acid, triterpenes, and carbohydrates (Stahl-Biskup 2007; Stahl-Biskup et al. 2009). Several thyme oils possess antifungal, antioxidant, insecticidal, and antibacterial activities (Daferera et al. 2000; Zarzuelo and Crespo 2002; Kalemba and Kunicka 2003; Nieto et al. 2010; Saad et al. 2010; Fadli et al. 2012; Ramchoun et al. 2012) and have many potential uses in the food industry as a dietetic supplement (Youdim and Deans 1999; Nieto et al. 2011). Furthermore, the use of thyme as a food preservative and as an aromatic ingredient for seasoning various dishes are of considerable importance (Zarzuelo and Crespo 2002; Burt 2004). Thymus species have complex chemical compositions (Sáez 2001; Martinez et al. 2005) as a consequence of ecological variability. Thymus hyemalis Lange (winter thyme) is a wild plant; it is widely collected because its essential oil, with a high proportion of phenols, is greatly valued by exporters (Sáez 1995). Thymol, carvacrol, borneol, and linalool are the most abundant compounds in these plants (Sáez 1998; Goodner et al. 2006; Rota et al. 2008; Bektas et al. 2011). The essential oil from wild T. hyemalis of the southeastern Iberian Peninsula shows great chemical variability (Sáez 1995; Sotomayor 1998; Jordán et al. 2006). T. hyemalis is an important commercial thyme, and effective propagation protocols are needed to produce a large quantity of plants from which chemicals of interest can be extracted, thus preventing the exploitation of wild populations.
In vitro culture techniques offer a viable tool for mass multiplication and conservation of rare, endangered, and threatened medicinal plants (Prakash et al. 1999; Tiwari et al. 2006). In addition, plant cell and tissue culture methods are used for secondary metabolite production (Rout et al. 2000; Guo et al. 2007; Shinde et al. 2010). Somatic embryogenesis has been used for micropropagation (Gastaldo et al. 1994; Jayanthi and Mandal 2001; Gopi and Ponmurugan 2006), genetic transformation (Hassig et al. 1987; Kiran et al. 2005), artificial seed production (Jayanthi and Mandal 2001; Kumar and Thomas 2012), and to study plant development (Anita et al. 2012). In medicinal plants, there is a growing interest in developing somatic embryogenesis systems as useful and efficient method for the production of secondary metabolites (Shohael et al. 2006).
Due to the high demand for thyme essential oil, we evaluated T. hyemalis for micropropagation via somatic embryogenesis (SE), which has not previously been reported. We present data on the effects of explant type, genotype, and growth regulator type and concentration on the induction of somatic embryogenesis and regeneration of whole plants of T. hyemalis.
Materials and Methods
Plant material.
In vitro shoots of T. hyemalis were maintained for about 8 mo on Murashige and Skoog (1962) (MS) basal salts and vitamins without plant growth regulators (PGRs), enriched with 3% (w/v) sucrose (Duchefa Biochemie, Haarlem, The Netherlands) and solidified with 0.4% (w/v) gellan gum (KALYS, Saint-Ismier, France) (pH 5.8). Shoots were subcultured every 4 wk and grown under a 16-h light photoperiod using cool white fluorescent lamps (Sylvania Sylfast SSE T5, Germany) at 60 μmol m–2 s–1 photon flux, at a temperature of 23 ± 2°C. In this study, these axenic in vitro-propagated shoots were used as the explant donor.
Callus induction.
The effects of explant type and PGRs in the medium were first evaluated on callus induction of T. hyemalis. The explants consisted of nodes (2–3 mm long, the third level from the bottom) and young leaves transversely divided into small pieces (2–3 mm2 area) and placed with the abaxial surface in contact with the medium. Both types of explants (nodes and leaves) were cultured on MS medium supplemented with combinations of 0.0, 0.5, 1.8, 4.5, or 10.0 μM 2,4-dichlorophenoxyacetic acid (2,4-D) (Duchefa) and 0.0, 0.5 or 1.8 μM kinetin (KIN) (Duchefa). For callus induction using different growth regulators, each treatment was replicated three times and each replication consisted of five explants.
To study the effect of genotype on callus induction, three genotypes of T. hyemalis designated as G1, G2, and G3 were used. For each genotype, nodal explants were placed horizontally on MS medium supplemented with 1.8 μM 2,4-D and 0.5 μM KIN. Five replicates with five explants per replicate per genotype were used. After 4 wk, explants were examined for callus induction (number of explants with calli per total number of explants cultured × 100) weighed.
Callus proliferation.
Calli derived from nodal explants of G1 were excised, and any dead, dark-brown tissues were removed. Calli pieces (2–3 mm diameter) were subcultured three times (3-wk interval) on the same induction medium, in order to obtain a sufficient amount of tissue for somatic embryo induction.
Somatic embryo production and conversion to plantlets.
To evaluate the effects of growth regulators on production of somatic embryos, calli derived from nodal explants, obtained on medium with 1.8 μM 2,4-D and 0.5 μM KIN were transferred to either PGR-free MS medium or to medium supplemented with either 4.44, 8.88 or 13.32 μM 6-benzylaminopurine (BAP) (Duchefa), 0.54 μM naphthalene acetic acid (NAA) (Duchefa), or 0.93, 2.32, and 4.65 μM KIN. After 5 wk, data on embryogenic potential (%) of callus and number of somatic embryos were recorded. Four replicates with five calli per replicate were used. Somatic embryos >5 mm long were counted and then transferred onto conversion medium (MS basal salts without PGRs). After 5 wk, the results were recorded as the rate of somatic embryo conversion to whole plantlets.
Culture media and conditions.
For all experiments, MS basal salt medium contained 3% sucrose (w/v) and was solidified with 0.4% gellan gum (w/v). The pH of each medium was adjusted to 5.8 with NaOH or HCl prior to autoclaving for 15 min at 121°C. Media were dispensed into sterile 9 cm Petri dishes (25 ml per dish). Cultures were kept at 25 ± 1°C in the dark except for the conversion step, when the cultures were incubated at 23 ± 2°C with a 16 light/8 h photoperiod at light intensity of 60 μmol m–2 s–1 provided by white fluorescent tubes (Sylvania Sylfast SSE T5, Germany).
Acclimatization and transfer to soil.
When the plantlets had grown adequately and had sufficient root systems, they were removed from the in vitro containers and washed carefully in running tap water to remove the media adhering to roots. The plantlets were subsequently transplanted into plates with wells 3 cm in diameter, filled with peat and vermiculite (2:1 v/v). The plants were covered with polyethylene bags and watered with tap water three times per day during the first week of acclimatization. Plants were also watered with a diluted solution of MS basal medium (1/10 MS) once per week. After 21 d, the polyethylene bags were removed and well-established plants were transferred to a greenhouse.
Statistical analysis.
Statistical analysis of the data was performed with SPSS for Windows. Homogeneity was assessed by Levene’s test. Mean values were calculated and then compared by Duncan’s multiple range test at P ≤ 0.05.
Results and Discussion
Effect of explant type and growth regulators.
Explant type, PGR type, and the interaction of these factors significantly affected the percentage of explants producing callus (Table 1). No explants produced any callus when cultured on PGR-free MS medium (Fig. 1). When PGRs were included, nodal explants showed the earliest signs of callus formation after 2 wk of culture, whereas the leaf started to initiate callus from cut surfaces after 3 wk of culture. After 4 wk of culture, callus induction was as high as 100% and 66.66% for nodes and leaves, respectively (Fig. 1). The type of explant is important in determining the rate of callus induction (Martin 2004; Yan et al. 2009; Gonçalves and Romano 2013), which is likely due to their differential reactivity to medium components (Kouakou 2003).
Effect of explant type and plant growth regulator on callus induction of T. hyemalis during 4 wk of culture. Means within an explant type marked by the same letter are not significantly different (P ≤ 0.05). Lower-case letters correspond to leaf explant means; upper-case letters correspond to node explant means. Error bars indicate SE.
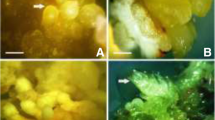
The presence of 2,4-D (0–10 μM) alone in the culture media was generally less effective than a combination of 2,4-D and KIN for callus induction (Fig. 1). The calli obtained from media with 2,4-D alone were small, brown, and hard (Fig. 2a ). Callus induction with 2,4-D alone in the medium may be less effective than when 2,4-D is used with a cytokinin (BAP) (Lee et al. 2009). Here, the highest frequency of callus induction was 100%, obtained with node explants cultured on MS medium supplemented with a combination of 1.8 μM 2,4-D and 0.5 μM KIN. No significant differences were observed between this combination and 0.5/0.5, 1.8/1.8, and 0/1.8 μM of 2,4-D/KIN. The highest percentage of callus formation from leaf explants was 66.66%, obtained on MS medium supplemented with only 1.8 μM KIN. This result is not significantly different from that obtained on MS medium with 4.5/0.5 μM of 2,4-D/KIN (Fig. 1). Furthermore, callus formation on MS medium with KIN alone was accompanied by shoot initiation at the end of 4 wk (data not shown). The callus obtained with 2,4-D and KIN combinations was off-white, friable, and globular, especially that obtained with 1.8 μM 2,4-D and 0.5 μM KIN (Fig. 2b ). This result is in agreement with Natasa et al. (2004) and Lin et al. (2011), who showed that a combination of 2,4-D and KIN was beneficial for embryogenic callus induction. The effects of PGRs on callus induction have been reported in several recent studies (Gonçalves and Romano 2013; Pérez-Jiménez et al. 2013). On the basis of our callus induction results, callus from nodes, cultured on MS with 1.8 μM 2,4-D and 0.5 μM KIN, were chosen to test the effect of genotype on callus induction in T. hyemalis.
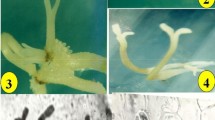
In vitro regeneration of plantlets through somatic embryogenesis of T. hyemalis. a, Callus developed from node explant on MS with 10 μM 2,4-D. b, Proliferated callus on MS with 1.8 μM 2,4-D and 0.5 μM KIN. c, Somatic embryos produced in MS with 4.44 μM BAP, 0.54 μM NAA, 4.65 μM KIN. d, Somatic embryos on MS PGR-free medium. e, Somatic embryos at stage of conversion to plantlets. f, Micropropagated plants after 21 d of acclimatization. Bars = 1.66 (a); 5 (b); 3.96 (c); 4.61 (d); 20.7 (e); 100 mm (f).
Effect of genotype.
Calli could be induced from all genotypes, but significant genotypic differences in callus induction response were observed among the three genotypes tested (Table 2). Callus formation was observed at 2 wk in genotype G1, whereas the induction of callus in G2 and G3 started after 3 wk. G1 clearly showed the best response (92%) followed by G2 (60%); the lowest response was observed in G3 (32%). In addition, G1 produced a higher fresh weight (96.01 mg) of callus, while no significant difference was observed between G2 (37.16 mg) and G3 (33.36 mg) (Table 2). Other reports also indicate that genotype has a significant effect on callus induction (Abe and Futsuhara 1986; Nhut et al. 2007).
Callus proliferation.
During three subcultures on the same callus induction medium, the callus growth on MS medium with 2,4-D (1.8 μM ) and KIN (0.5 μM) was slow at the beginning, but at the end of the third subculture onto the same medium, the fresh weight of the calli was about threefold. The callus structure became more organized with successive subcultures and had a yellowish color.
Somatic embryo production.
All the media (Table 3) allowed somatic embryo development, including the basal medium consisting of MS inorganic salts and vitamins without PGRs. However, the efficiency of somatic embryo production varied with PGR type and concentration. The highest somatic embryo production (85%) and highest number of somatic embryos per 45 mg callus (27.00) were obtained on medium supplemented with 4.44 μM BAP, 0.54 μM NAA, and 4.65 μM KIN. The lowest response (4.75) occurred on MS medium with 13.32 μM BAP, 0.54 μM NAA, and 2.32 μM KIN. In a previous study, MS medium containing 4.44 μM BAP, 5.37 μM NAA, and 2.32 μM KIN induced maximum embryogenesis in Ocimum basilicum leaf callus culture (Gopi and Ponmurugan 2006). In the present study, MS medium with 1.8 or 0.5 μM KIN induced somatic embryogenesis on 65% or 35% of the callus, respectively, with 19.25 or 10.00 somatic embryos per 45 mg of callus, respectively (Table 3). The effects of cytokinin on somatic embryo production have also been observed in Corydalis yanhusuo (Sagare et al. 2000), where somatic embryos were induced in tuber-derived callus using cytokinins (zeatin, BAP, and KIN) without auxin. Similarly, Singh et al. (2003) reported that thidiazuron was a suitable cytokinin to produce somatic embryos in pigeonpea (Cajanus cajan).
In the present study, the production of somatic embryos was asynchronous: Somatic embryos at different morphological stages and of different sizes could be seen on the same explant at the same time (Fig. 2c ). Asynchronous somatic embryo development was also reported in Psidium guajava (Jayanthi and Mandal 2001; Rai et al. 2007).
Conversion to plantlets.
Following transfer of embryos onto conversion medium (Fig. 2d ), somatic embryo turned green after 1–2 wk, which indicated early embryo conversion (Fig. 2e ). Root elongation started spontaneously on the same conversion medium, beginning after 3 wk. After 5 wk of culture, all the somatic embryos that developed into complete plantlets were counted; the rate of conversion was 90%. Somatic embryo conversion using PGR-free medium has been obtained previously (Choi et al. 1998; Jayanthi and Mandal 2001; Rai et al. 2007).
Acclimatization and transfer to soil.
After 5 wk of in vitro culture in PGR-free MS medium, germinated somatic embryos were hardened and successfully established ex vitro (Fig. 2f ). The survival frequency was 80%, and the acclimatized plants were phenotypically similar to the mother plants.
Conclusion
The present report describes, for the first time, a regeneration procedure for T. hyemalis via somatic embryogenesis. The highest callus induction rate was obtained for nodal explants cultured on MS medium supplemented with 1.8 μM 2,4-D and 0.5 μM KIN. The genotype had a highly significant effect on callus induction. The maximum number of somatic embryos was obtained following transfer of callus to MS medium supplemented with 4.44 μM BAP, 0.54 μM NAA, and 4.65 μM KIN, with 90% conversion to plantlets. Plantlets were successfully acclimatized to ex vitro conditions. This protocol provides a successful and rapid technique that could be useful for commercial propagation, secondary metabolite production, synthetic seed production, and genetic transformation.
References
Abe T.; Futsuhara Y. Genotypic variability for callus formation and plants regeneration in rice. Theor App Genet 72: 3–10; 1986.
Anita W.; Agnieszka G.; Anna P.-B.; Norikazu T.; Sabina Z.; Rafal W.; Zbigniew P.; Stefan M.; Marcin F. Identification of genes up-regulated during somatic embryogenesis of cucumber. Plant Physiol Biochem 50: 54–64; 2012.
Bektas T.; Cengiz S.; Seyda B.; Ahmet A.; Askin A. H. Chemical composition, radical scavenging and antimicrobial activity of the essential oils of Thymus boveii and Thymus hyemalis. Rec Nat Prod 5: 208–220; 2011.
Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94: 223–253; 2004.
Choi Y. E.; Yang D. C.; Park J. C.; Soh W. Y.; Choi K. T. Regenerative ability of somatic single and multiple embryos from cotyledons of Korean ginseng on hormone-free medium. Plant Cell Rep 17: 544–551; 1998.
Daferera D. J.; Ziogas B. N.; Polissiou M. G. GC/MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J Agric Food Chem 48: 2576–2581; 2000.
Fadli M.; Saad A.; Sayadi S.; Chevalier J.; Mezrioui N.; Pagès J.-M.; Hassani L. Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection—bacteria and their synergistic potential with antibiotics. Phytomedicine 19: 464–471; 2012.
Gastaldo P.; Carli S.; Profumo P. Somatic embryogenesis from stem explants of Aesculus hippocastanum. Plant Cell Tissue Organ Cult 39: 97–99; 1994.
Gonçalves S.; Romano A. In vitro culture of lavenders (Lavandula spp.) and the production of secondary metabolites. Biotechnol Adv 31: 166–174; 2013.
Goodner K. L.; Mahattanataweea K.; Plotto A.; Sotomayor J. A.; Jordán M. J. Aromatic profiles of Thymus hyemalis and Spanish T. vulgaris essential oils by GC–MS/GC–O. Ind Crop Prod 24:264–268; 2006.
Gopi C.; Ponmurugan P. Somatic embryogenesis and plant regeneration from leaf callus of Ocimum basilicum L. J Biotechnol 126: 260–264; 2006.
Guo B.; Gao M.; Liu C. Z. In vitro propagation of an endangered medicinal plant Saussurea involucrata Kar. et Kir. Plant Cell Rep 26: 261–265; 2007.
Hassig B. L.; Nelson N. D.; Kidd G. H. Trends in the use of tissue culture in forest improvement. Nat Biotechnol 5: 52–59; 1987.
Jayanthi M.; Mandal P. K. Plant regeneration through somatic embryogenesis and RAPD analysis of regenerated plants in Tylophora indica. In Vitro Cell Dev Biol Plant 37: 576–580; 2001.
Jordán M. J.; Martínez R. M.; Goodner K. L.; Baldwin E. A.; Sotomayor J. A. Seasonal variation of Thymus hyemalis Lange and Spanish Thymus vulgaris L. essential oils composition. Ind Crop Prod 24: 253–263; 2006.
Kalemba D.; Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem 10: 813–829; 2003.
Kiran K. S.; Pooja B.-M.; Trevor A. T. Genetic transformation technology: status and problems. In Vitro Cell Dev Biol Plant 41: 102–112; 2005.
Kouakou T. H. Contribution à l’étude de l’embryogenèse somatique chez le cotonnier: évolution de quelques paramètres biochimiques au cours de la callogenèse et de la culture de suspension cellulaires. Thèse de doctorat N° 023/2003, Université de Cocody, Abidjan, Côte d’Ivoire; 2003.
Kumar G. K.; Thomas T. D. High frequency somatic embryogenesis and synthetic seed production in Clitoria ternatea Linn. Plant Cell Tissue Organ Cult 110: 141–151; 2012.
Lee C. Y.; Kim Y. K.; Kim Y. S.; Suh S. Y.; Lee S. Y.; Park S. U. Somatic embryogenesis and plant regeneration in Cnidium officinale Makino. J Med Plant Res 3: 096–100; 2009.
Lin G.; Zhao X.; Hong S. K.; Lian Y. Somatic embryogenesis and shoot organogenesis in the medicinal plant Pulsatilla koreana Nakai. Plant Cell Tissue Organ Cult 106: 93–103; 2011.
Martin K. P. Efficacy of different growth regulators at different stages of somatic embryogenesis in Eryngium foetidum L.––a rare medicinal plant. In Vitro Cell Dev Biol Plant 40: 459–463; 2004.
Martinez R. M.; Jordan M. J.; Quilez M.; Sotomayor J. A. Effects of edaphoclimatic conditions on Thymus hyemalis L. essential oil yield and composition. J Essent Oil Res 17: 614–618; 2005.
Murashige T.; Skoog F. A revised medium for the rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–479; 1962.
Natasa B.; Dunja L. L.; Sibila J. Rosmarinic acid synthesis in transformed callus culture of Coleus blumei Benth. Z Naturforsch C 59: 554–560; 2004.
Nhut D. T.; Truong T. T. A.; Nguyen T. D. H.; Nguyen T. D.; Nguyen T. H.; Nguyen Q. T.; Nguyen H. V. Effect of genotype, explant size, position, and culture medium on shoot generation of Gerbera jamesonii by receptacle transverse thin cell layer culture. Sci Hortic 111: 146–151; 2007.
Nieto G.; Bañón S.; Garrido M. D. Effect of supplementing ewes’ diet with thyme (Thymus zygis ssp. gracilis) leaves on the lipid oxidation of cooked lamb meat. Food Chem 125: 1147–1152; 2011.
Nieto G.; Díaz P.; Bañón S.; Garrido M. D. Effect on lamb meat quality of including thyme (Thymus zygis ssp. gracilis) leaves in ewes’ diet. Meat Sci 85: 82–88; 2010.
Pérez-Jiménez M.; López-Soto M. B.; Cos-Terrer J. In vitro callus induction from adult tissues of peach (Prunus persica L. Batsch). In Vitro Cell Dev Biol Plant 49: 79–84; 2013.
Prakash E.; Sha Valli Khan P. S.; Sairam Reddy P.; Rao K. R. Regeneration of plants from seed-derived callus of Hybanthus enneaspermus L. Muell., a rare ethnobotanical herb. Plant Cell Rep 18: 873–878; 1999.
Rai M. K.; Akhtar N.; Jaiswal V. S. Somatic embryogenesis and plant regeneration in Psidium guajava L. cv. Banarasi local. Sci Hortic 113: 129–133; 2007.
Ramchoun M.; Harnafi H.; Alem C.; Büchele B.; Simmet T.; Rouis M.; Atmani F.; Amrani S. Hypolipidemic and antioxidant effect of polyphenol-rich extracts from Moroccan thyme varieties. e-SPEN J 7: e119–e124; 2012.
Rota M. C.; Herrera A.; Martinez R. M.; Sotomayor J. A.; Jordan M. J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Cont 19: 681–687; 2008.
Rout G. R.; Samantaray S.; Das P. Manipulation and propagation of medicinal plants. Biotechnol Adv 18: 91–120; 2000.
Saad A.; Fadli M.; Bouaziz M.; Benharref A.; Mezrioui N.-E.; Hassani L. Anticandidal activity of the essential oils of Thymus maroccanus and Thymus broussonetii and their synergism with amphotericin B and fluconazole. Phytomedicine 17: 1057–1060; 2010.
Sáez F. Essential oil variability of Thymus hyemalis growing wild in Southeastern Spain. Biochem Syst Ecol 23: 431–438; 1995.
Sáez F. Variability in essential oils from populations of Thymus hyemalis Lange in Southeastern Spain. J Herbs Spices Med Plants 5: 65–76; 1998.
Sáez F. Volatile oil variability in Thymus serpylloides ssp. gadorensis growing wild in Southeastern Spain. Biochem Syst Ecol 29: 189–198; 2001.
Sagare A. P.; Lee Y. L.; Lin T. C.; Chen C. C.; Tsay H. S. Cytokinin-induced somatic embryogenesis and plant regeneration in Corydalis yanhusuo (Fumariaceae)—a medicinal plant. Plant Sci 160: 139–147; 2000.
Shinde A. N.; Malpathak N.; Fulzele D. P. Determination of isoflavone and antioxidant activity in Psoralea corylifolia L. callus cultures. Food Chem 118: 128–132; 2010.
Shohael A. M.; Ali M. B.; Yu K. W.; Hahn E. J.; Paek K. Y. Effect of temperature on secondary metabolites production and antioxidant enzyme activities in Eleutherococcus senticosus somatic embryos. Plant Cell Tissue Organ Cult 85: 219–228; 2006.
Singh N. D.; Sahoo L.; Sarin N. B.; Jaiwal P. K. The effect of TDZ on organogenesis and somatic embryogenesis in pigeonpea (Cajanus cajan L. Millsp). Plant Sci 164: 341–347; 2003.
Sotomayor J. A. Estudio sobre plantas aromàticas de los géneros Salvia y, espontáneas en el Sureste Ibérico, para su establecimiento como cultivo. Doctoral thesis, Departamento de Biologıa Vegetal (Botànica), University of Murcia, Murcia, Spain; 1998.
Stahl-Biskup E.; Hiller K.; Loew D. Thymian. In: Wichtl M. (ed) Teedrogen und Phytopharmaka. 5th ed. Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart, pp 664–666; 2009.
Stahl-Biskup E. Thymus. In: Blaschek W.; Elbel S.; Hackenthal E.; Holzgrabe U.; Keller K.; Reichling J.; Schulz V. (eds) Hagers Enzyklopädie. 6th ed. Springer-Verlag, Berlin, pp 729–759; 2007.
Stahl-Biskup E.; Sáez F. Thyme. The genus Thymus. Medicinal and aromatic plants—industrial profiles, vol 24. Taylor & Francis, London and New York; 2002.
Tiwari V.; Tiwari K. N.; Singh B. D. Shoot bud regeneration from different explants of Bacopa monniera (L.) Wettst. by trimethoprim and bavistin. Plant Cell Rep 25: 629–635; 2006.
Yan M. M.; Xu C.; Kim C. H.; Um Y. C.; Bah A. A.; Guo D. P. Effects of explant type, culture media and growth regulators on callus induction and plant regeneration of Chinese jiaotou (Allium chinense). Sci Hortic 123: 124–128; 2009.
Youdim K. A.; Deans S. G. Beneficial effects of thyme oil on age-related changes in the phospholipids C20 and C22 polyunsaturated fatty acid composition of various rat tissues. Biochim Biophys Acta 1438: 140–146; 1999.
Zarzuelo A.; Crespo E. The medicinal and non-medicinal uses of thyme. In: Stahl-Biskup E.; Sáez F. (eds) Thyme: the genus Thymus. Medicinal and aromatic plants—industrial profiles, vol 24. Taylor & Francis, New York, pp 263–292; 2002.
Acknowledgments
We thank the Bni Boufrah association for providing seeds and Dr. A. Ennabili for identification of the botanical name of Thymus species. We also thank Drs. S. M. Udupa and D. Iraqi for their help. This research program was supported by the National Institute of Medicinal and Aromatic Plants, Taounate, Morocco.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Frans Krens
Rights and permissions
About this article
Cite this article
Nordine, A., Tlemcani, C.R. & El Meskaoui, A. Regeneration of plants through somatic embryogenesis in Thymus hyemalis Lange, a potential medicinal and aromatic plant. In Vitro Cell.Dev.Biol.-Plant 50, 19–25 (2014). https://doi.org/10.1007/s11627-013-9577-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-013-9577-x