Abstract
We describe an efficient protocol for callus induction from adult tissues of Prunus persica (L.) Batsch. Three different commercial peach genotypes, Early May®, Zise May®, and UFO-3®, plus three other genotypes from hybrid crosses performed in February 2006, PS108, PS208, and PS708, were used in the study. Thirteen explant treatments were tested using nine different plant parts. Murashige and Skoog and woody plant medium salts were assayed with several concentrations of 2,4-dichlorophenoxyacetic acid (2,4-D), kinetin (KN), and thidiazuron, and two different photoperiods were tested, a 16-h photoperiod or continuous darkness. In terms of the quantitative response, two parameters were assessed: the number of d to callus induction and relative callus growth recorded after 30 d. Woody plant medium supplemented with 2,4-D and KN significantly increased the rates of callus induction in the majority of treatments. And no significant differences among the P. persica genotypes were found. The explants derived from stem and calyx produced up to 85 and 96% callus induction, respectively. The protocol described here could be used for efficient callus induction in a range of Prunus spp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Callus is an amorphous and dedifferentiated tissue composed of disorganized cells. It may be produced naturally in response to insect or microorganism attack or stress (George 1993). Several in vitro biotechnological techniques have been developed, all of which require a reliable protocol to produce a responsive cell mass. Unorganized cells of Prunus spp. have traditionally been cultured for protoplast fusion of different individuals, somatic hybridization (Hidano and Niizeki 1988), to obtain haploids (Peixe et al. 2004), or for induction of tolerance to low temperatures (Arora and Wisniewski 1995). Callus induction has also been used for genetic transformation (Scorza et al. 1994) and adventitious regeneration (Gentile et al. 2002), which is the initial phase in a transformation protocol.
Peach is one of the most widely consumed fruits in the world, but its recalcitrance in many biotechnological processes has hindered the advance of in vitro techniques. For most purposes, in vitro callus establishment is important as an intermediate step in peach biotechnology. Most of the advances made in peach have used embryo-derived explants. The main disadvantage of developing a protocol from seed-derived material is that each genotype is unique and not a clone of the parent (Abbott et al. 2008). Development of these biotechnological tools from mature tissues is important for the improvement of desirable commercial cultivars that have been selected for beneficial features, which may not be present in naturally produced seeds. Only a few authors have developed somatic regeneration protocols using adult peach material (Gentile et al. 2002; Pérez-Jiménez et al. 2012).
Many factors affect callus induction as well as its growth and development in vitro, namely, the type of explant tissue, quality and type of light and photoperiod conditions, plant growth regulators, culture media, gelling agent, pH, temperature, and many others. Plant growth regulators particularly influence callus induction; a phase in which auxins play a major role by inducing callus proliferation and development (Paris et al. 2004). Different types of callus are frequently developed for different purposes. A white and globular callus has been used for embryogenesis (López-Pérez et al. 2005; Hammerschlag et al. 1985), while green and nodular calli are used for organogenesis (Gentile et al. 2002; Pérez-Jiménez et al. 2012). Thus, a thorough study of all the factors involved is important for determining the choice of protocol for the type of callus required.
This work aimed to develop an efficient and reproducible protocol for obtaining callus from adult material in peach, by comparing different types of explant, culture media, growth regulators, and culture conditions. The results will be useful to develop further studies, to regenerate plants, protoplast cultures, and to obtain metabolites from in vitro cultures.
Materials and Methods
Plant material.
Plant tissues were collected from 4-yr-old peach (Prunus persica L. Batsch) trees grown at the Torreblanca experimental field station of the Instituto Murciano de Investigación y Desarrollo Agrario y Alimentario, Murcia, Spain. Three commercial varieties Early May® (nectarine), UFO-3® (flat peach), and Zise May® (peach) and another three preselected varieties from a peach breeding program using hybrid crosses PS108 (Early May® × N-292), PS208 (Early May® × UFO-3®), and PS708 (Zise May® × Early May®) were used as explant sources. The plants were watered daily using a drip irrigation system with 700 m3 ha−1/mo at the time of sampling. The samples for callus induction experiments were taken from young branches producing their first shoots after flowering in April 2010.
In vitro culture establishment.
Branches and flowers in the balloon stage from the selected genotypes were disinfected in a solution of 2% (v/v) sodium hypochlorite and 0.1% (v/v) Tween 20 for 2 h. After disinfection, the plant material was transferred to a laminar flow cabinet to separate the plant organs. Six sections of vegetative organs (fully expanded leaf blade, petiole, juvenile leaf, stem, terminal buds, and axillary buds) and three reproductive organs (calyx, petals, and stamens) were selected and cultured as follows: leaf explant with the abaxial leaf surface facing the medium, with and without midvein; leaf explant with the adaxial leaf surface facing the medium, with and without midvein; approximately 1 cm long stem explants plated in an upright or inverted position; juvenile leaf; terminal bud; axillary bud; petiole; calyx; petals; and stamens (Fig. 1).
Culture media.
The explants were cultivated on four different media: Murashige and Skoog medium (MS; Murashige and Skoog 1962) supplemented with 1.2 mg l−1 of 2,4-dichlorophenoxyacetic acid (2,4-D) and 1 mg l−1 of kinetin (KN, MS-DK); MS supplemented with 1.2 mg l−1 of 2,4-D and 1 mg l−1 thidiazuron (TDZ; MS-DT); woody plant medium (WPM; Lloyd and McCown 1980) with 1.2 mg l−1 of 2,4-D and 1 mg l−1 of KN (WPM-DK); and WPM supplemented with 1.2 mg l−1 of 2,4-D and 1 mg l−1 TDZ (WPM-DT). All media contained 3% (w/v) sucrose and 0.7% (w/v) of plant propagation agar (Pronadisa®), in Petri dishes (12 mm ø). The pH was adjusted to 5.7 with KOH (0.1 N) prior to autoclaving for 16 min at 1.1 kg cm−2 (122°C).
Culture conditions.
The cultures were incubated in a climatic chamber at 25 ± 1°C with two different light regimes. Half of the plates (selected randomly) with the same type and number of explants were kept under 16 h light (45 μmol m−2 s−1, GRO-LUX, Sylvania, Surrey, UK) photoperiod and the other half in constant (24 h) darkness.
Experiment design and data collection.
Data were collected from 28,080 explants. Four media were tested with 15 explants for each of the 13 types, 6 genotypes, and 2 different light conditions with three replications. During the study, the following parameters were measured: percentage of explants in which callus was induced, d to the appearance of callus, color and type of callus produced, and callus relative growth (CRG)—which refers to the percentage of explant covered by callus. Data were recorded each day to know exactly when callus started to appear, and at 30 d, CRG was recorded, as well as the number of explants and which part of the explant that the callus growth originated. No subcultures were performed during the experiment. Significance was determined by analysis of variance and the least significance (P ≤ 0.05) differences among mean values were estimated using Duncan's new multiple range test.
Results and Discussion
Callus was induced in all six genotypes of P. persica cultivated on four different media supplemented with 2,4-D, KN, or TDZ and two light regimes: 16 h light and constant darkness. Disinfection resulted in effective prevention of contamination and only 1.4% of explants died from the process. No significant genotypic differences were observed for any of the three parameters assessed for the eight different treatments (Table 1). A high percentage of callus formation was demonstrated for all the six genotypes, where the percentage of explants developing callus ranged from almost 80% in PS208 to a maximum of 91% in PS708. Generally, explants started to develop callus from 14 to 16 d after the experiment commenced (Table 1). The amount of callus induced was statistically the same in all the studied genotypes; therefore, further studies in callus induction did not discriminate between peach genotypes.
Thirteen treatments with a variety of explant types were tested for callus induction (Table 2). Statistical differences were observed between groups; however, all the genotypes provided a high level of callus induction for the different explants (above 80%), and all responded in a similar timeframe. The only exception was the stamen explants which were completely unresponsive to all media and conditions tested. Due to the small standard deviation, there were three statistical groups: the stamens and the other two in which the differences between both had a small statistical significance. The lack of response from anthers was unexpected since callus induction from anthers has been reported previously in Prunus species. Long et al. (1994) and Peixe et al. (2004) obtained callus from anthers in Prunus avium and Prunus armeniaca, respectively. Both authors used culture media different to the ones used in these experiments, with the exception of Peixe et al. (2004), who also used MS salts, although their best result was obtained with Nitsch and Nitsch medium. As regards the percentage of explants that produced callus, the rates ranged from 82.37 to 98.96%, and the average time when the callus started to develop ranged from 14.4 to 17.3 d, similar to the results obtained in almond by Işikalan et al. (2010).
Of the 13 explant sources and treatments tested, significant differences were also observed in CRG, which measured the percentage of explant covered by callus after 30 d of in vitro culture (Table 3). Of the floral tissues, the calyx surface showed the highest CRG (70–96%). Whereas for the vegetative tissues, the stem treatments (explants plated in either the upright or inverted positions) and the petiole gave the highest CRG. Stem explants plated in the upright position showed surface callus formation covering between 30.17 and 85.56% of the tissue (similar to the stem-in inverted position), while the petiole showed a CRG of 42.33 and 71.67%. It has been found that the explant source strongly influences the callus induction process. Previous reports have observed the same effect in other species, such as maize (Green and Phillips 1975) and sugarcane (Guiderdoni and Demarly 1988). Also, Declerck and Korban (1996) reported similar findings in peach, as did Ansley et al. (2000) for almond explants. In this study, explants consisting of leaves without a midvein presented a higher percentage of callus development than leaves with midvein. It could be due to the fact that leaves without midvein have a greater surface area of wounded tissue, which is known to be more conducive to callus growth.
With regard to the culture media, taking into account all the treatments, WPM salts were the most successful in all the tested explants except for calyx and stem (inverted position) explants, for which, MS salts produced the higher callus induction rate (Table 3). Zhou et al. (2010) also found WPM to be more favorable for callus initiation than MS in a regeneration study in peach rootstocks. WPM-DK was the most successful treatment, inducing substantial callus growth in either of the light conditions tested; 64.58% of the CRG in darkness and 38.78% under a 16-h photoperiod. MS-DT was the second most prolific medium for callus induction, with 43.52 and 35.81% CRG for 0 and 16 h light, respectively. Therefore, the results show that there is a combined effect induced by the culture medium and the plant growth regulators used.
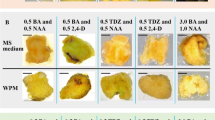
The callus obtained from explants exposed to the 16-h photoperiod had a green and very compact texture in vegetative tissues (Fig. 2A–C ), whereas in petals (Fig. 2F ) and calyx (Fig. 2G ), the calli were mainly pink in color. The stamens (Fig. 2E ) turned pink, but no callus was formed. The appearance of calli in green tissues varied slightly depending on the explant type, but in general, it was composed of small green globular structures (Fig. 2A, B ). In young leaf explants (Fig. 2A ), the presence of callus was found mainly in the petiole or along the cut surface. These results agree with the previous research of Zhou et al. (2010) where young leaves of the hybrid P. persica × Prunus davidiana were tested. In this study, under 16 h light conditions, the callus that developed from buds and stems differed from leaves, being limited by the outer structure of the stems (Fig. 2C ) and buds, while the callus on leaf tissue was unconstrained and globular. The appearance of all calli induced in the dark was the same, regardless of the explant type (Fig. 2D ) or the culture media. These calli were white to yellow, smooth, friable, and more voluminous than those obtained under light conditions. In all cultures, irrespective of exposure to light, the callus initially developed from cut tissue surfaces. Declerck and Korban (1996) tested different concentrations of auxins (2,4-D, dicamba) and cytokinins (BA, zeatin, kinetin, TDZ) in callus induction of peach. These authors maintained that in leaf tissues of P. persica, cytokinins are more likely to produce chlorophyllous and compact callus cultures, whilst auxins increase callus production, inducing friable callus. To the best of our knowledge, those typologies are produced by the light since, in our study, the same media have been used in both conditions and the results were the same in the 13 peach explants, genotypes, or treatments tested. Both typologies of peach callus we observed have been previously described as being embryogenic (white callus; Svircev et al. 1993) or organogenic (green callus; Gentile et al. 2002; Zhou et al. 2010; Pérez-Jiménez et al. 2012). Friable, white callus can be used effectively in cell suspension cultures due to its propensity to crumble (Bhansali et al. 1991).
Differences between treatments and explants in callus induction of peach after 30 d. (A) Calli induced in a young leaf under a 16-h light photoperiod. (B) Callus induced in a leaf explant without midvein with the abaxial leaf surface facing the medium under a 16-h light photoperiod. (C) Callus induced in a stem explant plated in upright position under a 16-h photoperiod. (D) Calli induced in leaves with midvein and the abaxial leaf surface facing the medium, in the dark. (E) Stamens with no callus. (F) Callus induced in petals under a 16-h photoperiod. (G) Callus induced in calyx under a 16-h photoperiod.
In summary, this study demonstrates that the growth of P. persica calli is greatly influenced by the type of explant, the combination of plant growth regulators, culture media, and light conditions. The media composed of WPM supplemented with 2,4-D and KN induced a higher percentage of callus than the other media tested. A 16-h light photoperiod or constant darkness can be applied for callus induction for different purposes. White to yellow and friable callus was obtained under dark conditions and green compact, nodular callus was produced when explants were cultured in the light. The calyx was the most productive explant with regards to callus induction, followed by the vegetative explants, buds, stems, and petioles. No callus was obtained from the anthers or filaments with the conditions used in this study.
References
Abbott AG, Arús P, Scorza R (2008) Genetic engineering and genomics. In: Layne DR, Bassi D (eds) The peach: botany, production and uses. CABI, Wallingford, pp 85–105
Ansley PJ, Collins GG, Sedgley M (2000) Adventitious shoot regeneration from leaf explants of almond (Prunus dulcis Mill.). In Vitro Cell Dev Biol Plant 36:470–474
Arora R, Wisniewski ME (1995) Ultrastructural and protein changes in cell suspension cultures of peach associated with low temperature-induced cold acclimation and abscisic acid treatment. Plant Cell Tiss Organ Cult 40:17–24
Bhansali RR, Driver JA, Durzan DJ (1991) Somatic embryogenesis in cell suspension cultures of Prunus persica (L.). J Hortic Sci 66:601–605
Declerck V, Korban SS (1996) Influence of growth regulators and carbon sources on callus induction, growth and morphogenesis from leaf tissues of peach (Prunus persica L. Batsch). J Hortic Sci 71:49–55
Gentile A, Monticelli S, Damiano C (2002) Adventitious shoot regeneration in peach (Prunus persica (L.) Batsch). Plant Cell Rep 20:1011–1016
George EF (1993) Plant propagation by tissue culture. Part 1. The technology. Exegetics Ltd, Edington, p 14
Green CE, Phillips RL (1975) Plant regeneration from tissue cultures of maize. Crop Sci 15:417–427
Guiderdoni E, Demarly Y (1988) Histology of somatic embryogenesis in cultured leaf segments of sugarcane plantlets. Plant Cell Tiss Organ Cult 14:71–88
Hammerschlag FA, Bauchan G, Scorza R (1985) Regeneration of peach plants from callus derived from immature embryos. Theor Appl Genet 70:248–251
Hidano Y, Niizeki M (1988) Protoplast culture of deciduous fruit tree. Sci Hortic 37:201–216
Işikalan Ç, Akbas F, Namli S, Başaram D (2010) Adventitious shoot development from leaf and stem explants of Amygdalus communis L. cv. Yaltinski. Plant Omics J 3:92–96
Lloyd G, McCown B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Proc Int Plant Propag Soc 30:421–427
Long CM, Mulinix CA, Iezzoni AF (1994) Production of a microspore-derived callus population from sweet cherry. HortSci 29:1346–1348
López-Pérez AJ, Carreño J, Martínez-Cutillas A, Dabauza M (2005) High embryogenic ability and plant regeneration of table grapevine cultivars (Vitis vinifera L.) induced by activated charcoal. Vitis 44:79–85
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Paris R, Pratesi D, Negri P (2004) In vitro morphogenic ability of mature or embryonic apricot tissues. Acta Horticult 663:487–490
Peixe A, Barroso J, Potes A, Pais MS (2004) Induction of haploid morphogenic calluses from in vitro cultured anthers of Prunus armeniaca cv. ‘Harcot’. Plant Cell Tiss Organ Cult 77:35–41
Pérez-Jiménez M, Carrillo-Navarro A, Cos-Terrer J (2012) Regeneration of peach (Prunus persica L. Batsch) cultivars and Prunus persica × Prunus dulcis rootstocks via organogenesis. Plant Cell Tiss Organ Cult 108:55–62
Scorza R, Ravelonandro M, Callahan AM, Cordts JM, Fuchs M, Dunez J, Gonsalves D (1994) Transgenic plums (Prunus domestica L.) express the plum pox virus coat protein gene. Plant Cell Rep 14:18–22
Svircev AM, Biggs AR, Miles NW (1993) Peach regeneration from callus derived from embryos of selected cultivars. Fruit Var J 47:13–16
Zhou H, Li M, Zhao X, Fan X, Guo A (2010) Plant regeneration from in vitro leaves of the peach rootstock ‘Nemaguard’ (Prunus persica × P. davidiana). Plant Cell Tiss Organ Cult 101:79–87
Acknowledgments
This research was supported by the Instituto Nacional de Investigaciones Agrarias (INIA) (RTA2008-00121-00-00) and by a fellowship provided by INIA to Margarita Pérez-Jiménez.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Forster
Rights and permissions
About this article
Cite this article
Pérez-Jiménez, M., López-Soto, M.B. & Cos-Terrer, J. In vitro callus induction from adult tissues of peach (Prunus persica L. Batsch). In Vitro Cell.Dev.Biol.-Plant 49, 79–84 (2013). https://doi.org/10.1007/s11627-012-9466-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-012-9466-8