Abstract
Saffron (Crocus sativus L.) is a monocotyledonous plant propagated via corms, but recently several alternative methods have been reported. To find the conditions suitable for saffron shoot formation from corms, the effect of different concentrations of the plant growth regulatory cytokinins N6-benzyladenine (BA) and N-phenyl-1, 2,3-thidiazol-5-ylurea, commonly known as thidiazuron (TDZ), were compared. In all corm explants, an average of 39.5 ± 5.1 shoots per corm were induced by 4.54 μM TDZ, whereas only 3.6-11.4% by BA. The outstanding result in the shoot formation stage is the generation of globular, translucent structures that are morphologically similar to globular embryos. To optimize the plant regeneration from the induced adventitious shoots obtained from the TDZ treatment, the shoots were transferred to MS and B5 media supplemented with different concentrations and combinations of NAA and BA. The highest rate of plant regeneration from developing shoots was observed in the B5 medium containing 2.22 μM NAA and 2.68 μM BA. With optimized hormonal conditions, an average of 19.55 ± 5.75 shoots and 3.18 ± 1.5 roots per explants were obtained. Based on this experiment, a simple, new and efficient protocol is presented to produce numerous plants from induced corm explants of saffron.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Saffron (Crocus sativus L.) belongs to the Iridaceae family. Its valuable dried spice that accumulates in the stigmas is widely used for coloring and flavoring many foods. The production of stigmas requires a large number of flowers: 160 kg of flowers are estimated to be needed to obtain 1 kg of dried stigmas (Tirillini et al. 2006). Currently, saffron is cultivated intensely in Iran, India, Greece, Morocco, Spain, Italy, Turkey, France, Switzerland, Pakistan, Azerbaijan, China, Egypt, the United Arab Emirates, Japan, and recently in Australia (Tasmania). The world’s total annual saffron production is estimated at 205 t per year, of which Iran produces 80% (160 t); the Khorasan province alone accounts for 46,000 ha and 137 t (Fernández 2004).
Saffron is a triploid plant conventionally propagated by corm. Yield and quality improvement of saffron through breeding have not been possible because of sterility problems (Dhar et al. 1988). In vitro culture is greatly advantageous for mass propagation and development of disease-resistant cultivars, mainly for vegetatively propagated crops such as saffron. Plant growth regulatory substances play a crucial role in the different stages of the plant micropropagation; for example, addition of cytokinins such as N6-benzyladenine (BA) and kinetin to the culture medium is essential for sprouting and formation of multiple shoots from in vitro explants. N-phenyl-1,2,3-thidiazol-5-ylurea or thidiazuron (TDZ), a substituted phenylurea, is another potent bioregulator of in vitro morphogenesis of plants (Huetteman and Preece 1993; Singh et al. 2003).
There are several reports on the effects of TDZ and BA alone or in combination on shoot formation in different plants (Nielsen et al. 1995; Wilhelm 1999). In some plants, TDZ is more effective than adenine-based compounds for inducing adventitious shoot regeneration [e.g., indirectly in Vitis vinifera cvs. (Reisch and Martens 1988) and directly in Rhododendron sp. (Imel and Preece 1988) and Malus sp. (Elobeidy and Korban 1988)]. TDZ can also be highly effective in inducing axillary shoot formation in shoot cultures [e.g., in Rorippa sp. (Gilby and Wainwright 1989), Rhododendron sp. (Fellman et al. 1987), and Malus domestica (Fasolo et al. 1988a, b)]. Although many shoots can be formed, their elongation might not be sufficient. Effective shoot proliferation was seen in Acer fremanii with 1 μM BA and 0.01 μM TDZ (Kerns and Meyer 1986) and in Pyrus communis with 5 μM BA and 0.4 mM TDZ (Singha and Bhatia 1988), but the induced shoots could not be used for further propagation.
Plant regeneration and shoot formation through organogenesis have been shown to be induced in saffron by 2,4-dichlorophenoxyacetic acid (2,4-D) and zeatin. Shoot growth and callus proliferation can be obtained by combinations of NAA and BA (Ilahi et al. 1987), while the combined effects of 2,4-D, BA, and kinetin in shoot regeneration have been reported as well (Isa and Ogasawara 1988). So far, no data are available on the effect of TDZ compared to that of BA on shoot formation of saffron plants.
One of the research lines of our laboratory is the regeneration of shoots and corms from different parts of saffron by using several combinations of 2,4-D, BA, or NAA. The optimal conditions for shoot regeneration were (MS (Murashige and Skoog; 1962)) medium supplemented with 1 mg/l NAA and 1 mg/l BA (Ebrahimzadeh et al. 1996). Shoot regeneration from microcalli formed from protoplast division (Ebrahimzadeh et al. 2000) is another topic.
Here, we study in detail the effects of TDZ and BA on callus and shoot induction and optimize the use of these plant growth regulators for developing a new procedure for micropropagation of corm explants of saffron.
Materials and Methods
Plant media.
The basal media MS, including vitamins and B5 (Gamborg et al. 1968), were used. Plant media were supplemented with 30 g/l (3% w/v) sucrose and solidified with 7 g/l (0.7% w/v) (agar-agar (Merck, Darmstadt, Germany)). The pH was adjusted to 5.7 with 1 M NaOH. The media were autoclaved at 120 °C for 20 min, whereafter plant hormones in a dimethyl sulfoxide (DMSO) stock solution were added. All plant media, growth regulators, and DMSO were purchased from Duchefa (Haarlem, The Netherlands) and Merck (Darmstadt, Germany). Depending on the experiment, plant growth regulators were added to the two media. For callus induction, the culture media were supplemented with 1.13, 4.54, or 9.08 μM TDZ and 2.22, 8.87, or 17.75 μM BA. For callus proliferation, shoot growth, and multiplication, the MS and B5 media contained 0.05% activated charcoal with the following concentrations of plant hormones: 2.22 μM NAA + 2.68 μM BA, 4.44 μM NAA + 5.37 μM BA, and 8.88 μM NAA + 10.74 μM BA.
Plant materials.
Healthy resting corms were collected between August and October 2006 from the research farm of the Faculty of Science, University of Tehran (Mardabad, Karaj, Iran). Corms were washed in running water for 30 min. After surface disinfection with detergent (dish-washing liquid), they were soaked in hygen (1% benzalconium chloride) for 10 min, then rinsed in tap water. Corms explants were transferred into a sterile laminar air flow cabinet, incubated first in 70% ethanol for 2 min, then in 20% (v/v) commercial bleach containing 1% sodium hypochlorite for 15 min, and rinsed three times in distilled sterile water. A rectangular section from the central meristematic region of each corm was cut as starting material. Experiments were done in two series. For each experiment, 25 corm explants per treatment were placed on shoot-inducing media and incubated in the dark at 25 ± 3 °C for 14 wk to allow callus induction. Explants with induced shoots were then transferred into shoot-growth medium-containing jars and maintained under a 16-h/8-h photoperiod for further growth.
Light microscopy.
The translucent nodular callus structures on the corm explants were isolated and prefixed for 2 h with 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer (PBS) at pH 7.4. After the calluses had been washed twice with PBS and fixed with 1% osmium tetraoxide (OSO4), they were dehydrated in graded acetone and embedded in epoxy 812 resin (TAAB, Berkshire, UK). With an ultra-microtome apparatus (Leica Ultracut-UCT), 500-nm-thick blocks were sectioned. The semi-thin sections were stained in 1% toluidine blue and 1% borax and observed with an Olympus BH-2 light microscope.
Scanning electron microscopy.
To optimize the quality of the sample fixation and the scanning electron microscopy (SEM) photographs, two methods were tested. (a) Corm explants with nodular calluses and/or developed shoots from a culture on B5 medium and 9.08 μM TDZ were fixed with 2.5% glutaraldehyde in 0.1 M PBS for 90 min at 4 °C and rinsed twice (15 min each) in the same buffer. The fixed samples were postfixed in 2% OSO4 for 1 h, followed by rinsing with PBS buffer for 5–10 min, and dehydrated through a graded ethanol (25%, 50%, 70%, 90%, and 100%) series for 15 min. After dehydration, the tissues were air-dried and sputter-coated with gold. Samples were examined and photographed with a scanning electron microscope (Zeiss, Jena, Germany). (b) In the alternative method, corm explants with nodular calluses grown on the B5 medium containing 9.08 μM TDZ were fixed in liquid nitrogen and coated with gold with the sputter coater (model SCD005; BAL-TEC, Balzers, Liechtenstein). Samples were photographed with a Philips XL30 scanning electron microscope.
Statistical analysis.
The significance of the differences in the callus induction frequency was calculated by cross tabulation and Pearson χ 2. The factor interaction was recognized by the general linear model with the Bonferroni test. The analysis of variance between averages was calculated, and significant differences were found by the Duncan multiple range test at P < 0.05 (with the SPSS 14.0 for Windows evaluation Version; SPSS, Chicago, IL).
Results
Callus induction.
The production of calluses was optimized with different cytokinins in the MS or B5 media. The maximal and minimal number of calluses was formed in MS medium supplemented with 4.54 μM TDZ and 8.87 μM BA, respectively. Indeed, all explants produced calluses on MS medium containing 4.54 μM TDZ, while the lowest callus production (3.6%) was obtained on MS medium containing 8.87 μM BA (Table 1). According to the χ 2 test, the callus induction differences were significant between the media containing BA or TDZ as well as between the different TDZ concentrations in the MS medium. The interaction of different concentrations of TDZ in B5 an MS media was shown in Fig. 1.
Effect of TDZ on callus induction and shoot formation.
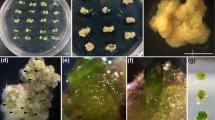
TDZ, one of the most active cytokinin-like substances, is frequently used in plant tissue culture. On TDZ-containing MS or B5 media, shoot buds were formed on corm explants with an intermediate nodular and embryo-like callus structure (Fig. 2), whose detailed morphological structures are visible on the SEM images (Fig. 2). The multiple shoot buds that were formed on the corm explants had a globular-to-leaf shape or looked similar to shoots, but no root pole was observed (Fig. 2). To identify the embryo-like structures, the semi-sections from the globular shapes were studied by light microscopy (Fig. 2). Histological examination showed the presence of a leaf primordium and meristematic dome (Fig. 2). Vascular elements occurred in some sections (Fig. 2) and parenchymatous cells with young amyloplasts appeared in most sections (Fig. 2). Data analysis indicates that the average of the induced shoots from corm explants grown on B5 medium was higher than that of those grown on MS medium, although the maximum shoots per explant (39.5 ± 5.1) and the maximum number of shoots per cubic centimeter (26.1 ± 7.6) had been found in explants on MS medium containing 4.54 μM TDZ (Table 1). The number of regenerating structures from explants that had been kept on B5 or MS media containing 4.54 or 9.08 μM TDZ (Table 1) differed significantly. At 4.54 μM TDZ, the number of shoots per explant in the MS medium was twice that on the B5 medium, but at 9.08 μM TDZ, the number of shoots per explant was approximately fivefold higher in the B5 than in the MS medium. However, the number of induced shoots per explant did not correlate with the different concentrations of the growth regulators. In other words, augmenting the concentration of the plant growth regulators in both media did not increase the number of shoots (Table 1).
In vitro manipulation and microscopical analysis of saffron. (a) morphology of BA-induced shoot formation, (b) globular-to-heart, translucent, embryo-like structures induced by TDZ, (c) SEM of translucent, embryo-like structures, (d) globular-to-leaf structures induced by TDZ. (e) SEM of globular structures, (f) semi-section of one of the globules observed under light microscopy showing meristematic dome and leaf primordium, (g) rectangular cells with xylem, (h) peripheral cells of medullar meristem, full of young amyloplasts, (i) multiple shoot formation induced by TDZ, (j) SEM of shoots, (k) regeneration after 25 wk on B5 medium containing a combination of NAA and BA; from left to right: 2.22 μM NAA + 2.68 μM BA; 4.44 μM NAA + 5.37 μM BA; and 8.88 μM NAA + 10.74 μM BA), (l) regeneration after 25 wk on MS medium containing a combination of NAA and BA; from left to right: 2.22 μM NAA + 2.68 μM BA; 4.44 μM NAA + 5.37 μM BA; and 8.88 μM NAA + 10.74 μM BA, (m) regenerated plants, and (n) transfer of plants to pots.
Effect of BA.
On corm explants treated with BA, shoots were formed, but embryo-like structures and translucent nodular tissues were not observed (Fig. 2). The nodular organogenic calluses appeared on the surface of those explants that were able to develop white shoots (Fig. 2). Shoot induction and the number of shoots per explant were higher in all ranges of BA concentration (2.22, 8.87, and 17.75 μM) on the B5 than on the MS medium, but differences were not statistically significant (Table 1). The rates of organogenic callus induction on the explants grown on BA-containing MS or B5 media ranged from 3.6% to 11.4%. The maximum shoot induction (5.7 ± 2.8) was seen in explants placed on the B5 medium, whereas the number of shoots per explant (0.4 ± 0.4) was the lowest on the MS medium containing the same concentration of BA (17.75 mM).
Plant regeneration.
No significant differences were observed in shoot and root regeneration from explants growing either on the plant hormone-containing MS or B5 media (Table 2). Regarding the different combinations of NAA and BA, the maximum number of roots (19.55 ± 5.75) was formed on B5 media containing 2.22 μM NAA and 2.68 μM BA.
Microscopical analysis.
SEM was used to analyze the globular shape, the oval shape, and the morphology of developed shoots (Fig. 2). Although the appearance of the globular structures resembled that of globular embryos, the multiple shoot structure was obviously distinct from individual mature embryos loosely connected to the basal tissues (Fig. 2).
To identify the structures in more detail, translucent nodules were examined with light microscopy. Similar structures were found at different frequencies in all treatments. Three corm explants with nodular structures grown from samples on medium containing 9.08 μM TDZ were fixed and observed by light microscopy. Although the morphology of these structures corresponded to that of the globular stage of embryos (Fig. 2), the histological analysis showed a similarity between the regenerated globular structures and the shoot buds (Fig. 2). Further observations indicated a continuous vascular supply from the shoot explants to the vasculature of the callus (Fig. 2). For the histological analysis with the light microscope, three nodule-like embryos were fixed and sectioned. No embryogenic tissues were found, and the vast majority of cells was parenchymatous, i.e., the cytoplasm was strongly vacuolated with large amyloplasts. In addition, spiral vessels (elements of the xylem bundle) were visible (Fig. 2). These nodules developed into shoots during further incubation (Fig. 2).
Discussion
Hormones.
When compared to that of BA, the impact of TDZ on multiple shoot formation has been shown to be superior in many plant species (Karam and Al-Majathoub 2000; D’Onofrio and Morini 2005; Geneve 2005). At concentrations higher than 1 μM, TDZ can stimulate the formation of calluses, adventitious shoots, or somatic embryos (Huetteman and Preece 1993). The synchronous effect of TDZ on organogenesis and somatic embryogenesis was observed in many plants; for example, in pigeonpea (Cajanus cajan L.) at low (0–5 μM) and high (10–20 μM) concentrations, TDZ caused shoot organogenesis and somatic embryogenesis, respectively (Singh et al. 2003). Some reports support the effects of low concentrations of TDZ on shoot formation; for instance, in soybean (Glycine max), the optimal concentration of TDZ for organogenesis was 2–10 μM in the B5 medium (Yoshida 2002) and in taro (Colocasia esculenta (L.) Schott), organogenesis was induced by 2 μM TDZ (Verma et al. 2004). These results confirm our observations on shoot induction upon usage of TDZ at various concentrations (1.13, 4.54, and 9.08 μM). Based on this experiment, a practical protocol was set up to use TDZ for highly efficient shoot induction in saffron (see Appendix).
That TDZ affects embryogenic callus formation and somatic embryogenesis has been reported (Sheibani et al. 2007) for which 2.27 μM TDZ has been found to be the most effective concentration. Here, the TDZ concentrations used were 1.13, 4.54, and 9.08 μM. We observed that application of TDZ alone at concentrations below 10 μM induced shoots instead of somatic embryos. In the case of regeneration through organogenesis, the maximum number of shoots per explant was 12 (Ilahi et al. 1987), in contrast to our experiment in which it was 39.5 ± 5.1. Whereas, 85.7% of the calluses had been reported to regenerate shoots after transfer to 4.44 μM NAA plus 0.53 μM BA (Isa and Ogasawara 1988), in our study, 100% of the explants responded and formed shoots when the MS medium was supplemented with 1.13 μM TDZ. We also obtained approximately the same result for both media in all treatments, except at 17.75 μM BA (Fig. 1), meaning that the B5 and MS media had no significant influence on shoot formation and type and that the concentration of the plant growth regulators is more important than the medium (Fig. 1).
Morphology.
At all TDZ concentration levels and in all treatments, we observed nodular calluses that consisted of translucent nodular structures. Although the appearance of these structures was very similar to that of somatic embryos, our data suggest that they are not. In the many available reports on somatic embryogenesis of saffron (George et al. 1992; Ahuja et al. 1994; Blázquez et al. 2004; Karamian 2004; Darvishi et al. 2006; Chaloushi et al. 2007; Sheibani et al. 2007), the different stages have been demonstrated. In another experiment, different stages of somatic embryogenesis were displated by semi-thin sections of the nodular structures (Blázquez et al. 2004). We believe that the observed process was shoot organogenesis and not somatic embryogenesis because the medullar meristem is surrounded by parenchymatous cells with large amyloplasts that are uncharacteristic of somatic embryos. The structures that have been indicated as monopolar somatic embryos are indeed shoots. Although different hormonal treatments were used, the morphology and histology of the observed structures are similar. In contrast to the differentiated epidermal and subepidermal layers, vacuolized cells, medullar meristem, and parenchymatous tissues surrounded with mature amyloplasts (Blázquez et al. 2004); characteristic features of embryos, such as meristematic cells with condensed cytoplasm, small nucleus, small vacuole, and absence of amyloplasts and vascular connections were not seen.
Morphohistological studies of TDZ-induced somatic embryo-like structures in Pelarganium sp. (Haensch 2004a, b) indicated that intermediate structures were formed of globular somatic embryos, but, based on microscopical analysis, were, in fact, shoots, as confirmed by our work on saffron. We believe that a resemblance between the external appearance of the regenerating structures and embryos is insufficient to conclude on the presence of embryogenic structures. It will be necessary to confirm histologically that these regenerated tissues develop into structures with clear bipolarity through stages similar to those of zygotic embryogenesis (Haensch 2004a).
Conclusion
We have shown that TDZ is a more potent bioregulator than BA to induce organogenesis in saffron, and its effect is similar in both the B5 and MS media because no correlation was found between the TDZ concentration and the medium. The interesting point in TDZ treatments was the formation of globular embryo-like structures on organogenic calluses. Induction of callus and shoot formation was slightly better in the B5 than in the MS medium, although the difference was not significant. The root formation by BA was the highest on the B5 media containing 2.22 μM NAA and 2.68 μM BA. Based on this experiment, a reproducible protocol was obtained for in vitro mass shoot production in saffron, in which 100% of the explants formed organogenic calluses instead of the reported 85.7% (6/7) of corm explants that regenerated into calluses (Isa and Ogasawara 1988). Shoot production via this new protocol was threefold more efficient than that of Ilahi et al. (1987). This optimized protocol (see “Appendix” section) is now routinely used in our laboratory.
References
Ahuja A.; Koul S.; Ram G.; Kaul B. L. Somatic embryogenesis and regeneration of plantlets in saffron, Crocus sativus L. Indian J. Exp. Biol. 32: 135–140; 1994.
Blázquez S.; Olmos E.; Hernández J. A.; Hellin E.; Fernández J.-A.; Piqueras A. Somatic embryogenesis in saffron (Crocus sativus L.): Morphological differentiation and the role of the antioxidant enzymatic system. Acta Hort 650: 261–267; 2004.
Chaloushi B.; Zarghami R.; Abd-Mishani C.; Omidi M.; Agayev Y. M.; Pakdaman Sardood B. Effects of different hormonal treatments on the callus production and plantlet regeneration in saffron (Crocus sativus L.). Pak. J. Biol. Sci. 10: 1625–1631; 2007.
Darvishi E.; Zarghami R.; Mishani C. A.; Omidi M.; Sarkhosh A. In vitro production of pathogen-free plantlets via meristem culture in saffron (Crocus sativus L.). Biotechnology 5: 292–295; 2006.
Dhar A. K.; Sapru R.; Rekha K. Studies on saffron in Kashmir. 1: Variation in natural population and its cytological behaviour. Crop Improv 15: 48–52; 1988.
D’Onofrio C.; Morini S. Development of adventitious shoots from in vitro grown Cydonia oblonga leaves as influenced by different cytokinins and treatment duration. Biol. Plant. 49: 17–21; 2005.
Ebrahimzadeh H.; Karamian R.; Noori-Daloii M. R. In vitro regeneration of shoot and corm from the different explants of Crocus sativus L. J. Sci. I. R. Iran 7: 1–7; 1996.
Ebrahimzadeh H.; Karamian R.; Noori-Daloii M. R. Shoot regeneration from saffron protoplasts immobilized in Ca-alginate beads. J. Sci. I. R. Iran 11: 69–72; 2000.
Elobeidy A.; Korban S. S. The effect of thidiazuron on shoot regeneration from apple leaf discs. HortScience: 23: 755; 1988.
Fasolo F.; Zimmerman R. H.; Fordham I. Adventitious shoot formation on excised leaves in vitro cultured apple cultivars. HortScience 23: 676; 1988a.
Fasolo F.; Zimmerman R. H.; Fordham I.; Falbo L. Adventitious shoot formation on excised leaves of in vitro cultured apples: effects of cytokinin, auxin, and leaf age. HortScience 23: 755; 1988b.
Fellman C. D.; Read P. E.; Hosier M. A. Effects of thidiazuron and CPPU on meristem formation and shoot proliferation. HortScience 22: 1197–1200; 1987.
Fernández J.-A. Biology, biotechnology and biomedicine of saffron. In: Pandalai S. G. (ed) Recent Research Developments in Plant Science, vol. 2. Research Signpost, Trivandrum, pp 127–159; 2004.
Gamborg O. L.; Miller R. A.; Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50: 151–158; 1968.
Geneve R. Comparative adventitious shoot induction in Kentucky coffeetree root and petiole explants treated with thidiazuron and benzylaminopurine. In vitro Cell. Dev. Biol.-Plant 41: 489–493; 2005.
George P. S.; Visvanath S.; Ravishankar G. A.; Ventkaraman L. V. Tissue culture of saffron (Crocus sativus L.): Somatic and shoot regeneration. Food Biotechnol 6: 217–223; 1992.
Gilby A. C.; Wainwright H. Use of tissue culture in improvement of watercress (Rorippa nasturtium aquaticum L. Hayek). Acta Hort 244: 105–113; 1989.
Haensch K.-T. Morpho-histological study of somatic embryo-like structures in hypocotyls cultures of Pelargonium hortorum Bailey. Plant Cell Rep. 22: 376–381; 2004a.
Haensch K.-T. Thidiazuron-induced morphogenetic response in petiole cultures of Pelargonium hortorum and Pelargonium domesticum and its histological analysis. Plant Cell Rep. 23: 211–217; 2004b.
Huetteman C. A.; Preece J. E. Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult. 33: 105–119; 1993.
Ilahi I.; Jabeen M.; Firdous N. Morphogenesis with saffron tissue culture. J. Plant. Physiol. 128: 227–232; 1987.
Imel M. R.; Preece J. E. Adventitious shoot formation from recultured leaves of rhododendron. HortScience 23: 760; 1988.
Isa T.; Ogasawara T. Efficient regeneration from the callus of saffron (Crocus sativus). Jpn. J. Breed. 38: 371–374; 1988.
Karam N. S.; Al-Majathoub M. In vitro shoot regeneration from mature tissue of wild Cyclamen persicum Mill. Sci. Hortic. 86: 323–333; 2000.
Karamian R. Plantlet regeneration via somatic embryogenesis in four species of Crocus. Acta Hort. 650: 253–257; 2004.
Kerns H. R.; Meyer M. M. Tissue culture propagation of Acer freemanii using thidiazuron to stimulate shoot tip proliferation. HortScience 21: 1209–1210; 1986.
Murashige T.; Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497; 1962.
Nielsen J. M.; Hansen J.; Brandt K. Synergism of thidiazuron and benzyladenine in axillary shoot formation depends on sequence of application in Miscanthus X ogiformis ‘Giganteus’. Plant Cell Tissue Organ Cult. 41: 165–170; 1995.
Reisch B. I.; Martens M. H. R. Shoot regeneration from grape leaf, petiole and tendril tissues. HortScience 23: 807; 1988.
Sheibani M.; Nemati S. H.; Davarinejad G. H.; Azghandi A. V.; Habashi A. A. Induction of somatic embryogenesis in saffron using thidiazuron (TDZ). Acta Hort. 739: 259–267; 2007.
Singh N. D.; Sahoo L.; Sarin N. B.; Jaiwal P. K. The effect of TDZ on organogenesis and somatic embryogenesis in pigeonpea (Cajanus cajan L. Millsp). Plant Sci 164: 341–347; 2003.
Singha S.; Bhatia S. K. Shoot proliferation of pear cultures on medium containing thidiazuron and benzylaminopurine. HortScience 23: 803; 1988.
Tirillini B.; Pagiotti R.; Menghini L.; Miniati E. Volatile organic compounds from tepals and anthers of saffron flowers (Crocus sativus L.). J. Essent Oil Res 18: 298–300; 2006.
Verma V. M.; Cho J. J.; Aikne J.; David J. High frequency plant production of taro (Colocasia esculenta (L.) Schott) by tissue culture. 4th International Crop Science Congress (ICSC), Brisbane, Australia; 26th September-1st October 2004.
Wilhelm E. Micropropagation of juvenile sycamore maple via adventitious shoot formation by use of thidiazuron. Plant Cell Tissue Organ Cult. 57: 57–60; 1999.
Yoshida T. Adventitious shoot formation from hypocotyl sections of mature soybean seeds. Breed. Sci 52: 1–8; 2002.
Acknowledgments
This paper is the first part of author’s dissertation that will be presented to the Faculty of Science (University of Tehran) in partial fulfillment of the requirements for the PhD degree. The authors thank the technicians for the SEM and transmission electron microscopy at the Universities of Tehran and of Tarbiat Modares and Dr. Kristiina Himanen and Dr. Martine De Cock (VIB, Ghent University, Belgium) for suggestions and critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: D. T. Tomes
Appendix
Appendix
Optimized protocol for micropropagation through multiple shoot induction in saffron
-
1.
Wash the healthy saffron corms for 30 min in running water, disinfect them with detergent, and rinse with water.
-
2.
Incubate the corms for 10 min in 1% benzalconium chloride and rinse them with tap water.
-
3.
Surface sterilize the corms in 70% ethanol for 2 min and in 20% bleach for 15 min; rinse them three times with sterile distilled water.
-
4.
Cut off blocks of the central meristematic regions of the corms, place on the MS medium containing 4.54 μm TDZ, and transfer them into a growth cabinet at 25 °C and in the dark for 14 wk (two subcultures on the same medium).
-
5.
Transfer all the explants with induced shoots into jars containing the B5 medium plus 2.22 μM NAA and 2.68 μM BA for further growth for 11 wk.
-
6.
Transfer the rooted shoots into soil.
Rights and permissions
About this article
Cite this article
Sharifi, G., Ebrahimzadeh, H., Ghareyazie, B. et al. Globular embryo-like structures and highly efficient thidiazuron-induced multiple shoot formation in saffron (Crocus sativus L.). In Vitro Cell.Dev.Biol.-Plant 46, 274–280 (2010). https://doi.org/10.1007/s11627-009-9264-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-009-9264-0