Abstract
Somatic embryo-like structures were produced from the hypocotyls of ten cultivars of Pelargonium × hortorum using the protocols of Marsolais et al. (1991; Can J Bot 69:1188–1193) and Slimmon et al. (1991; Plant Cell Rep 10:587–589) and their embryonic natures evaluated. Nine cultivars responded, and 937 structures were formed. Regeneration corresponded well with published data. The somatic embryo-like structures were globular- to leaf-shaped or similar to shoots. A root pole was never visible. Histological examinations confirmed the lack of bipolarity and revealed vascular connections to the explant in the more developed structures. Therefore, these structures cannot be classified as somatic embryos. The importance of these results is discussed in terms of evaluating published protocols for the propagation of these pelargoniums by somatic embryogenesis from hypocotyls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelargoniums are among the world’s most popular ornamental plants. Mass propagation is from cuttings or seed (Berninger 1993), which is very expensive. The use of cuttings involves problems with several bacterial and viral diseases (Daughtrey and Wick 1993; Nameth and Adkins 1993), and propagation from seed is only practical for cultivars bred for this purpose (Craig 1993; Lange and Horn 1996). The application of shoot-tip culture for large-scale propagation is unsuitable because the multiplication rates are very low (Appelgren et al. 1991). Regeneration via somatic embryogenesis has the potential to facilitate more efficient propagation techniques.
The most desirable explants for such purposes are those that are available from adult plants following quality testing. In Pelargonium × hortorum, the embryogenic response of those explants is very low. Average regeneration rates of up to 0.1 to 0.5 somatic embryos per explant have been achieved using petioles (Marsolais et al. 1991; Haensch 1999). Therefore, most of the published work on somatic embryogenesis in Pelargonium × hortorum is based on explants of very young seedlings, especially hypocotyls (Marsolais et al. 1991; Slimmon et al. 1991; Visser et al. 1992; Qureshi and Saxena 1992; Gill et al. 1993, 1994; Hutchinson and Saxena 1996a, 1996b; Hutchinson et al. 1996a, 1996b, 1997a, 1997b, 2000; Murthy et al. 1996, 1999; Wilson et al. 1996; Croke and Cassels 1997; Senaratna et al. 1999, 2002; Madakadze and Senaratna 2000; Madakadze et al. 2000; Murch and Saxena 2001). Somatic embryos are defined, with general acceptance (Brown et al. 1995), as new individuals that develop both a shoot apex and a discrete radicular end (Haccius 1978). The embryo-like structures from hypocotyl cultures of Pelargonium × hortorum have been classified as somatic embryos, although no distinct and unambiguous root pole could be demonstrated (Marsolais et al. 1991; Visser et al. 1992; Qureshi and Saxena 1992; Gill et al. 1993, 1994; Hutchinson and Saxena 1996b; Murthy et al. 1996; Croke and Cassels 1997). The uncertainty in the classification of these regenerants is suggested by the fact that even Croke and Cassels (1997) used terms such as “putative somatic embryos” and “somatic embryo-like structures”. Histological examinations were rarely made, and when they were, the classification of the regenerating structures as somatic embryos was unconvincing insofar as the more advanced stages did not appear to be clearly separated from the maternal explant, and their bipolarity was not verified (Gill et al. 1993; Hutchinson et al. 1996a). The aim of the investigation reported here was, therefore, to produce embryo-like structures on the hypocotyls of Pelargonium × hortorum using two published protocols (Marsolais et al. 1991; Slimmon et al. 1991) and to check the embryonic nature of these structures.
Materials and methods
In vitro regeneration
Hypocotyl cultures were established according to the methods of Marsolais et al. (1991) or Slimmon et al. (1991) with minor modifications. An assortment of ten cultivars of Pelargonium × hortorum were studied, two of which, White Orbit and Violet Orbit, had also been used by Marsolais et al. (1991) (Table 1). Seeds were surface-sterilized by shaking for 20 min in a 1.4% solution of sodium hypochlorite and rinsing five times in sterile distilled water. Each seed was incubated on distilled water solidified with 6.5 g/l agar, at 22°C, in the dark, in a 50-ml test tube sealed with plastic film. After 4–5 days, the hypocotyls of the germinated plants reached lengths of 1.0–2.5 cm. At this time, the hypocotyls were cut into 0.5-cm-long segments. The segments were placed on 25 ml medium in 90×15-mm petri dishes that were subsequently wrapped with plastic film and incubated in a growth chamber at 24°C under a 16/8-h (day/night) photoperiod (white fluorescent light supplied at an intensity of 46 μmol m-2 s-1). Two different media were compared: (1) GCM medium supplemented with 1 μmol/l indole-3-acetic acid (IAA), 2 μmol/l 6-benzylaminopurine (BAP), 30 g/l sucrose, and 6.5 g/l agar (Serva, Heidelberg, Germany), pH 5.6 (Marsolais et al. 1991); (2) MS (Murashige-Skoog 1962) medium supplemented with 1 μmol/l IAA, 8 μmol/l BAP, 30 g/l sucrose, and 2.5 g/l Gelrite (Duchefa, Haarlem, The Netherlands), pH 5.0 (Slimmon et al. 1991). In each medium, iron was provided as ethylenediaminetetraacetic acid iron salt. In the MS medium, the iron content was lowered to 86.9 μM. Each petri dish contained ten explants and the treatments were replicated four times. After 28 days, the cultures were assessed for the number and kinds of structures formed by means of a stereomicroscope.
Histological evaluation
Thirty-one structures derived from cvs. White Orbit and Scarlet Orbit were evaluated histologically. All of these were formed on MS medium as modified by Slimmon et al. (1991).
Explants with regenerated structures were fixed in a solution of formalin, alcohol and acetic acid (FAA), 100 ml of which contains 5.4 ml formalin (37%), 65.6 ml ethanol (96%), 5 ml glacial acetic acid, and 24 ml distilled water (Gerlach 1984). Parts of the explants with representative structures were cut and embedded in hydroxyethylmethacrylate (Histo-Technique-Set Technovit 7100; Kulzer, Wehrheim, Germany). At the beginning of this process, the specimens were dehydrated in 2-h steps through a graded series of ethanol (70%, 90%, 96%, and 100%). The samples were then pre-infiltrated overnight with a mixture of equal parts of 100% ethanol and Technovit 7100 base liquid. The explants were then transferred into an infiltration solution of 100 ml Technovit 7100 base liquid and 1 g hardener I for 1 day. A vacuum was established for 30 min at the start of the last two processes. Explants were embedded in Teflon moulds with a mixture of 15 parts infiltration solution and one part hardener II. The samples were polymerized for 1 h at room temperature and for a further 6 h at 37°C. Specimens were mounted on block-holders with Technovit 3040. Slices (6 μm) were cut at room temperature using a Jung CM 1800 microtome equipped with type 818 disposable microtome blades (both from Leica Instruments, Nussloch, Germany). Slices were stretched on a bath of distilled water and mounted on slides. They were then stained with 0.05% toluidine blue O (Serva, Heidelberg, Germany) dissolved in 1% sodium tetraborate decahydrate buffer (Hutchinson et al. 1996a), rinsed in distilled water, dried, and covered with Entellan (Merck, Darmstadt, Germany) and a cover slip. This procedure stains the cytoplasm and unlignified cell walls red and the DNA-containing structures and lignified cell walls blue (Gerlach 1984). Microscopic analysis was performed using a Leitz DMR microscope (Leica, Wetzlar, Germany) with a Wild MPS 48/52 camera (Leica, Heerbrugg, Switzerland).
Results
In vitro regeneration
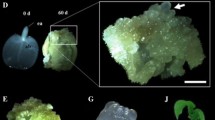
Regenerating structures were observed on the hypocotyl explants of nearly all cultivars. These structures occurred on the epidermis of the long sides of the explants and were globular- to leaf-shaped or similar to shoots, greenish, and partly covered with hairs (Fig. 1). No root pole was visible. An unambiguous classification of these structures was not possible without histological evaluation. Therefore, they were designated “somatic embryo-like structures” for the interim. In total, 937 structures were regenerated from 675 surviving explants. Cultivar White Orbit showed the highest response on GCM and MS media, with mean somatic embryo-like structures per surviving explant of 0.65 and 7.73 respectively, whereas there was no regeneration of the PAC Sitta cultivar on either medium (Table 1). Cultivar Violet Orbit did not form somatic embryo-like structures on GCM medium and showed only a weak response on MS medium. Of the two media, the MS medium as modified by Slimmon et al. (1991) produced much better results in terms of the number of responsive genotypes and the number of regenerating structures.
Histological evaluation
Histological slices confirmed the initial globular shape of the regenerants (Fig. 2). No root pole could be demonstrated in the more developed structures (Fig. 3a). Although these structures could be assigned to the globular or heart stage on the basis of their appearance at the beginning of regeneration, the vast majority of their cells were parenchymatous, i.e., with a strongly vacuolized cytoplasm. In addition to the absence of a root meristem, spiral vessels (i.e., elements of the xylem bundle) were visible. These vascular strands in the regenerating structures stretched to the parenchymal cells of the maternal explant (Fig. 3b) or even into the tissue of the explant (Fig. 3c, d).
Longitudinal sections of more developed somatic embryo-like structures on hypocotyl segments of Pelargonium × hortorum Scarlet Orbit (a, b) and White Orbit (c, d). In each case, the tissue of the regenerant is predominantly parenchymatous, and there is no root pole. a Vascular strands are present and extend to the explant tissue (×92), b Fig. 3a in detail, showing vascular strands (×366). c A vascular connection is established into the tissue of the explant (×92), d Fig. 3c in detail (×366)
Discussion
From the several publications that have reported on somatic embryogenesis in hypocotyl cultures of Pelargonium × hortorum (Marsolais et al. 1991; Slimmon et al. 1991; Visser et al. 1992; Qureshi and Saxena 1992; Gill et al. 1993, 1994; Hutchinson and Saxena 1996a, 1996b; Hutchinson et al. 1996a, 1996b, 1997a, 1997b, 2000; Murthy et al. 1996, 1999; Wilson et al. 1996; Croke and Cassels 1997; Senaratna et al. 1999, 2002; Madakadze and Senaratna 2000; Madakadze et al. 2000; Murch and Saxena 2001), we selected two reliable protocols to generate somatic embryo-like structures and to check their embryonic nature: (1) GCM medium supplemented with 1 μmol/l IAA and 2 μmol/l BAP, which was used to screen cultivars by Marsolais et al. (1991); (2) MS medium supplemented with 1 μmol/l IAA and 8 μmol/l BAP, which was used as a control by Slimmon et al. (1991).
It was possible to reproduce the regeneration of the structures that were classified as somatic embryos by Marsolais et al. (1991) and Slimmon et al. (1991). This result is supported by a correspondence in the morphological appearance of these structures (Fig. 1) as well as by the similarity of the quantitative responses (Table 1). Cultivar White Orbit responded best to both of the protocols used. This is consistent with the assessment of Marsolais et al. (1991), who evaluated this cultivar as the fourth best in terms of the number of somatic embryos produced from an assortment of 30 cultivars of Pelargonium × hortorum. Similarly, there was a correspondence between the lack of regeneration shown by cv. Violet Orbit on GCM medium in this study and its weak response in the study of Marsolais et al. (1991).
Although the regenerated structures superficially resembled somatic embryos, it was not possible to confirm their embryonic nature. Somatic embryos are individual bipolar structures with no vascular connection to the mother plant at any time of their life (Haccius 1978) and which develop from somatic cells through characteristic embryological stages (Williams and Maheswaran 1986). The regenerating structures in the present study did have a globular shape at the beginning of their development, but the majority of their cells were parenchymatous, i.e., vacuolized (Fig. 2). Globular somatic embryos normally consist of small cells with dense cytoplasm and large nuclei, as has been observed in other species (Daucus, Schiavone and Cooke 1985; Nigella, Banerjee and Gupta 1976; Panicum, Lu and Vasil 1985). More importantly, and in contradiction to the definition cited above, none of the 937 somatic embryo-like structures in the present experiment showed a root pole (Fig. 1). This lack of bipolarity was confirmed by histological examination. Furthermore, histology revealed vascular connections near or into the explant in the more developed structures (Fig. 3). On the basis of our findings, the structures regenerated on the hypocotyls of Pelargonium × hortorum on GCM medium supplemented with 1 μmol/l IAA and 2 μmol/l BAP (Marsolais et al. 1991) or on MS medium supplemented with 1 μmol/l IAA and 8 μmol/l BAP (Slimmon et al. 1991) cannot be classified as somatic embryos, predominantly because they are not bipolar. According to the criteria formulated by Haccius (1978), some of these regenerants are shoots or shoot-like structures because they have a shoot meristem and a vascular connection into the explant (Fig. 3c, d). Other regenerants can only be regarded as leaf-like structures because their tissue is parenchymatous, contains partially vascular strands, and has no shoot or root meristem (Figs. 2, 3a, b).
These results indicate that the published protocols for the propagation of Pelargonium × hortorum via somatic embryogenesis from hypocotyls must be evaluated critically. A resemblance between the external appearances of the regenerating structures and embryos is insufficient justification for the conclusion of identity. It is necessary to show histologically that these regenerating structures develop via stages similar to those of zygotic embryogenesis into individual structures with clear bipolarity. The only two publications that present histological evidence of somatic embryogenesis from hypocotyls of Pelargonium × hortorum are not convincing. Gill et al. (1993) showed only a single regenerant classified as a “heart-shaped somatic embryo”, but the structure was connected to the maternal explant over its total width and the transition between the cell types of the explant and the structure was gradual instead of constituting a sharp boundary. Hutchinson et al. (1996a) published the development of these structures more comprehensively, but again the more advanced structures, such as the “cotyledonary-stage embryos”, were broadly linked to the explant, and there was a gradual transition of cell types between the maternal tissue and the structures. These advanced structures showed only a shoot meristem, and not a root pole. Not one bipolar structure was presented.
On the basis of these criticisms of published histological results and the results of the present experiment, we conclude that there has been as yet no proof of somatic embryogenesis from the hypocotyls of Pelargonium × hortorum, although several papers have already dealt with this matter.
References
Appelgren M, Hunter CS, Paludan N, Reuther G, Theiler-Hedrich R (1991) COST 87—Pelargonium micropropagation and pathogen elimination—A report of the Pelargonium Working Group. Commission of the European Communities, pp 1–126.
Banerjee S, Gupta S (1976) Embryogenesis and differentiation in Nigella sativa leaf callus in vitro. Physiol Plant 38:115–120
Berninger LM (1993) Status of the industry. In: White JW (ed) Geraniums IV—The grower’s manual. Ball Publ, Geneva, Ill., pp 1–2
Brown DCW, Finstad KI, Watson EM (1995) Somatic embryogenesis in herbaceous dicots. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer, Dordrecht, pp 345–415
Craig R (1993) Breeding geraniums for 2000 and beyond. In: White JW (ed): Geraniums IV—The grower’s manual. Ball Publ, Geneva, Ill., pp 373–388
Croke J, Cassels AC (1997) Dark induction and genetic stability of somatic embryos of zonal geraniums (Pelargonium x hortorum Baily). J Appl Bot 71:119–124
Daughtrey M, Wick RL (1993) Vascular wilt diseases—bacterial blight. In: White JW (ed): Geraniums IV—The grower’s manual. Ball Publ, Geneva, Ill., pp 237–242
Gerlach D (1984) Botanische Mikrotechnik. Georg Thieme, Stuttgart
Gill R, Gerrath JM, Saxena PK (1993) High-frequency direct somatic embryogenesis in thin layer cultures of hybrid seed geranium (Pelargonium x hortorum). Can J Bot 71:408–413
Gill R, Senaratna T, Saxena PK (1994) Thidiazuron-induced somatic embryogenesis enhances viability of hydrogel-encapsulated somatic embryos of geranium. J Plant Physiol 143:726–729
Haccius B (1978) Question of unicellular origin of non-zygotic embryos in callus cultures. Phytomorphology 28:74–81
Haensch KT (1999) Somatic embryogenesis in vitro from adult plants of Pelargonium: influence of genotype and basal medium. Gartenbauwissenschaft 64:193–200
Hutchinson MJ, Saxena PK (1996a) Role of purine metabolism in thidiazuron-induced somatic embryogenesis of geranium (Pelargonium x hortorum) hypocotyl cultures. Physiol Plant 98:517–522
Hutchinson MJ, Saxena PK (1996b) Acetylsalicylic acid enhances and synchronizes thidiazuron-induced somatic embryogenesis in geranium (Pelargonium x hortorum Bailey) tissue cultures. Plant Cell Rep 15:512–515
Hutchinson MJ, KrishnaRaj S, Saxena PK (1996a) Morphological and physiological changes during thidiazuron-induced somatic embryogenesis in geranium (Pelargonium x hortorum Bailey) hypocotyl cultures. Int J Plant Sci 157:440–446
Hutchinson MJ, Murch SJ, Saxena PK (1996b) Morphoregulatory role of thidiazuron: evidence of the involvement of endogenous auxin in thidiazuron-induced somatic embryogenesis of geranium (Pelargonium x hortorum Bailey). J Plant Physiol 149:573–579
Hutchinson MJ, KrishnaRaj S, Saxena PK (1997a) Inhibitory effect of GA3 on the development of thidiazuron-induced somatic embryogenesis in geranium (Pelargonium x hortorum Bailey) hypocotyl cultures. Plant Cell Rep 16:435–438
Hutchinson MJ, Murr D, KrishnaRaj S, Senaratna T, Saxena PK (1997b) Does ethylene play a role in thidiazuron-regulated somatic embryogenesis of geranium (Pelargonium x hortorum Bailey) hypocotyl cultures? In Vitro Cell Dev Biol Plant 33:136–141
Hutchinson MJ, Senaratna T, Sahi S, Saxena PK (2000) Light mediates endogenous plant growth substances in thidiazuron-induced somatic embryogenesis in geranium hypocotyl cultures. J Plant Biochem Biotechnol 9:1–6
Lange P, Horn W (1996) Geraniaceae. In: Horn W (ed) Zierpflanzenbau. Blackwell Wissenschafts-Verlag, Berlin, pp 335–348
Lu CY, Vasil IK (1985) Histology of somatic embryogenesis in Panicum maximum (Guinea Grass). Am J Bot 72:1908–1913
Madakadze RM, Senaratna T (2000) Effect of growth regulators on maturation of geranium (Pelargonium x hortorum) somatic embryos. Plant Growth Regul 30:55–60
Madakadze RM, Krochko JE, Senaratna T (2000) Identification and characterization of storage proteins in zygotic and somatic embryos of geranium (Pelargonium x hortorum). J Am Soc Hortic Sci 125:525–529
Marsolais AA, Wilson DPM, Tsujita MJ, Senaratna T (1991) Somatic embryogenesis and artificial seed production in zonal (Pelargonium x hortorum) and regal (Pelargonium x domesticum) geranium. Can J Bot 69:1188–1193
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murch SJ, Saxena PK (2001) Molecular fate of thidiazuron and its effects on auxin transport in hypocotyls tissues of Pelargonium × hortorum Bailey. Plant Growth Regul 35:269–275
Murthy BNS, Singh RP, Saxena PK (1996) Induction of high-frequency somatic embryogenesis in geranium (Pelargonium x hortorum Bailey cv. Ringo Rose) cotyledonary cultures. Plant Cell Rep 15:423–426
Murthy BNS, Vettakkorumakankav NN, KrishnaRaj S, Odumeru J, Saxena PK (1999) Characterization of somatic embryogenesis in Pelargonium x hortorum mediated by a bacterium. Plant Cell Rep 18:607–613
Nameth ST, Adkins ST (1993): Viral diseases. In: White JW (ed) Geraniums IV—The grower’s manual. Ball Publ, Geneva, Ill. pp 267–275
Qureshi JA, Saxena PK (1992) Adventitious shoot induction and somatic embryogenesis with intact seedlings of several hybrid seed geranium (Pelargonium x hortorum Bailey) varieties. Plant Cell Rep 11:443–448
Schiavone FM, Cooke TJ (1985) A geometric analysis of somatic embryo formation in carrot cell cultures. Can J Bot 63:1573–1578
Senaratna T, Dixon K, Bunn E, Touchell D (1999) Smoke-saturated water promotes somatic embryogenesis in geranium. Plant Growth Regul 28:95–99
Senaratna T, Bunn E, Bishop A (2002) Triazole treatment of explant source provides stress tolerance in progeny of geranium (Pelargonium hortorum Bailey) plants regenerated by somatic embryogenesis. Plant Growth Regul 36:169–174
Slimmon T, Qureshi JA, Saxena PK (1991) Phenylacetic acid-induced somatic embryogenesis in cultured hypocotyl explants of geranium (Pelargonium x hortorum Bailey). Plant Cell Rep 10:587–589
Visser C, Qureshi JA, Gill R, Saxena PK (1992) Morphoregulatory role of thidiazuron—substitution of auxin and cytokinin requirement for the induction of somatic embryogenesis in geranium hypocotyl cultures. Plant Physiol 99:1704–1707
Williams EG, Maheswaran G (1986) Somatic embryogenesis: factors influencing coordinated behavior of cells as an embryogenic group. Ann Bot 57:443–462
Wilson DPM, Sullivan JA, Marsolais AA, Tsujita MJ, Senaratna T (1996) Improvement of somatic embryogenesis in zonal geranium. Plant Cell Tissue Organ Cult 47:27–32
Acknowledgements
This investigation was undertaken with the support of the Ministries of Agriculture of the Federal Republic of Germany and of the states of Brandenburg and Thüringen. The author would like to thank Mrs. Barbara Weinlich for her excellent technical assistance. The author also wishes to acknowledge Erfurter Samenzucht Weigelt & Co, Walluf, Walz Samen GmbH, Stuttgart, and Elsner pac Jungpflanzen, Dresden, for the supply of cultivars.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Lörz
Rights and permissions
About this article
Cite this article
Haensch, KT. Morpho-histological study of somatic embryo-like structures in hypocotyl cultures of Pelargonium × hortorum Bailey. Plant Cell Rep 22, 376–381 (2004). https://doi.org/10.1007/s00299-003-0726-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0726-2