Abstract
Including lipoic acid (LA) in culture media during Agrobacterium transformation processes of four crop species has significantly improved the transformation methods of the crops, even for previously recalcitrant genotypes. Plant transformation efficiency of soybean was significantly increased from 0.6% to 3.7% and tomato from 29.8% to 87.0%. Transformation efficiency was doubled from 2.8% to 5.7% in wheat. The frequency of glyphosate-resistant embryos had a significant increase from 41.4% to 61.2% in cotton. Regeneration of non-transgenic shoots under selection (“shoot escapes”) was significantly reduced in tomato from 91.5% to 46.2% while in soybean from 92.0% to 72.0% under optimal conditions. This study also demonstrated that the increase of transformation efficiency in tomato was accompanied by as much as a significant 2-fold reduction in severity of browning of Agrobacterium-infected plant tissues and up to a significant 3-fold increase in the percentage of explants with a high level of transient gene expression. LA application in plant transformation has enabled the resolution of three common problems in plant transformation: browning or necrosis of the transformed cells or tissues, difficulty in regenerating transformed cells or tissues, and shoot escapes, which severely limit the number of transgenic plants that can be regenerated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant transformation plays a critical role in agricultural biotechnology and the application of genomic sciences to plant biology. Genetically modified crops have been in use commercially around the world for over a decade to improve agricultural, nutritional, and food processing traits, such as insect, herbicide, and virus resistance, vitamin enrichment, and controlled fruit ripening (James 2006). Functional genomics in plants involves the use of high-throughput methods for functional analysis of many genes (Jeon et al. 2000, Pereira 2000, Lagudah et al. 2001, Lee et al. 2004, Ostergaard and Yanofsky 2004). With genome sequences for plant species becoming rapidly available, efficient plant transformation systems are essential for the application of genomic sciences to understand physiological, biochemical, and molecular mechanisms of metabolic pathways (Tyagi and Mohanty 2000, Olhoft et al. 2003, Yang et al. 2004). The technology of plant transformation is only moderately or marginally successful in many important cultivars of crops, which can be a major limiting factor for the biotechnological exploitation of economically important plant species and the wider application of genomic science. Therefore, more efficient and reliable transformation methodology applicable to a wide range of species and cultivars could greatly exploit biotechnology and improve the application of genomic technologies.
The production of transgenic plants requires that some of the cells residing within an explant or tissue be transformed with a gene of interest and then induced to regenerate into a whole plant. With the exception of in planta Agrobacterium-mediated plant transformation, which has only been successfully applied to a few species, the most effective current method for transforming plants requires that cells or tissues be inoculated with Agrobacterium tumefaciens, then maintained on culture media for several weeks or months for co-cultivation, selection of transformed cells or tissues, and regeneration of transgenic plants. Many plants, particularly commercially important ones, suffer necrosis or recalcitrance during Agrobacterium-mediated transformation process (Perl et al. 1996, Olhoft et al. 2003, Zheng et al. 2005). Also, numerous crop transformation methods that use neonmycin phosphotransferase II selection have a common problem of regenerating non-transgenic shoots even under stringent selection conditions. Regeneration of shoot escapes from approximately 40% to 95% have been reported for apple (James et al. 1989), orange (Moore et al. 1992, Pena et al. 1995a), banana (May et al., 1995), pear (Mourgues et al. 1996), grapevine (Perl et al. 1996), sweet orange (Pena et al. 1995b, Cervera et al. 1998), lime (Pena et al. 1997), and cauliflower (Stipic et al. 2000). High frequency of the shoot escapes demands greater efforts in screening putative transgenic shoots. These three common problems of tissue browning or necrosis, recalcitrance, and shoot escapes severely limit the number of transgenic plants that can be regenerated. One possibility for the degeneration of plant tissue in culture media is that the tissues or cells are stressed when excised and inoculated with A. tumefaciens, thus, limiting their growth potential in tissue culture media. Potential stressors include production of free radicals or reactive oxygen species, which damage cells or activation or alteration of metabolic pathways. Oxidative stress, free radicals, or reactive oxygen species can dramatically influence the outcome of plant cell culture and transformation. Perl et al. (1996) reported that very short exposures of embryogenic calluses of Vitis vinifera cv. Superior seedless grape plants to diluted cultures of Agrobacterium resulted in plant tissue necrosis and subsequent cell death. The cell death seemed to be oxygen-dependent and correlated with elevated levels of peroxides. Therefore, the effects on necrosis of various combinations of antioxidants during and after grape-Agrobacterium co-cultivation were studied. The combination of polyvinylpolypyrrolidone and dithiothreitol was found to improve plant viability. Tissue necrosis was completely inhibited by these antioxidants, while Agrobacterium virulence was not affected. These antioxidants enabled the recovery of stable transgenic grape plants resistant to hygromycin. The antioxidant, glutathione (GSH), promoted callus growth and shoot development in a shoot tip culture of apple (Nomura et al. 1998). The effect of ascorbic acid and cysteine were evaluated on the viability of rice stem sections taken from in vitro rice plantlets and on their interaction with A. tumefaciens. Both ascorbic acid and cysteine significantly decreased necrosis with respect to controls after 6 h of treatment and improved rice transformation (Enríquez-Obregón et al. 1999). Glutathione increased plant regeneration and Agrobacterium-mediated transformation of a desiccation-tolerant plant, Craterostigma plantagineum (Toldi et al. 2002). l-cysteine, dithiothreitol, and sodium thiosulfate were reported to improve soybean transformation (Olhoft et al. 2003). Antioxidants such as ascorbic acid (Ostergaard and Yanofsky 2004), sodium selenite (Se), DL-α-tocopherol (TOC), and GSH were used during the peanut regeneration and co-cultivation with A. tumefaciens. GSH, TOC, and Se not only eliminated the formation of H2O2 produced in wound tissue during preparation of leaflets and the co-cultivation with A. tumefaciens but also decreased malondialdehyde formation and enhanced superoxide dismutase and catalase activities. Therefore, GSH, TOC, or Se increased the frequency of plant regeneration and transformation efficiency of peanut explants by A. tumefaciens (Zheng et al. 2005).
Lipoic acid (LA) is a sulfur-containing compound involved in several multienzyme complexes such as pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, branched-chain keto acid dehydrogenase, and glycine decarboxylase complex. In animals, free LA and dihydrolipoic acid are metabolic antioxidants that are able to scavenge reactive oxygen species to recycle other antioxidants such as vitamin C, glutathione, and vitamin B and to increase the expression of genes involved in the regulation of normal growth and metabolism as well as redox regulation of gene transcription (Packer et al. 1995, Packer and Tritschler 1996, 1997). We hypothesize that (1) LA reduces browning of Agrobacterium-transformed cells or tissues and subsequently the death of the cells or tissues, (2) LA increases the survivability of Agrobacterium-transformed cells or tissues, resulting in an increased transient expression level and plan transformation efficiency, and (3) LA reduces escapes through promoting the differentiation, proliferation and regeneration of the transformed cells or tissues and overcoming the growth of non-transformed cells or tissues under selective pressure.
In this paper, we report the first successful application of LA to plant tissue culture and transformation and demonstrate its utility and efficacy in plant transformation across four different plant species: soybean, tomato, cotton, and wheat.
Materials and Methods
Terminology.
A transgenic plant regenerated from a single poked wound of a cotyledon was considered to be from a single independent transformation event. Only one regenerated transgenic plant from a single poked wound was counted as an independent transgenic event to ensure that each regenerate represented an independent transgenic event. Transformation efficiency (TE) was determined as the percentage of independent plant transgenic events produced per explant. Transformation frequency (TF) was determined as the percentage of transgenic plants produced per explant. An independent plant transgenic event refers to a particular genomic insertion of the desired gene into a specific plant. A shoot escape refers to a shoot that survives the selection process without having the gene encoding for resistance to the selectable marker stably transformed into the plant genome.
MicroTom transformation.
Concentrations of 0, 5, 10, 50, and 100 µM of LA were examined in the selection stage of an Agrobaterium-mediated MicroTom transformation protocol (Dan et al. 2006) using plasmid pMON15715 (Table 1). The plasmid pMON15715 contains a β-glucuronidase gene (GUS)-intron (ST-LS1) chimeric gene (Dan et al. 2006). No GUS activity is detected in agrobacteria containing this intron gene due to the lack of a eukaryotic splicing apparatus in prokaryotes (Vancanneyt et al. 1990). LA was dissolved in ethanol to make 100 mg/ml stock. Three independent experiments were conducted with approximately 150 explants tested per treatment. Data were statistically analyzed for ANOVA using Statistix 9 (Analytical Software, Tallahassee, FL 32317–2185).
About 5 d after selection, 39 to 43 explants were randomly selected from each treatment of the three independent experiments to measure their severity of browning within and around the poked wounds (usually six poked wounds made on each cotyledon) under a dissecting microscope. Low severity of browning was determined as less than 30% of the poked wounds made on each cotyledon explant that were browning. The high severity of browning or necrosis was determined as more than 30% of the poked wounds on each explant that were browning. At the same time, the severity of browning was measured and transient expression of GUS of the same explants was scored. High transient GUS expression was defined as an explant having more than 30% of the poked wounds producing blue spots. Low transient expression was defined as an explant having less than 30% of the poked wounds producing blue spots. Non-transient expression was determined as an explant having no blue spot. TE and TF were calculated from 43 to 49 explants of each treatment of the three independent experiments.
To determine the effect of LA on shoot escape production at the shoot stage, shoots were screened using root screening assay by culturing them at the stages of shoot length more than 1 cm on a selection medium with 40 mg/l kanamycin (Dan et al. 2006). The shoots that did not root on the selection medium were determined as shoot escapes. The shoots, which rooted on the selection medium and the rooted shoots were confirmed by GUS staining, were determined as transgenic shoots (Dan et al. 2006). According to Dan et al. (2006), 95% of shoots that passed the rooting screening assay were transgenic plants, which were confirmed by GUS staining, Southern blot analysis, and R1 phenotype segregation.
Wheat transformation.
Immature embryos of wheat (Triticum aestivum L) cv. Bobwhite were isolated from the immature caryopsis 13–15 d after pollination and cultured on M7 media (Dan et al. 2004) for 3–4 d in the dark at 25°C. Embryos without embryogenic callus were selected for Agrobacterium inoculation. Plasmids pMON42071, pMON42072, and pMON66350 were used for transformation (Table 1). LA at the concentrations of 0, 5, 10, 30, 50, and 100 µM each was investigated in all stages of delay, selection, and the first regeneration media using an Agrobacterium-based wheat transformation protocol (Dan et al. 2004). Two different concentrations of 25 and 50 µM LA were also tested in combinations at the stages of delay, selection, and the first regeneration media, respectively. LA was dissolved in ethanol to make 100 mg/ml stock. Two independent experiments were conducted and approximately 308 to 599 explants were tested for each treatment. Data were statistically analyzed for ANOVA using Statistix 9 (Analytical Software, Tallahassee, FL 32317–2185). Transgenic plants were confirmed by a routine rooting screening assay that was used to produce transgenic wheat (Dan et al. 2004).
Soybean transformation.
LA at the concentrations of 0, 5, 10, 50, 100, 250, and 500 µM was applied in the co-culture medium of an Agrobacterium-mediated soybean transformation protocol (Dan et al. 2004) using plasmid pMON15737 (Table 1). Soybean genotype of A3244 was used. LA was dissolved in either ethanol or potassium hydroxide to make a 100 mg/ml stock. The reason to dissolve LA in potassium hydroxide vs. in ethanol was to test any possible ethanol effect on soybean transformation when ethanol was used as a solvent. Explants were incubated in the co-culture media at 23°C in the dark for 3 d, and then transgenic plants were generated following the Agrobacterium-mediated soybean transformation protocol (Dan et al. 2004). Each concentration was tested on 170–490 explants, and data were statistically analyzed using Statistix 9 (Analytical Software, Tallahassee, FL 32317–2185). Transgenic plants were confirmed by a routine rooting screening assay that was used to produce transgenic soybean and GUS staining assay.
Cotton transformation.
For cotton transformation, LA at the concentrations of 0, 5, 10, 50, and 100 µM was applied in the selection medium UMSEL according to an Agrobacterium-mediated cotton transformation protocol (Dan et al. 2004) using plasmids pMON40507, pMON45373, and pMON52061 (Table 1). Cotton cultivar of C312 was used. Three independent experiments were conducted with 190 to 292 explants for each concentration tested, and data were statistically analyzed for ANOVA using Statistix 9 (Analytical Software, Tallahassee, FL 32317–2185).
GUS assay.
Transgenic tissue materials were assayed for histochemical GUS expression according to Jefferson (1987) but using half strength of the reagents. The tissues were incubated at 37°C overnight.
Results and Discussions
Effect of LA on browning of Agrobacterium-transformed cells or tissues in MicroTom transformation.
MicroTom explants treated at concentrations of 0, 5, 10, 50, and 100 µM LA had 38.5%, 72.1%, 65.9%, 63.2.0%, and 45.2% of explants having low severity of tissue browning, respectively (Table 2 and Fig. 1) while they had 61.5%, 27.9%, 34.2%, 36.8%, and 54.8% of explants having high severity of tissue browning, respectively. LA at 5 µM significantly reduced 2.2-fold of the explants having high severity of tissue browning while it significantly increased 1.9-fold of explants having low severity of tissue browning compared with the treatment without LA when the explants were cultured 5 d after on selection medium (Table 2). Previous studies showed that other antioxidants of ascorbic acid, cysteine, polyvinylpolypyrrolidone, and Dithiothreitol-reduced tissue browning in sugarcane, rice, and grape Agrobacterium-mediated transformation (Perl et al. 1996; Enriquez et al. 1997, 1999; Mozsar et al. 1998).
Effect of LA on transient GUS expression in MicroTom transformation.
The treatments with LA at concentrations of 0, 5, 10, 50, and 100 µM produced 17.1%, 44.1%, 51.4%, 43.2%, and 36.8% of explants having high transient expression, respectively, and they generated 82.9%, 55.9%, 48.7%, 56.8%, and 63.2% of explants having low transient expression, respectively (Table 3 and Fig. 1). LA at the concentrations of 10 µM significantly increased 3-fold of the explants having high level of transient expression compared with the treatment without LA when it was used in the selection stage of the transformation (Table 3). The increase of transient GUS expression seemed to concur with the reduction of browning of Agrobacterium-transformed cells or tissues (Tables 2, 3). This result suggested that transient GUS expression enhanced by LA was due to the prevention of Agrobacterium-transformed cells or tissues from browning by LA.
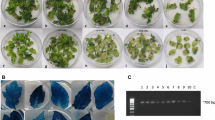
Effect of LA on tissue browning, transient expression and stable expression during MicroTom transformation. a GUS transient expression of the explants from LA treatments at the concentrations of 0, 5, 10, 50, and 100 µM 5 d after selection on 100 mg/l kanamycin. b Browning or death on the explant poked regions, which were required for Agrobacterium infection and from which transgenic plants were derived, 5 d after selection on 100 mg/l kanamycin. c GUS stable expression of shoots/buds derived from explants treated with LA at the concentrations of 0, 5, 10, 50, and 100 µM 5 d after selection on 100 mg/l kanamycin
Effect of LA on transgenic plant production in MicroTom transformation.
The LA at the concentrations of 5 and 10 µM significantly increased TE from 29.8% (without LA) to 80.0% and 87%, respectively, while LA at 10 µM had significantly increased TF from 42.6% (without LA) to 165.2% (Table 4). TF and TE increased with the reduction of browning and the increase of transient GUS expression by using LA (Table 2, 3, 4). These results seemed similar to reports of other antioxidants, including sodium selenite, DL-α-tocopherol, glutathione, cysteine, sodium thiosulfate, polyvinylpolypyrrolidone, and dithiothreitol, which enhanced plant transformation in peanut, corn, soybean, grape, Craterostigma planntagineum, and Ramonda myconi Agrobacterium-mediated transformation (Perl et al. 1996, Toldi et al. 2002, Olhoft et al. 2003, Zheng et al. 2005).
To determine if LA had any negative effect on transgenic plant production, a total of 83 transgenic plants derived from the LA treatments at all the concentrations tested were assayed by GUS staining of whole plants. One hundred percent of the plants derived from the LA treatments were GUS-positive, and 95.6% of 163 transgenic plants derived from the treatment without LA (standard MircoTom transformation protocol) were GUS-positive. This demonstrated that LA had no negative effect on transgenic plant production after it was used during the selection stage of MicroTom transformation. LA at the concentrations of 5, 10, 50, and 100 µM significantly reduced the frequency of shoot escapes from 91.5% (without LA) to 59.7%, 65.1%, 65.6%, and 46.2%, respectively (Table 4).
Effect of LA on non-transgenic and transgenic shoot development in MicroTom transformation.
To determine the effect of LA on the number of non-transgenic and transgenic shoot developed under selection pressure, the shoot growth at the stage of shoot length more than 1 cm was measured. Number of total shoots produced per explant at the concentrations of 0, 5, 10, 50, and 100 µM LA were not significantly different (Table 5). However, the percentages of transgenic shoots among the total shoots produced at the concentrations of 5, 10, 50, and 100 µM LA had significant 4.7-, 4.0-, 4.1-, and 6.3-fold increases, respectively, compared with the treatment without LA (Table 5). Also, LA at 5, 10, 50, and 100 µM significantly reduced the percentage of non-transgenic shoots produced among the total shoots from 91.5% (without LA) to 59.7%, 65.1%, 65.6%, and 46.2%, respectively. These results indicated that LA had no significant effect on total shoot development at all concentrations tested, and it significantly increased TE and TF by significantly increasing transgenic shoot production and decreasing non-transgenic shoot production. These results suggested that within a population of Agrobacterium-transformed and non-transformed cells or tissues, LA promoted the differentiation, proliferation, and regeneration of the transformed cells or tissues under appropriate selection pressure by preventing the transformed cells or tissues, which were stressed by Agrobacterium and any unfavorable in vitro culture conditions, from browning and dying at an early stage of development and subsequently enhanced its transient GUS expression. Therefore, the growth of non-transformed cells or tissues was overwhelmed by the promoted growth of the transformed cells or tissues by LA under the selective competition, resulting in a significant decrease of shoot escapes and subsequent increase of TF and TE in MicroTom transformation. Zheng et al. (2005) reported a similar result with the antioxidants, sodium selenite, DL-α-tocopherol, and glutathione, increasing the percentage of GUS-positive shoots without altering the total number of shoots regenerated in peanut Agrobacterium-mediated transformation.
This study demonstrated that using LA in MicroTom transformation resulted in a significant 2-fold reduction of browning of Agrobacterium-transformed cells or tissues and a significant 3-fold increase of the percentage of the explants having a high transient GUS expression. Subsequently, LA enabled significant 2.9- and 3.8-fold increases in TE (from 29.8% to 87.0%) and TF (from 42.6% to 165.2%), respectively, compared with the treatment without LA. The frequency of shoot escapes was significantly reduced from 91.5% without LA to 46.2% when LA was used. Utilizing LA in the standard MicroTom transformation protocol resulted in the highest TF across all the previous transformation methods for the cultivars of tomato, Lycopersicon esculentum species, based on published literatures.
Wheat transformation.
Among the LA concentrations each tested through the delay, selection, and first regeneration media, the best concentration was 50 µM, which increased the percentage of responding embryogenic calluses from 53.5% (without LA) to 83.5% and TE from 2.8% (without LA) to 5.7% (Table 6). Among the levels tested at each stage, 25 µM in the delay medium, 50 µM in the selection medium, and 50 µM in the regeneration were the most effective with a TE of 6.4% compared to 3.2% without LA (Table 7). Practically, these increases of TE were important for a commercial transgenic production to reduce the production cost and time although they were not statistically significant (P > 0.05).
Soybean transformation.
LA at the concentration of 250 µM significantly increased 6-fold TE (3.7% versus 0.6% without LA, Table 8). The low levels of LA (5 to 100 µM), which were effective in MicroTom transformation, were not particularly effective with the soybean transformation protocol used in these experiments (Table 8). No significant effect on TE was observed whether the LA was dissolved in ethanol or potassium hydroxide at the concentrations of 5, 10, 50, and 100 µM (Table 8). In addition, the frequencies of shoot escapes were reduced in soybean from 92% to 72% under the optimal conditions (data not shown). Similar results were reported using antioxidants, cysteine, to enhance transformation frequency in soybean Agrobacterium-mediated transformation (Olhoft et al. 2003).
Cotton transformation.
LA at a concentration of 50 or 100 µM in UMSEL medium at the selection stage of cotton transformation significantly increased the frequency of explants producing glyphosate-resistant embryogenic callus formation from 41.4% (without LA) to 61.2% and 56.6%, respectively (Table 9).
Suitable LA concentrations for plant transformation.
The amount of LA to include in plant transformation media varied from plant species and transformation system being employed. In our Agrobacterium-mediated MicroTom transformation, LA was most beneficial in the shoot induction media at the selection stage of the transformation at the concentrations of 5 or 10 µM. However, a higher LA concentration of 500 µM was significantly effective when included in the co-culture media using our Agrobacterium-mediated soybean transformation. With our Agrobacterium-mediated wheat transformation system, LA at the concentrations of either 50 µM or 25, 50, and 50 µM in the delay, selection, and first regeneration media, respectively, were most effective. The inclusion of LA at the concentrations of 50 or 100 µM in the selection media for our Agrobacterium-based cotton transformation system significantly increased the frequency of glyphosate-resistant embryogenic callus production. LA was added to media at various stages of the plant transformation in four different plant species to optimize its use for the particular plant species. Although the same effect was not seen for each plant species and its particular transformation process, an overall dramatically positive result was seen for each species in terms of improving plant transformation efficiency. The inclusion of LA in plant transformation medium seemed to be most beneficial during the stages of co-cultivation and selection where the plant tissues were exposed to plant stress conditions such as explant excision or Agrobacterium inoculation.
The use of LA in plant transformation has dramatically resolved the three common problems in plant transformation: browning or possible necrosis of the transformed cells or tissues, difficulty to regenerate transformed cells or tissues and shoot escapes, which severely limit the number of transgenic plants that can be regenerated. LA has some unique features compared with other antioxidants reported in plant transformation. It enhanced plant transformation efficiency across four different crop species and functioned in all four terms such as reducing tissue browning, increasing transient gene expression, increasing plant transformation efficiency, and reducing shoot escapes. It has not been reported that any other antioxidant has its all functions in plant transformation.
References
Cervera M.; Juarez J.; Navarro A.; Pina J. A.; Duran-Vila N.; Navarro L.; Pena L. Genetic transformation and regeneration of mature tissue of woody fruit plants bypassing the juvenile stage. Transgenic Res 7: 51–59; 1998.
Barry G.; Kishore G.; Padgette S.; Taylor M.; Kolacz K.; Weldon M.; Re D.; Eichholtz D.; Fincher D.; Hallas L. Inhibitors of amino acid biosynthesis: strategies for imparting glyphosate tolerance to crop plants. In: Sinch B. K.; Flores H. E.; Shannon J. C. (eds) Biosynthesis and molecular regulation of amino acids in plants. American Society of Plant Physiologists, Rochville, pp139–145; 1992.
Dan Y.; Munyikawa T.; Rayford K.; Rommens C. Use of lipoic acid in plant culture media. US Patent Pub. No.: US 2004/0133938 A1; (http://appft1.uspto.gov/netacgi/nph-Parser?Sect1 = PTO2&Sect2 = HITOFF&p = 1&u = %2Fnetahtml%2FPTO%2Fsearch-bool.html&r = 1&f = G&l = 50&co1 = AND&d = PG01&s1 = %22Dan+Yinghui%22&OS=“Dan+Yinghui”&RS=“Dan+Yinghui”)
Dan Y.; Yan H.; Munyikwa T.; Dong J.; Zhang Y.; Armstrong C. L. MicroTom—a high-throughput model transformation system for functional genomics. Plant Cell Rep 25: 432–441; 2006.
Enriquez-Obregon G. A.; Vazquez-Padron R. I.; Prieto-Samsonov D. L.; Perez M.; Selman-Housein G. Genetic transformation of sugarcane by Agrobacterium tumefaciens using antioxidants compounds. Biotechno. Appl 14: 169–174; 1997.
Enríquez-Obregón G.; Prieto-Samsónov D.; Riva G.; Pérez M.; Selman-Housein G.; Vázquez-Padrón R. I. Agrobacterium-mediated japonica rice transformation: a procedure assisted by an antinecrotic treatment. Plant Cell Tiss Organ Cult 59: 159–168; 1999.
James C. Global status of commercialized biotech/GM crops: 2005. In: ISAAA Briefs 34–2006. 2006.
James D. J.; Passey A. J.; Barbara D. J.; Bevan M. Genetic transformation of apple (Malus pumila Mill) using a disarmed Ti-binary vector. Plant Cell Rep 7: 658–661; 1989.
Jefferson R. A. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5: 387–405; 1987.
Jeon J.; Lee S.; Jung K.; Jun S.; Jeong D.; Lee J.; Kim C.; Jang S.; Lee S.; Yang K.; Nam J.; An K.; Han M.; Sung R.; Choi H.; Yu J.; Choi J.; Cho S.; Cha S.; Kim S.; An G. T-DNA insertional mutagenesis for functional genomics in rice. Plant J 22: 561–570; 2000.
Lagudah E.; Dubcovsky J.; Powell W. Wheat genomics. Plant Physiol Biochem 39: 335–344; 2001.
Lee M.; Kim H.; Kim J.; Kim S.; Park Y. Agrobacterium-mediated transformation system for large-scale production of transgenic Chinese cabbage (Brassica rapa L. ssp pekinensis) plants for insertional mutagenesis. J Plant Biol 47: 300–306; 2004.
May G. D.; Afza R.; Mason H. S.; Wiecko A.; Novak F. J.; Arntzen C. J. Generation of transgenic banana (Musa acuminata) plants via Agrobacterium-mediated transformation. Bio/Technology 13: 486–492; 1995.
Moore G. A.; Jacono C. C.; Neidigh J. L.; Lawrence S. D.; Cline K. Agrobacterium-mediated transformation of citrus stem segments and regeneration of transgenic plants. Plant Cell Rep 11: 238–242; 1992.
Mourgues F.; Chevreau E.; Lambert C.; Bondt A. Efficient Agrobacterium-mediated transformation and recovery of transgenic plants from pear (Pyrus communis L.). Plant Cell Rep 16: 245–249; 1996.
Mozsar J.; Viczian O.; Sule S. Agrobacterium-mediated genetic transformation of an interspecific grapevine. Vitis 373: 127–130; 1998.
Nomura K.; Matsumoto S.; Masuda K.; Inoue M. Reduced glutathione promotes callus growth and shoot development in a shoot tip culture of apple root stock M.26. Plant Cell Rep 178: 597–600; 1998.
Olhoft P. M.; Flagel L. E.; Donovan C. M.; Somers D. A. Efficient soybean transformation using hygromycin B selection in the cotyledonary-node method. Planta 216: 723–35; 2003.
Ostergaard L.; Yanofsky M. Establishing gene function by mutagenesis in Arabidopsis thaliana. Plant J 39: 682–696; 2004.
Packer L.; Tritschler H. Alpha-lipoic acid: The metabolic antioxidant. Free Radical Biol Med 20: 625–626; 1996.
Packer L.; Tritschler H.; Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radical Biol Med 22: 359–378; 1997.
Packer L.; Witt E.; Tritschler H. Alpha-lipoic acid as a biological antioxidant. Free Radical Biol Med 19: 227–250; 1995.
Pena L.; Cervera M.; Juarez J.; Navarro A.; Pina J. A.; Duran-Vila N.; Navarro L. Agrobacterium-mediated transformation of sweet orange and regeneration of transgenic plants. Plant Cell Rep 14: 616–619; 1995a.
Pena L.; Cervera M.; Juarez J.; Navarro A.; Pina J. A.; Navarro L. Genetic transformation of lime (Citrus aurantifolia Swing.): factors affecting transformation and regeneration. Plant Cell Rep 16: 731–737; 1997.
Pena L.; Cervera M.; Juarez J.; Ortega C.; Pina J. A.; Duran-Vila N.; Navarro L. High-efficiency Agrobacterium-mediated transformation and regeneration of citrus. Plant Sci 104: 183–191; 1995b.
Pereira A. A. Transgenic perspective on plant functional genomics. Transgenic Res 9: 245–260; 2000.
Perl A.; Lotan O.; Abu-Abied M.; Holland D. Establishment of an Agrobacterium-mediated transformation system for grape (Vitis vinifera L.): the role of antioxidants during grape-Agrobacterium interactions. Nat Biotechnol 14: 624–628; 1996.
Rogers S. G. Promoter for transgenic plants. US Patent No. 05378619; 1990.
Stipic M.; Rotino G. L.; Piro F. Regeneration and genetic transformation attempts in the cauliflower ‘Tardivo di Fano’. Italus Hortus 7: 20–26; 2000.
Toldi O.; Tóth S.; Pónyi T.; Scott P. An effective and reproducible transformation protocol for the model resurrection plant Craterostigma plantagineum Hochst. Plant Cell Rep. 211: 63–69; 2002.
Tyagi A.; Mohanty A. Rice transformation for crop improvement and functional genomics. Plant Sci 158: 1–18; 2000.
Vancanneyt G.; Schmidt R.; O’Connor-Sanchez A.; Willmitzer L.; Rocha-Sosa M. Construction of an intron-containing marker gene: Splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol Gen Genet 220: 245–250; 1990.
Yang Y.; Peng H.; Huang H.; Wu J.; Ha S.; Huang D.; Lu T. Large-scale production of enhancer trapping lines for rice functional genomics. Plant Sci 167: 281–288; 2004.
Zheng Q. S.; Ju B.; Liang L. K.; Xiao X. H. Effects of antioxidants on the plant regeneration and GUS expressive frequency of peanut (Arachis hypogaea) explants by Agrobacterium tumefaciens. Plant Cell Tiss Org Cult 811: 83–89; 2005.
Acknowledgement
The authors thank Dr. Caius M. Rommens for his helpful idea and encouragement of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Ranch
Rights and permissions
About this article
Cite this article
Dan, Y., Armstrong, C.L., Dong, J. et al. Lipoic acid—an unique plant transformation enhancer. In Vitro Cell.Dev.Biol.-Plant 45, 630–638 (2009). https://doi.org/10.1007/s11627-009-9227-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-009-9227-5