Abstract
An efficient somatic embryogenesis and plant regeneration system was developed from shoot apex explants of finger millet, Eleusine coracana. Eight genotypes, CO 7, CO 9, CO 13, CO 14, GPU 26, GPU 28, GPU 45, and GPU 48, were assessed in this study. The maximum somatic embryogenic induction, at 98.6%, was obtained from explants cultured on Murashige and Skoog medium supplemented with 18.0 μM dichlorophenoxyacetic acid and 2.3 μM kinetin. The highest number of shoot induction (26) was observed after transfer of embryonic callus to regeneration medium supplemented with 4.5 μM thidiazuran and 4.6 μM kinetin. Significant differences were observed between genotypes for somatic embryogenesis and plant regeneration. GPU 45 gave the best response, while CO 7 was the least responsive under the culture conditions tested in this study. Regenerated plants were successfully rooted and grown to maturity after hardening in soil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Finger millet (Eleusine coracana (L.) Gaertn) is a versatile grain that can be used in many different types of food and forms an essential component of the staple diet in Africa and India (Malleshi and Desikachar 1986). It is usually converted into flour and made into cakes, bread, and other bakery products. The sprouted seeds are also nutritious and easily digested. The grain may also be malted and a flour of the malted grain used as a nourishing food for infants (Magonja et al. 2007).
Finger millet production is significantly affected by fungal disease (Nicholas 2003). Integration of fungal resistant genes into finger millet is needed to enhance the fungal resistance and improve the yield (Radjacommare et al. 2004). Transgenic modification offers one method for the introduction of fungal resistance genes but requires first the development of efficient in vitro plant regeneration systems. The first in vitro study in finger millet was reported in 1976 by Rangan (1976). The induction of somatic embryogenesis in finger millet has been reported in which immature inflorescences and shoot apical meristems were induced to form somatic embryos on Murashige and Skoog (MS; Murashige and Skoog 1962) basal medium containing 2,4-d dichlorophenoxyacetic acid (2,4-d) and kinetin (KN) (Sivadas et al. 1990; Latha et al. 2005) and also from medium supplemented with picloram and kinetin (George and Eapen 1990; Eapen and George 1990).
The morphogenic potential of in vitro culture depends on the physiological status of the explant material (Caswell et al. 2000), medium composition, culture conditions (Saharan et al. 2004), and the genotype of the donor plants. Screening of genotypes for response to in vitro culture is a vital requirement for development of efficient multiplication and regeneration protocols for any given crop (Nicolle et al. 2004). To date, no detailed report is available describing the effect of the genotype on somatic embryogenesis in finger millet. This study was undertaken to examine this phenomenon and to develop an efficient somatic embryogenesis and plant regeneration system from shoot apex explants of this species.
Shoot apical meristems have been used effectively to develop regeneration systems across the cereals, and the use of apical meristem explants have been successfully employed as starting material to recover stably transformed maize, wheat, rice, oat, barley, sorghum, and millet (Sticklena and Orabya 2005). Transgenic finger millet expressing an antifungal protein has been developed by Latha et al. (2005), in which embryogenic callus was induced from the shoot apex and used as the target tissue for transgene insertion. In this study, we have obtained somatic embryos from shoot apex explants cultured on MS basal medium containing 2,4-d and KN with subsequent plant regeneration achieved on MS basal medium containing benzylaminopurine (BAP) and KN. We also report efficacy of thidiazuran (TDZ) in the embryogenic process.
We report here application of the regeneration technique into eight Indian genotypes of finger millet. These genotypes (CO 7, CO 9, CO 13, CO 14, GPU 26, GPU 28, GPU 45, and GPU 48) were selected due to their economic importance and frequency of cultivation in different geographical and climatic zones of India. CO 7, CO 9, CO 13, and CO 14 are hybrid varieties grown across 1.67 million hectares in India with an average productivity of 1,477 kg/ha (John et al. 2005). These are mainly cultivated by the farmers in the Indian states of Tamilnadu, Karnataka, and Andarapradesh. GPU 28, GPU 26, GPU 45, and GPU 48 are fungal blast-resistant, hybrid cultivars, recently released by the University of Agricultural Sciences, Bangalore, India and cultivated in the above-mentioned regions of India (Prabu 2006).
Materials and Methods
Plant material. The eight varieties of E. coracana selected for this study were: CO 7, CO 9, CO 13, CO 14, GPU 26, GPU 28, GPU 45, and GPU 48. Seeds of CO 7, CO 9, CO 13, and CO 14 were procured from Tamilnadu Agricultural University, Coimbatore, India, and GPU 26, GPU 28, GPU 45, and GPU 48 were obtained from University of Agricultural Sciences, Bangalore, India.
Seeds were de-husked and rinsed in 70% alcohol for 30 s and washed with sterile distilled water. They were then immersed in 0.1% HgCl2 for 6 min for surface sterilization and washed a further three times for 5 min each with distilled water before transferring to 100 ml Erlenmeyer flasks (30 seeds in each flask) containing MS (Murashige and Skoog 1962) basal medium supplemented with 3% (w/v) sucrose (Hi-media, Mumbai, India) and solidified with 0.6% (w/v) agar (bacteriological grade, Hi-media, Mumbai, India) for germination. The flasks were incubated at 25 ± 2°C in the dark for 3 d. Shoot tips, consisting of the apex, 4–6 mm in size, were excised inside the laminar airflow hood with the help of a scalpel blade from aseptically grown, 3-d-old seedlings. The pH of this and all medium used in the study was adjusted to 5.8 using 0.1 M NaOH before autoclaving for 15 min at 121°C and 1.3 kg/cm3 pressure. All the experiments were performed in 100 ml Erlenmeyer flasks. Inoculated cultures were incubated in the dark for induction of somatic embryogenesis and in the light (16 h) with the light intensity of 50 μm−2s−1 photosynthetic photon flux density for regeneration at 25 ± 2°C.
Induction of somatic embryogenesis. The excised explants were transferred to MS medium supplemented with 0.3% (w/v) sucrose and 2,4-d, 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) or 1-naphthaleneacetic acid (NAA) at 9.0, 18.0, or 36.0 μM to determine the optimum concentration and type of auxin for the induction of somatic embryogenesis. To determine the combined effect of auxin and cytokinin on somatic embryogenesis, MS medium containing 18.0 μM 2,4-d was further supplemented with 0.46, 2.30, and 9.20 μM KN or zeatin (ZN). These cultures were incubated at 25 ± 2°C in the dark. The resulting callus were subcultured routinely every 2 wk onto fresh medium with the same composition, and the callus was observed closely at 2-d intervals for any change in the morphology. Total number of callus producing somatic embryos was determined after 7 wk of incubation.
Plant regeneration. Well-developed somatic embryogenic callus with nodular structures containing coleoptiles, root, and shoot poles were obtained from medium supplemented with 18.0 μM 2,4-d and 2.3 μM KN and were transferred to regeneration medium consisting of MS medium supplemented with 3% (w/v) sucrose but devoid of growth regulators or MS medium containing KN or BAP at 0.46, 4.6, and 9.2 μM.
The effect of combining TDZ with KN or BAP on shoot induction was also tested by inclusion of TDZ at 2.25, 4.5, and 9.0 μM to MS medium containing 4.6 μM KN or BAP. The cultures were incubated in the light at 25 ± 2°C. Total number of shoots produced by each embryogenic callus was counted after 4 wk of incubation.
Data collection and statistical analysis. Each experiment was repeated three times with each experiment consisting of three replicates. In somatic embryogenesis, 20 shoot apex explants were used per treatment; mean percentage of somatic embryogenesis calculated after 4 wk of incubation and mean number of somatic embryos produced was counted after 7 wk of incubation. In plant regeneration, ten somatic embryogenic explants per treatment were used with mean percentage of shoot induction and mean number shoots calculated after 4 wk of culture. Mean values were calculated, and the significance of differences among means was carried out using Fisher’s least significant difference (LSD) test.
Results
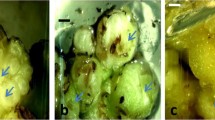
Induction of somatic embryogenesis. Mature seeds of all finger millet genotypes germinated and produced initial shoots after 3 d of culture on growth regulator-free MS medium. Shoot tips excised from these seedlings served as the initial explants for callus induction and somatic embryogenesis and were cultured on MS medium supplemented with different concentrations of 2,4-d, 2,4,5-T, or NAA. Callus induction was observed developing on the cut ends of shoot apex explants after 1 wk of incubation in the dark (Fig. 1 A). Initially, all callus from all genotypes were white in color and non-regenerative in nature, being instead, soft, friable with unorganized morphology, and devoid of nodular structures. This callus was subcultured every 2 wk onto fresh medium of the same composition, after which most of the callus became yellow, nodular, and embryogenic in nature after a total of 5 wk (Fig. 1 B), depending on the auxin type and the concentration in the medium. Only this latter type of tissue was investigated further and reported in this study.
Somatic embryogenesis and plant regeneration in finger millet. (A) Initiation of callus from excised shoot apex explants after 1 wk incubation in the dark on MS medium supplemented with 18.0 μM 2,4-d of genotype CO 14. (B) Somatic embryogenic tissue formation after 5 wk of incubation in the dark on MS medium supplemented with 18.0 μM 2,4-d and 2.3 μM kinetin of genptype GPU 45. (C) Shoot and root induction from a somatic embryo after 2 wk of culture in the light on MS medium supplemented with 4.60 μM kinetin of genotype GPU 26. (D) Shoot elongation on MS medium supplemented with 4.60 μM kinetin plus 4.5 μM thidiazuron of variety GPU 26. (E) Hardened plant of variety GPU 45. (F) Maturation and seed setting of soil grown plant of genotype CO 14.
The average percentage of induction of this embryogenic tissue ranged from 9.0% to 70.8% based on the type of auxin, concentration of auxin, and genotype of finger millet (Table 1). All three auxins tested were capable of inducing the production of embryogenic callus at the three different concentrations tested (9.0, 18.0, and 36.0 μM). Efficient somatic embryogenesis was observed from tissues cultured on MS medium supplemented with 2,4-d, while medium containing 2,4,5-T produced 60% somatic embryogenesis, and NAA-added medium produced the least response, at 53% somatic embryogenesis in GPU 45 (Table 1). Forty-six percent of the callus induced on NAA-supplemented medium remained non-embryogenic even after 5 wk of incubation. Although callus was induced at all three concentrations of auxins, not all callus converted into embryogenic tissues. The medium containing 18.0 μM 2,4-d gave the best somatic embryogenesis response in all the eight genotypes tested (Table 1: CO 7, 35%; CO 9, 46%; CO 13, 48%; CO 14, 63%; GPU 26, 61%; GPU 28, 55%; GPU 45, 70%; GPU 48, 60%).
Effect of KN and ZN with 2,4- d on somatic embryogenesis. Inclusion of cytokinins in the medium increased the percentage somatic embryogenesis as well as maturation of somatic embryos. Addition of KN or ZN to medium containing 18.0 μM 2,4-d was the most effective for the induction of somatic embryogenesis, with 98% somatic embryogenesis in GPU 45 from shoot apex explants, the maximum achieved; percentage of somatic embryogenesis was also increased in the remaining genotypes. The optimum concentration of KN and ZN was 2.3 μM with 18.0 μM 2,4-d for somatic embryogenesis (Table 2). KN produced more percentage embryogenesis than ZN at all the three concentrations. Percentage somatic embryogenesis of eight genotypes at 2.3 μM KN with 18.0 μM 2,4-d was CO 7, 53%; CO 9, 68%; CO 13, 70%; CO 14, 90%; GPU 26, 86%; GPU 28, 78%; GPU 45, 98%; GPU 48, 85%.
The percentage of embryogenic callus-producing shoots was determined by subculturing ten somatic embryogenic callus with nodular structures containing coleoptiles, root, and shoot poles derived from each treatment onto regeneration medium consisting of MS basal medium without growth regulators, MS medium supplemented with KN, and MS containing BAP (Table 3). Shoot induction response varied among genotypes but in all cases was highest on MS medium containing 4.60 μM KN. GPU 45 regenerated at the highest rate (85%) when cultured on this medium. Shoot induction ranged from 27% to 66% in the remaining seven genotypes with CO 7 being the least responsive (Table 3). Inclusion of BAP was less effective than KN for induction of shoot regeneration with MS medium alone being less effective than when a cytokinin was present (Table 3).
The rate of somatic embryogenesis was dependent on the genotypes in all treatments tested. For induction of embryogenic tissues 2,4-d was the most effective auxin, inducing the best response for all the genotypes when added to MS medium at 18.0 μM. On this medium, GPU 45 showed the greatest response, at 70.8% of the explants forming embryogenic tissues, while CO 7, at 35.0%, was the least responsive genotype tested (Table 1). In a separate experiment, KN and ZN were added to 18.0 μM 2,4-d in order to increase the frequency of somatic embryogenesis. Addition of KN or ZN to 18.0 μM 2,4-d increased the frequency of somatic embryogenesis in all the eight genotypes compared to the use of auxin alone (Table 2), such that supplementation of the medium with 2,4-d at 18.0 μM with KN at 2.3 μM increased somatic embryogenesis to 98% in the GPU 45 genotype (Table 2).
Plant regeneration. Well-developed somatic embryogenic callus possessing green cotyledons and obtained from MS medium containing 18.0 μM 2,4-d and 2.3 μM KN were transferred to regeneration medium. Embryogenic callus obtained from this medium alone produced more shoots in the regeneration medium compared to the callus obtained from the other somatic embryogenesis media tested (results not shown). Callus obtained from this medium was preferred for the remaining investigation. Initially, three types of media were used for regeneration, MS basal medium without growth regulators, MS medium supplemented with KN, and MS containing BAP. MS medium containing KN or BAP produced more shoots than growth regulator-free MS medium. In the GPU 45 genotype, MS medium without growth regulators produced an average of eight shoots per somatic embryogenic callus subcultured, MS with 4.6 μM BAP produced 12 shoots per somatic embryogenic callus, and MS containing 4.6 μM KN induced almost 16 shoots per somatic embryogenic callus. Plant regeneration also varied among the genotypes. CO 7 formed the fewest shoots (seven shoots per somatic embryogenic callus) and other six genotypes produced medium responses of shoot induction (CO 9, 9%; CO 13, 10%; CO 14, 14%; GPU 26, 11%; GPU 28, 12%; GPU 48, 13%) on MS medium containing 4.6 μM KN. The optimum concentration of both BAP and KN for regeneration was 4.6 μM. MS medium supplemented with KN was found to be superior to that containing BAP for the induction of shoot regeneration from the somatic embryos. MS medium containing KN produced the better response across all the genotypes compared to BAP (Table 4).
Effect of TDZ used in combination with KN or BAP on regeneration. Combining TDZ with BAP or KN in the shoot regeneration medium increased the mean number of shoots recovered in all the genotypes tested. Three concentrations (2.25, 4.50, and 9.0 μM) of TDZ were added to MS medium supplemented with 4.6 μM KN or BAP. The combinations of TDZ with KN gave a superior response compared to that obtained when embryos were cultured on medium containing TDZ with BAP (Table 5). Increasing the concentration of TDZ from 2.25 to 4.5 μM also correspondingly increased the number of shoots regenerated from 17 to 22 per somatic embryogenic callus in the responsive genotype GPU 45 (Table 5). Increased concentration of TDZ also increased the number of shoots regenerated in the remaining genotypes, with earlier shoot induction. Further increase in the concentration of TDZ (9.0 μM) adversely affected shoot regeneration (Table 5).
Effect of genotypes on plant regeneration. Plant regeneration varied greatly among the genotypes studied in this investigation. GPU 45 produced the highest number of somatic embryos (Table 6) and shoots (Table 5), while CO 7 produced the least number of somatic embryos. GPU 45 produced an average of 45 somatic embryos and 26 shoots per somatic embryogenic callus, while CO 7 produced only seven somatic embryos and 16 shoots (Tables 5 and 6) per somatic embryogenic callus, the other remaining six genotypes producing intermediate levels of responses (Tables 6 and 5).
Acclimatization and transfer to soil. Regenerated plants were rooted on the same regeneration medium consisting of MS basal media supplemented with cytokinins (Table 4 and 5) and, after 6 wk of incubation in the light, were transferred to the field after hardening with a 90% survival rate. There was no significant difference among genotypes in survival rate through the soil establishment phase. Regenerants grew well and did not show any variation in morphology and growth characteristics compared to control. Flower and seed settings, panicle morphology, maturation of panicle and yield of the grain were also similar to control plants (Fig. 1 F).
Discussion
In this study, mature seeds of finger millet were germinated in culture and used to obtain shoot apex explants for the successful induction of embryogenic tissues and subsequent plant regeneration. While this confirms previous reports (Tyagi et al. 2001; Latha et al. 2005), we have shown that this regeneration system can be extended into a range of agronomically important Indian varieties of finger millet. Explants derived from mature seeds are considered an excellent source material for biotechnological application due to easy storage and accessibility to large amounts of uniform quality explant material (Jiang et al. 2000; Sudhakar et al. 2004).
In order to induce somatic embryogenesis in finger millet, the three auxins, 2 4-d, 2,4,5-T, and NAA, were tested separately and in combination with KN or ZN. 2,4-d was found to be the most effective source of auxin when used at 18.0 μM. The induction of callus in cereals and millets is commonly achieved by 2,4-d (Mohanty et al. 1985; Chandra and Kothari 1995). However, in this study, addition of KN or ZN to medium containing 18.0 μM 2,4-d increased the percentage of somatic embryogenic induction from shoot apex explants, with the optimal response achieved on medium supplemented with KN at 2.3 μM. Pius et al. (1994) also used 2,4-d and KN for somatic embryogenesis on two of these genotypes (CO 9 and CO13), while Eapen and George (1990) obtained somatic embryos when combining KN and picloram in MS medium. 2,4-D and KN have also been used for callus induction and somatic embryogenesis in the related species Eleusine indica (Yemets et al. 2003).
In order to achieve plant regeneration from the embryogenic tissues, three types of media were used (MS alone, MS + KN, and MS + BAP). Although shoot recovery was achieved from all three media types, inclusion of cytokinin was found to be significantly beneficial, with KN inducing more shoots than BAP (Table 4). Addition of plant growth regulators in the medium seems to be essential for efficient regeneration in finger millet. Mohanty et al. (1985) used a lower level (1.1 μM) of 2,4-d for regeneration. KN and BAP have also been used for efficient shoot induction and plant regeneration in pearl millet (Mythili et al. 2001; Srivastav and Kothari 2002), kodo millet (Nayak and Sen 1989), and foxtail millet (Xu et al. 1984).
Addition of TDZ to KN or BAP in the regeneration medium increased the mean number of shoots recovered in all genotypes tested. TDZ is a substituted phenylurea which shows high cytokinin activity (Mok et al. 1982), possibly by stimulating conversion of cytokinin nucleotides to more biologically active nucleotides (Capelle et al. 1983) and promoting accumulation of endogenous purine cytokinins (Thomas and Katterman, 1986). In this study, the highest rate of shoot regeneration occurred on medium supplemented with 4.5 μM TDZ and 4.6 μM KN (Table 5). Franklin et al. (2004) found that addition of TDZ to other cytokinins had a synergistic effect and produced more shoots in sorghum. In this study, addition of TDZ to KN produced more shoots compared to the addition of TDZ to BAP.
The effect of genotypes on morphogenic potential in vitro has been reported in cereals and millets including wheat (Zale et al. 2004; Filippov et al. 2006), barley (Hanzel et al. 1985; Bregitzer 1992), and pearl millet (Mythili et al. 1997). Screening of varieties for their response in culture is important since it allows the identification of genotypes that are more amicable for manipulation within biotechnological applications. In this study, eight genotypes of finger millet have been screened for their capacity to undergo somatic embryogenesis and plant regeneration. All varieties tested were able to undergo somatic embryogenesis and plant regeneration under the culture conditions tested; however, the degree of morphogenic response was found to be genotype dependent. GPU 45 was the most responsive in all treatments, while CO 7 was consistently the least responsive.
In conclusion, a system for somatic embryogenesis and plant regeneration has been developed which is effective across a range of Indian finger millet varieties. This was achieved by combining auxin and cytokinin at appropriate concentrations and combinations to stimulate somatic embryogenesis from seedling-derived explants and the use of TDZ in the plant regeneration stages. This protocol may form the basis of transgenic technologies for this important crop species.
References
Bregitzer P. Plant regeneration and callus type in barley: effects of genotype and culture medium. Crop Sci 32: 1108–1112; 1992.
Capelle S.; Mok D.; Kirchner S.; Mok S. Effects of thidiazuran on cytokinin autonomy and metabolism of N6 (Δ2-isopentyl)(8- 14C) adenosine in callus tissues of phaseolus linatus. L. Plant Physiol 73: 796–802; 1983.
Caswell K.; Leung N.; Chibbar R. N. An efficient method for in vitro regeneration from immature inflorescence explants of Canadian wheat cultivars. Plant Cell Tissue Organ Cult 60: 69–73; 2000. doi:10.1023/A:1006357501737.
Chandra N.; Kothari S. L. Advances in tissue culture and genetic transformation of cereals. J. Indian Bot. Soc 74: 323–342; 1995.
Eapen S.; George L. Influence of phytohormones, carbohydrates, amino acids, growth supplements and antibiotics on somatic embryogenesis and plant differentiation in finger millet. Plant Cell Tissue Organ Cult 22: 87–93; 1990. doi:10.1007/BF00043683.
Filippov M.; Miroshnichenko D.; Vernikovskaya; Sergey The effect of auxins, time exposure to auxin and genotypes on somatic embryogenesis from mature embryos of wheat. Plant Cell Tissue Organ Cult 84: 100192–100201; 2006. doi:10.1007/s11240–005–9026–6.
Franklin G.; Carpenter L.; Davis E.; Reddy C. S.; Al-Abed D.; Abou Alaiwi W.; Parani M.; Smith B. Factors influencing regeneration of soybean from mature and immature cotyledons. Plant Growth Regul 43: 73–79; 2004. doi:10.1023/B:GROW.0000038359.86756.18.
George L.; Eapen S. High frequency plant regeneration through direct shoot development and somatic embryogenesis in immature inflorescence cultures of Finger millet (Eleusine coracana (L.) Gaertn). Euphytica 48: 269–274; 1990. doi:10.1007/BF00023660.
Hanzel J. J.; Miller J. P.; Brinkman M. A.; Fendos E. Genotype and media effects on callus formation and regeneration in barley. Crop Sci 25: 27–3; 1985.
Jiang J.; Steve D. L.; Wang J.; James H. O. High efficiency transformation of U.S. rice lines from mature seed-derived Calli and segregation of glufosinate resistance under field conditions. Crop. Sci 40: 1729–1741; 2000.
John J. A.; kumaravadivel N.; Nirmalakumari A.; Senthil N.; Mohanasundaram K.; Raveendran T. S.; Mallikavangamudi V. A high yielding Finger millet variety CO (Ra) 14. Madras Agric. J 92: 375–380; 2005.
Kothari S. L.; Kumar S.; Agarwal K. In vitro induction and enlargement of apical domes and formation of multiple shoots in Finger millet, Eleusine coracana (L). Gaertn. and Crow foot grass, Eleusine indica (L). Gaertn. Curr Sci 81: 1482–1485; 2001.
Latha M. A.; Dasvantha Reddy V.; Madavi latha A.; Venkateswara Rao K. Production of transgenic plants resistant to leaf blast disease in finger millet (Eleusine coracana (L.) Gaertn.). Plant Sci 169: 657–667; 2005. doi:10.1016/j.plantsci.2005.05.009.
Magonja, M. A.; Lenne, J. M.; Manyasa, E.; Sreenivasaprasad, S. (eds.). Finger Millet Blast Management in East Africa. Creating opportunities for improving production and utilization of finger millet. Proceedings of the First International Finger Millet Stakeholder Workshop, Projects R8030 & R8445 UK Department for International Development—Crop Protection Programme. International Crops Research Institute for the Semi-Arid Tropics. 196 pp. ISBN: 978–92–9066–505–2. 2007.
Malleshi N. G.; Desikachar H. S. R. Studies on comparative malting characteristics of some tropical cereals and millets. J Inst Brew 92: 174–176; 1986.
Mohanty B. D.; Gupta S. D.; Ghosh P. D. Callus initiation and plant regeneration in ragi (Eleusine coracana (L). Gaertn). Plant Cell Tissue Organ Cult 5: 147–150; 1985. doi:10.1007/BF00040311.
Mok M. C.; Mok D. w.; Amstrong D. J.; Shudo K.; Isogai Y.; Okamanto T. Cytokinin activity of N-phenyl-N’-1,2,3-thiadiazol-5-urea(thidiazuron). Phytochemistry 21: 1509–1511; 1982.
Murashige T.; Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15: 473–497; 1962. doi:10.1111/j.1399–3054.1962.tb08052.x.
Mythili P.; Madhavi A.; Reddy V. D.; Seetharam N. Efficient regeneration of pearl millet Pennisetum glacum (L.) from shoot tip cultures. Indian J. Exp. Biol 39: 1274–1279; 2001.
Mythili P.; Satyavati V.; Pavankumar G.; Rao M. V.; Manga V. Genetic analysis of short term callus culture and orphogenesis in pearl millet, Pennisetum glacum. Plant Cell Tissue Organ Cult 50: 171–178; 1997. doi:10.1023/A:1005919306646.
Nayak P.; Sen S. K. Plant regeneration through somatic embryogenesis from suspension cultures of a minor millet, Paspalum scorbiculatum (L.). Plant Cell Rep 8: 296–299; 1989. doi:10.1007/BF00274134.
Nicholas J. T. On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea. Annu Rev Microbiol 57: 177–202; 2003. doi:10.1146/annurev.micro.57.030502.090957.
Nicolle H.; Randal L.; Nelson L.; Schuyler S. Influence of media components, pH on somatic embryo induction in three genotypes of soybean. Plant Cell Tissue Organ Cult 77: 157–163; 2004.
Pius J.; George J.; Eapen S. Influence of genotype and phytohormones on somatic embryogenesis and plant regeneration in finger millet. Proc Indian Natl Sci Acad B Biol Sci 601: 53–56; 1994.
Prabu, M. J. New finger millet varieties resistant to fungal attacks. The Hindu: Online edition of India’s National Newspaper: April 06. 2006
Radjacommare R.; Ramanathan A.; Kandan A.; Sible G. V.; Harish S.; Samiyappan R. Purification and anti-fungal activity of chitinase against Pyricularia grisea in finger millet. World. J. Micro. Biotech 20: 251–256; 2004. doi:10.1023/B:WIBI.0000023829.98282.0f.
Rangan T. S. Growth and plant regeneration in tissue culture of some Indian millets;Papsalum scorbiculatum (L.); Eleusine coracana (L.) Gaertn. and Pennisetum typhoideum Pers. Pflawzenphysiol 78: 208–216; 1976.
Saharan V.; Yadav R. C.; Yadav R. N.; Chapagain B. High frequency plant regeneration from desiccated calli of indica river (Oryyza Sativa L.) Afr J Biotechnol 3: 256–259; 2004.
Sivadas P.; Kothari S. L. Chandra, N. High frequency embryoid and plantlet formation from tissue culture of the Finger millet- Eleusine coracana (L). Gaertn. Plant Cell Rep 9: 93–96; 1990.
Srivastav S.; Kothari S. L. Embryonic callus induction and high frequency plant regeneration in pearl millet. Cer. Res. Commun 30: 69–74; 2002.
Sticklena M. B.; Orabya H. F. Shoot apical meristem: a sustainable explant for genetic transformation of cereal crops. In vitro Cell Dev. Biol. Plant 41: 187–200; 2005.
Sudhakar D.; Duc L. T.; Bui Ba Bong B. B.; Tinjuangjun P.; Maqbool S. B.; Valdez M.; Jefferson R.; Christou P. An efficient rice transformation system utilizing mature seed-derived explants and a portable, inexpensive particle bombardment device. Transgenic Res 7: 289–294; 2004.
Thomas J. C.; Katterman F. R. Cytokinin activity induced by thidiazuran. Plant Physiol 81: 681–683; 1986.
Tyagi K.; Gupta P.; Raguranshi S. Assessment of the efficiency of various gene promoters via Biolostics in leaf and regenerating seed callus of millets, Eleusine coracana and Echinochola crusgalli. Plant Biotechnol 18: 275–282; 2001.
Xu Z.; Wang D.; Yang L.; Wei Z. Somatic embryogenesis and plant regeneration in callus cultured immature inflorescence of Seturia italica. Plant Cell Rep 3: 144–150; 1984. doi:10.1007/BF00270210.
Yemets A.; Klimkina L.; Tarassenko L.; Blume Y. Efficient callus formation and plant regeneration of goose grass (Eleusine indica (L.) Gaertn.). Plant Cell Rep 21: 503–510; 2003.
Zale J. M.; Borchand H.; Kidwell K. K.; Stebber C. M. Callus induction and plant regeneration from mature embryo of diverse set of wheat genotypes. Plant Cell Tissue Organ Cult 76: 277–281; 2004.
Acknowledgements
The authors acknowledge Dr. K.T. Krishne Gowda, Project Coordinator, Small Millets, University of Agricultural Sciences, Bangalore for kindly providing finger millet seed varieties and Mr. P. Kannan & Mr. A. Prem Kumar of Entomology Research Institute, Loyola College, Chennai for their support in carrying out this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Nigel James Taylor
Rights and permissions
About this article
Cite this article
Antony Ceasar, S., Ignacimuthu, S. Efficient somatic embryogenesis and plant regeneration from shoot apex explants of different Indian genotypes of finger millet (Eleusine coracana (L.) Gaertn.). In Vitro Cell.Dev.Biol.-Plant 44, 427–435 (2008). https://doi.org/10.1007/s11627-008-9153-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-008-9153-y