Abstract
The repair of skeletal defects is the main goal of bone tissue engineering. Recent literature highlighted various regulatory roles of microRNAs in stem cell fate determination. In addition, the role of porous hydroxyapatite/polycaprolacton (nHA/PCL) as a bioactive scaffold which enhances adipose tissue-derived mesenchymal stem cells (AT-MSCs) growth and osteogenic differentiation has been proved. The aim of the present study was to investigate the synergistic potential of both down-regulating miR-221 and nHA/PCL scaffold seeding in osteogenic potential of AT-MSCs. After isolation and characterization of AT-MSCs, the transfection of anti-miR-221 was performed into the cells using lipofectamine 2000 and the transfected cells were seeded into a synthesized nHA/PCL scaffold. The DAPI staining confirmed the presence of AT-MSCs on nHA/PCL scaffold. Quantitative expression of osteoblast marker genes, Runx2, and osteocalcin of the transfected cells in the scaffold were evaluated. Interestingly, significant upregulation of transcribed Runx2 and osteocalcin genes (P < 0.01) were observed in miR-221-inhibited nHA/PCL seeded cells. Also, alkaline phosphatase activity (ALP) was significantly higher (P < 0.01) in miR-221-inhibited AT-MSCs seeded on nHA/PCL than those seeded on nHA/PCL or transfected with anti-miR-221, individually. The results of this combination suggest a valuable method for enhancing osteogenesis in AT-MSCs. This method could be applicable for gene-cell therapy of bone defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans are exposed to a range of diseases and impairments related to bone tissue. These defects can be treated with conventional medicine. Some of the osteogenic impairments occur due to muscular atrophy, reflex sympathic dystrophy, osteoporosis, cancer, and other diseases. In these cases, an alternative strategy to accelerate bone re-struction is osteocunductive substitutes such as cell therapy and tissue engineering.
Current studies on bone fracture healing impairments are mainly related to synthetic bone implants, auto and allo-grafting. Since applying cell-based regenerative medicine have faced several limitations, in clinical trials setting of safe and efficient cell therapies need more substantial efforts (Gómez-Barrena et al. 2015). Adipose tissue-derived mesenchymal stem cells (AT-MSCs) are known as a multi-potent adult-derived stem cell which can be isolated from bone marrow, ligaments, muscle, heart, liver, skin, umbilical cord blood, placenta, embryonic cells, and adipose tissue (Vats et al. 2002; Zuk et al. 2002; Le Blanc and Pittenger 2005). The adipose stem cells have the potential of quick growing, easier harvesting from small volumes of adipose tissue, and lower donor site morbidity (Ahn et al. 2009). Moreover, the adipose stem cells have a high proliferative capacity, which can therefore be applied for more passages (Cai et al. 2012). Parker et al. (2007) reported spontaneous proliferation and transformation for 4 to 5 mo in particular adipose stem cells whose phenotypes have not been changed (Parker et al. 2007). Finally, it was approved that the adipose stem cells have similar characteristics to the bone marrow stem cells, which need a less-invasive isolation method and can obtain large quantities of cells in small amount of tissue (Zuk et al. 2002; Prante et al. 2006). In this study, AT-MSCs were obtained from adipose tissue with regard to the mentioned advantages from previous studies.
Bone is an active tissue containing both organic and inorganic ceramic composites. Organic collagen fibrils, as an elastic protein is embedded in an inorganic chemical material known as calcium hydroxylapatite. This compound is the osseous tissue, which gives rigidity to bones (Hellmich and Ulm 2003; Poinern et al. 2009). In a laboratory scale, the osteoconductivity quality can be obtained from HA particle on a nanometric scale (nHA) (Hench et al. 1998). The nHA has some biologic advantages, including specific affinity to many adhesive proteins, direct involvement in the bone cell differentiation, and mineralization. Therefore, nHA has been attracted for bone regeneration (Habraken et al. 2007). On the other hand, polycaprolactone (PCL) is a biodegradable polymer with significant biocompatibility and toughness (Chastain et al. 2006; Xin et al. 2007). It is a semi-crystalline aliphatic polymer with a remarkable mechanical strength, slow degradation, and higher fraction energy among all the biocompatible polymers (Elfick 2002). Moreover, PCL can be dissolved in chloroform, dimethylformamide (DMF), hexafluoroisopropanol (HFIP), dichloromethane (DCM), and tetrafluoroethylene (TFE) solvent. However, the PCL is hydrophobic; the nHA can combine with it properly (Serra et al. 2013).
When the PCL is used as a polymer matrix in some inorganic composite like HA, bone regeneration will be promoted. The biomechanical characteristic of this scaffold is higher due to avoidance of poor surface wetting and interaction with biological fluid (Shor et al. 2007; Wutticharoenmongkol et al. 2007). Therefore, combing nHA/PCL allows the scaffold to hold the mechanical properties for a proper time as part of a tissue (Wang et al. 2010). Furthermore, the polymer solution concentration and electrospinning parameter investigated the sufficient effect of nHA/PCL composite (Venugopal et al. 2010). It was indicated that the incorporation of 10% (w/w) nHA with 7.5% (w/v) PCL solution is able to produce sufficient submicron-sized beadless fibers (Hassan et al. 2014).
MicroRNAs (miRNAs) are a group of tiny (∼22 nt) RNAs (Bauersachs and Thum 2011). They have major regulatory functions in cell differentiation (Alizadeh et al. 2015) especially at post-transcriptional level. MiRNAs operate by binding to the messenger RNA (mRNA) through base-pairing mechanism, which can therefore break down or switch off the expression of a target gene. Almost 60% of cellular mRNAs are regulated by miRNAs (Friedman et al. 2009). Schindeler and Little (2006) indicated that 80% of relative genes in AT-MSCs committed to an osteogenic fate are regulated and matched with miRNAs (24). Hence, it can be concluded that miRNAs play important roles in osteogenesis. Furthermore, other studies indicate that miRNAs regulate the translation of more than one third of human mRNAs (Bartel 2004; Bentwich 2005). According to the study by Bakhshandeh et al. (2012a, b), miR-221 is one of the most important miRNAs in osteogenesis pathway, but its specific function is still unknown (Bakhshandeh et al. 2012a). Several successful transfections of collagen I, bone morphogenetic protein 2, and four genes have been performed on AT-MSCs for musculoskeletal therapeutic goals (Damme et al. 2002; Reiser et al. 2005). Studies on the effects of using biocompatible scaffolds as externally supporting tools for improving bone tissue engineering as well as internal direction of osteogenic commitment by artificially developed miRNAs may help in optimizing cell-based therapy of bone defects. In this study, we investigated the effect of miR-221 inhibition in osteogenic potential of AT-MSCs independently and also in combination with nHA/PCL scaffold seeding.
Materials and Methods
Isolation of AT-MSCs
The subcutaneous abdominal fats were obtained by liposuction from contributors. This project was approved by the University ethics committee before any samplings, and written informed consent was signed by each contributor in the Center of Endocrinology and Metabolism Taleghani Hospital, Tehran, Iran. The adipose tissue samples were extensively washed with sterile phosphate-buffered saline (PBS) containing 1000 U/ml penicillin and 1000 μg/ml streptomycin. Then specimen was cut carefully into 1 × 3 mm pieces. The extracellular matrix was digested with 0.1% collagenase type I (Gibco, Invitrogen, Paisley, UK) at 37°C and shaken vigorously for 60 min to separate the stromal cells from primary adipocytes (Alizadeh et al. 2014a, b). An equal volume of low-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, South Logan, USA) containing 10% fetal bovine serum (FBS; Gibco; Carlsbad, CA) was added to neutralize collagenase activity. Dissociated tissue was filtered and centrifuged at 1500 rpm for 10 min. The supernatant containing lipid droplets was discarded and the cell pellet was resuspended and washed twice. An osmotic buffer was used to lyse erythrocytes, and the remaining cells were plated into 6-well plate at a density of 1 × 106 cell/ml. Plating and expansion medium consisted of l-DMEM with 10% FBS (Gibco), 100 U/ml penicillin, and 100 mg/l streptomycin. Once the adherent cells were more than 80% confluent, they were detached with 0.25% trypsin–0.02% EDTA and re-plated at a dilution of 1:3.
Flow cytometry
Flow cytometry was performed to characterize isolated stem cells. About 2 × 105 AT-MSCs were incubated with primary antibodies against human CD105, CD45, CD73, and CD90 (Biolegend, London, UK) for 30 min at room temperature. Also, same-species same-isotype-irrelevant antibodies as negative control were used. Then cells were washed twice in PBS and incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (1:50 dilution) for 30 min at 4°C. After two washing steps, cells were resuspended in 300 μl PBS for flow cytometry analysis and analyzed by fluorescein-activated cell sorting (FACS) Calibur (BD Biosciences, San Jose, CA, USA).
Fabrication of nHA/PCL
For preparation of nHA/PCL, 0.009 g hydroxyapatite nanocrystals (Sigma, St Louis, MO, USA) was dissolved in 2 cm3 DMF (N,N-dimethylformamide) and then 8 cm3 chloroform (Sigma-Aldrich) under magnetic stirring for 15 min. Afterward, it was exposed to ultrasound for 15 min and again 15 min under magnetic stirring to insure breakdown of all stacks. Subsequently, 1.2 g PCL (Sigma) was added to HA suspension. It was placed on a hot plate for 2.30 h (115 rpm, 25°C) and under magnetic stirring for 10 min, sonication for 10 min, and finally, stirring for 10 min. This solution was placed into two syringes (5 cm3 each syringe) and injected to the collector for collecting electrospun fibers.
Cell culture and differentiation
AT-MSCs were expanded and maintained undifferentiated in growth medium containing high glucose DMEM (Gibco), supplemented with 10% FBS (Gibco) and 1× antibiotics (penicillin/streptomycin). Cells were cultured under humidified atmosphere of 95% air/5% CO2 at 37°C in 24-well tissue culture polystyrene (TCP) with initial cell density of 104 cm−1. Cells were divided into five groups, including growth medium containing osteogenic supplement (positive control) and without osteogenic supplements (negative control); other groups were treated with nHA/PCL and anti-miR-221 in combination and individually. All groups (except negative control) were cultured in differentiation medium and supplemented with 250 nM dexamethasone, 50 μg/ml ascorbic acid 2-phosphate/ml, and 10 mM beta-glycerol phosphate (Sigma).
MicroRNA transfection
AT-MSCs were reverse transfected (Bakhshandeh et al. 2012a) with anti-hsa-miR-221 (inhibitor of hsa-miR-221, Qiagene) using siPORT™ NeoFX™ Transfection Agent (Invitrogen) according to the manufacturer’s instruction. After 72 h incubation, the cells were washed and harvested for further analysis. Transfection efficiency was checked using a FAM-labeled scrambled RNA.
Alizarin red staining
Alizarin red was used to stain calcium deposition in differentiated AT-MSCs. For this purpose, cells were washed by PBS then fixed with 4% paraformaldehyde (1 h at 25°C). After that, samples were rinsed in PBS and stained with 1% Alizarin red S (pH 4.1, 10 min at 25°C). The excess stain was removed by 0.1% HCL solution. Finally, light microscope was used to observe calcium precipitation.
DAPI staining
After PBS washing, add 4% cold paraformaldehyde for 20 min at 4°C and 5 min at room temperature (RT), and then incubate with 0.4% Triton X-100 for 10–30 min at RT. For nuclear staining, add DAPI (1 μg/ml) for 10–60 s. The images were taken by a fluorescent microscope (Olympus, Center Valley, PA , USA).
Real-time PCR
Total RNA of treated AT-MSCs from each group was extracted 21 d after differentiation using RNX™ Plus (CinnaGen Co.,Tehran, Iran). Extraction from nHA/PCL scaffold was performed by immersion of cellular scaffold directly in RNA lysis solution according to the manufacturer’s instructions. Non-treated AT-MSCs were used as negative control. Reverse transcription of extracted RNA samples was performed using Revert Aid kit (Thermo scientific, Waltham, MA, USA) as recommeneded by the manufacturer. Real-time PCR reactions were run using Maxima SYBR Green/ROX qPCR Master Mix (Amplicon, Brighton, UK) and monitored in the Rotor-gene Q real-time analyzer (Corbett, Sydney, Australia). Data were normalized to an endogenous control gene (GAPDH). All experiments were performed in triplicate, and relative expression was calculated using the comparative ΔΔ Ct method. The primer sequences used for real-time PCR reactions are shown in Table 1.
Alkaline phosphatase activity measurement
Levels of alkaline phosphatase activity were determined in cell lysates using p-nitrophenyl phosphate as the substrate (Sigma-Aldrich). The procedure was carried out according to the manufacturer’s instruction. Briefly, the cells growing in 24-well plates (Orang Scientific, Braine-l’Alleud, Belgium) were washed twice with PBS and incubated with 1% Triton X-100 (Sigma) for 30 min at 37°C. The resulting lysate was homogenized and then incubated with the substrate and the amount of liberated p-nitrophenol (pNP) was measured at 405 nm (Biotek plate reader, Winooski, United States). Alkaline phosphatase activity values were normalized to the total protein content.
Statistical analysis
One-way ANOVA was used to determine whether there is a significant difference between the groups. Statistical significance was defined at P < 0.05. The data were presented as mean ± SD.
Results
AT-MSCs were isolated and characterized
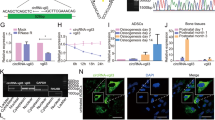
The isolated AT-MSCs cultivated and reached to the third passage (Fig. 1a ), then subjected to flow cytometry to evaluate their surface marker expression. The results showed that AT-MSCs express CD73, CD90, and CD105, which are known as mesenchymal stem cells markers, while lacking CD45 expression as a hematopoietic marker (Fig. 1b ).
AT-MSCs were cultured in expansion and differentiation medium as negative and positive controls, respectively. The group 3 cells were transfected individually by anti-miR-221, group 4 cells seeded on nHA/PCL scaffold, and combination of anti-miR-221 with nHA/PCL scaffold seeding was performed for group 5 in differentiation media (Tables 1 and 2).
In this research, AT-MSCs were transfected with anti-miR-221 using lipofectamine reagent. Validation of miRNA transfection was checked by a FAM-labeled scrambled RNA (Fig. 2).
Figure 3a shows the structure of nHA/PCL scaffold before the seeding of the cells. To confirm the presence of AT-MSCs on nHA/PCL scaffold, the cells were stained by DAPI. The stained cells were observed under a fluorescent microscope (Fig. 3b ).
After 21 d of induction, the osteogenic differentiation was investigated
Alizarin red staining was used to determine biomineralization in differentiated AT-MSCs. Figure 4b represents the Alizarin red staining which reflected the biological function of osteoblast in AT-MSCs before and after treatment. However, AT-MSCs on day 1 did not show any calcium accumulation (Fig. 4a ).
To evaluate osteogenic induction, quantitative transcriptional evaluation was performed by real-time PCR for osteocalcin and Runx2 genes, which are common osteogenic differentiation markers. Figure 5 indicates the relative transcriptional levels of osteogenic markers (osteocalcin and Runx2) after 21 d of differentiation in five groups.
Real-time analysis of RUNX2 (a) and osteocalcin (b) relative expression in treated AT-MSCs by anti-miR-221, scaffold, and scaffold + anti-miR-221. Values are shown as mean ± SD. Statistically different values (determined compared with the day 21 of differentiation): *P < 0.05; **P < 0.01; ***P < 0.001.
A significant difference in the level of the genes RUNX2 and osteocalcin expression showed that combination of anti-miR-221 and nHA/PCL scaffold can induce the osteogenesis most effective among all treatments
Runx2 expression in group anti-miR-221 inhibited then nHA/PCL scaffold seeded had increased 8.24- and 6.83-fold in comparison with groups 1 and 2, respectively. The expression level of osteocalcin in miR-221-inhibited AT-MSCs seeded on nHA/PCL scaffold were 14 and 5 times higher than miR-221-inhibited AT-MSCs and scaffold seeded AT-MSCs, respectively (Fig. 5b ).
ALP activity results are summarized in Fig. 6. The highest ALP activity was detected in day 14 of anti-miR-221+ nHA/PCL group (group 5).These results emphasized that the downregulation of miR-221 and using nHA/PCL scaffold can cause the upregulation of osteogenesis marker genes.
ALP activity of lysates from four groups of the cells including: differentiating AT-MSCs, AT-MSCs seeded on nHA/PCL scaffold, anti-miR-221 transfected MSCs, and anti-miR-221 transfected nHA/PCL scaffold seeded AT-MSCs harvested on days 0, 7, and 14. Significant higher ALP activity was observed in nHA/PCL+ anti-miR-221 group (**P < 0.01).
Discussion
Providing osteoblast tissue from proper stem cells is one of the major challenges in bone defect regeneration. Several studies focused on the efficiency of AT-MSCs for bone defect treatment (Ohgushi et al. 1989; Bruder et al. 1994). AT-MSCs have various advantages compared with other stem cells, including immunosuppressive capabilities which make them adequate for clinical application (Undale et al. 2009). AT-MSCs do not need immunosuppression of host with respect to the description as histocompatibility class I positive and major histocompatibility class II negative and are known to lack expression of co-stimulatory molecules such as CD40, CD80, and CD86 (Javazon et al. 2004; D’Antò et al. 2013). With regard to all the potential clinical features and promises of AT-MSCs for repair of bone in skeletal diseases in this study, we used this type of stem cells.
The second step in bone tissue engineering is introduction of AT-MSCs into the appropriate matrices, which is one of the main challenges in tissue engineering. Several studies investigated that PCL can be an alternative synthesis polymeric scaffold in bone tissue engineering. While, the nHA/PCL composite scaffolds have shown greater proliferation (D’Antò et al. 2013; Osathanon et al. 2014). Alkaline phosphatase enzymatic activity and mineral deposition are important factors in bone tissue engineering, and Osathanon et al. (2014) reported that these two factors are higher on nHA/PCL compared with PCL. Therefore, nHA/PCL scaffolds may represent a new therapeutic strategy for repairing bone defects (Osathanon et al. 2014).
In osteogenesis, several factors contribute and miRNAs as intra-cellular regulators play a significant role. MiRNA are closely involved in regulating gene expression at the post-transcriptional level and differentiation into certain lineage. MiRNAs could have a significant influence on bone formation, therefore providing a potential treatment for bone disease and injuries. MiRNA can bind to the specific sequence at 3′-untranslated region (3′-UTR) through complementary base pairing and repress the gene expression. Furthermore, have a key role in many signaling pathways such as human musculokeletal system (Dong et al. 2012).
However, assistance of miRNAs have supported in miscellaneous physiological mechanism, and their key role in osteogenesis and its application in bone tissue engineering have not been considered. These studies can provide necessary information in therapeutic approaches. It has been reported that transfected unrestricted somatic stem cell (USSC) by anti-miR-1274a, syn-let-7a, syn-miR-624, anti-miR-376, syn-miR-30b, and anti-hsa-miR-221 showed osteogenic development (Bakhshandeh et al. 2012b).
MiRNA functions and their specific targets in bone development have not been fully determined. Recently, some new pieces of evidence in particular miRNA functions have been achieved. It has been shown that miR-23a, miR-cluster 23a-27a-24, miR-141, miR-200a, miR-135b, miR-208, miR-155, miR-206, miR-2861, miR-196a, and miR-125b are involved in osteocyte differentiation (Inose et al. 2009; Kim et al. 2009; Schaap-Oziemlak et al. 2009; Hassan et al. 2010; Itoh et al. 2010; Koh et al. 2010; Hu et al. 2011). In this regard, first we evaluated potential of nHA/PCL scaffold in osteogenic induction of AT-MSCs. Then the effect of anti-miR-221 and finally combination of scaffold and anti-miR-221 was investigated.
A combination of gene therapy with cell therapy has not been applied in three-dimensional environment so we are looking for the influence of this situation in bone tissue engineering.
Previous studies indicated that nHA/PCL have better bone regeneration and mechanical straightening compared with the other kinds of scaffolds and without it (Undale et al. 2009), and our results are in agreement with mentioned studies. Previous study showed that miR-221 downregulation triggers osteogenic commitment in AT-MSCs. In fact, miR-221 impresses some regulators and indicators in osteogenesis pathways such as IGFs, MAPKs, TCFs, and protocadherins (Schindeler and Little 2006; Arnsdorf et al. 2009; Chen et al. 2010; Luo et al. 2010; Guntur et al. 2012). Our results confirmed that downregulation of miR-221 can promote osteogenic differentiation. Relative expression of Runx2 and osteocalcin genes showed that osteogenic induction in anti-miR-221 group was lower than nHA/PCL scaffold. A combination of nHA/PCL scaffold and anti-miR-221 showed highest gene expression in our groups. Altogether, our study showed gene therapy accompanied with cell therapy has superior effects in bone regeneration. Therefore, the development of this combination therapy might bring preferable option in bone tissue engineering. Anti-miRNA treatment can have many benefits because of the small size, easy synthesis, targeted, and quick transfer. Meanwhile, other in vivo researches are needed to confirm the use of both anti-miR-221and nHA/PCL as an option to treat patients who suffer from disorders and diseases of the bone.
Conclusions
The in vitro studies of anti-miR-221 and scaffold showed obvious acceleration in osteogenesis. Altogether, our results yield considerable theoretical and applicable outcomes. These results could help for development of future cell-gene therapy methods in bone tissue engineering. Accordingly, the exploration of high influence of combined anti-miR-221 and nHA/PCL on differentiated AT-MSCs may offer further studies for clarification of miRNA osteogenesis pathway. Moreover, it is recommended to look for the effect of anti-miR-221 on the other kind of scaffolds and also osteogenesis capability of different types of miRNAs on various scaffolds and stem cells.
References
Ahn HH, Kim KS, Lee JH et al (2009) In vivo osteogenic differentiation of human adipose-derived stem cells in an injectable in situ–forming gel scaffold. Tissue Eng A 15:1821–1832
Alizadeh E, Akbarzadeh A, Eslaminejad MB et al. (2014). Up-regulation of liver enriched transcription factors (HNF4a and HNF6) and liver specific microRNA (miR-122) by inhibition of Let-7b in mesenchymal stem cells. Chem biol drug des.
Alizadeh E, Zarghami N, Eslaminejad MB et al. (2014). The effect of dimethyl sulfoxide on hepatic differentiation of mesenchymal stem cells. Artificial Cells, Nanomedicine, Biotechnol 1–8.
Alizadeh E, Eslaminejad MB, Akbarzadeh A et al. (2015). Up-regulation of MiR-122 via trichostatin a treatments in hepatocyte like cells derived from mesenchymal stem cells. Chem Biol Drug Des.
Arnsdorf EJ, Tummala P, Jacobs CR (2009) Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One 4:e5388
Bakhshandeh B, Hafizi M, Ghaemi N et al (2012a) Down-regulation of miRNA-221 triggers osteogenic differentiation in human stem cells. Biotechnol Lett 34:1579–1587
Bakhshandeh B, Soleimani M, Hafizi M et al (2012b) MicroRNA signature associated with osteogenic lineage commitment. Mol Biol Rep 39:7569–7581
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Bauersachs J, Thum T (2011) Biogenesis and regulation of cardiovascular microRNAs. Circ Res 109:334–347
Bentwich I (2005) Prediction and validation of microRNAs and their targets. FEBS Lett 579:5904–5910
Bruder SP, Fink DJ, Caplan AI (1994) Mesenchymal stem cells in bone development, bone repair, and skeletal regenaration therapy. J Cell Biochem 56:283–294
Cai X, Li G, Wang J et al. (2012) Osteogenesis of adipose-derived stem cells. INTECH Open Access Publisher.
Chastain SR, Kundu AK, Dhar S et al (2006) Adhesion of mesenchymal stem cells to polymer scaffolds occurs via distinct ECM ligands and controls their osteogenic differentiation. J Biomed Mater Res A 78:73–85
Chen L, Jiang W, Huang J et al (2010) Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J Bone Miner Res 25:2447–2459
Damme A, Driessche T, Collen D et al (2002) Bone marrow stromal cells as targets for gene therapy. Curr Gene Ther 2:195–209
D’Antò V, Raucci MG, Guarino V et al. (2013). Behaviour of human mesenchymal stem cells on chemically synthesized HA–PCL scaffolds for hard tissue regeneration. J Tissue Eng Regen Med.
Dong S, Yang B, Guo H et al (2012) MicroRNAs regulate osteogenesis and chondrogenesis. Biochem Biophys Res Commun 418:587–591
Elfick A (2002) Poly (ε-caprolactone) as a potential material for a temporary joint spacer. Biomaterials 23:4463–4467
Friedman RC, Farh KK-H, Burge CB et al (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19:92–105
Gómez-Barrena E, Rosset P, Lozano D et al (2015) Bone fracture healing: cell therapy in delayed unions and nonunions. Bone 70:93–101
Guntur AR, Rosen CJ, Naski MC (2012) N-cadherin adherens junctions mediate osteogenesis through PI3K signaling. Bone 50:54–62
Habraken W, Wolke J, Jansen J (2007) Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev 59:234–248
Hassan MQ, Gordon JA, Beloti MM et al (2010) A network connecting Runx2, SATB2, and the miR-23a∼27a∼24–2 cluster regulates the osteoblast differentiation program. Proc Natl Acad Sci 107:19879–19884
Hassan MI, Sun, T, Sultana N (2014). Fabrication of nanohydroxyapatite/poly (caprolactone) composite microfibers using electrospinning technique for tissue engineering applications. J Nanomaterials 2014.
Hellmich C, Ulm F-J (2003) Average hydroxyapatite concentration is uniform in the extracollagenous ultrastructure of mineralized tissues: evidence at the 1–10-μm scale. Biomech Model Mechanobiol 2:21–36
Hench LL, Wheeler DL, Greenspan DC (1998) Molecular control of bioactivity in sol–gel glasses. J Sol-Gel Sci Technol 13:245–250
Hu R, Liu W, Li H et al (2011) A Runx2/miR-3960/miR-2861 regulatory feedback loop during mouse osteoblast differentiation. J Biol Chem 286:12328–12339
Inose H, Ochi H, Kimura A et al (2009) A microRNA regulatory mechanism of osteoblast differentiation. Proc Natl Acad Sci 106:20794–20799
Itoh T, Takeda S, Akao Y (2010) MicroRNA-208 modulates BMP-2-stimulated mouse preosteoblast differentiation by directly targeting V-ets erythroblastosis virus E26 oncogene homolog 1. J Biol Chem 285:27745–27752
Javazon EH, Beggs KJ, Flake AW (2004) Mesenchymal stem cells: paradoxes of passaging. Exp Hematol 32:414–425
Kim YJ, Bae SW, Yu SS et al (2009) miR-196a regulates proliferation and osteogenic differentiation in mesenchymal stem cells derived from human adipose tissue. J Bone Miner Res 24:816–825
Koh W, Sheng CT, Tan B et al (2010) Analysis of deep sequencing microRNA expression profile from human embryonic stem cells derived mesenchymal stem cells reveals possible role of let-7 microRNA family in downstream targeting of hepatic nuclear factor 4 alpha. BMCGenomics 11(Suppl 1):S6–S6
Le Blanc K, Pittenger M (2005) Mesenchymal stem cells: progress toward promise. Cytotherapy 7:36–45
Luo J, Tang M, Huang J et al (2010) TGFβ/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J Biol Chem 285:29588–29598
Ohgushi H, Goldberg VM, Caplan AI (1989) Repair of bone defects with marrow cells and porous ceramic: experiments in rats. Acta Orthop 60:334–339
Osathanon T, Chuenjitkuntaworn B, Nowwarote N et al (2014) The responses of human adipose-derived mesenchymal stem cells on polycaprolactone-based scaffolds: an in vitro study. Tissue Eng Regen Med 11:239–246
Parker A, Shang H, Khurgel M et al (2007) Low serum and serum-free culture of multipotential human adipose stem cells. Cytotherapy 9:637–646
Poinern GE, Brundavanam RK, Mondinos N et al (2009) Synthesis and characterisation of nanohydroxyapatite using an ultrasound assisted method. Ultrason Sonochem 16:469–474
Prante C, Bieback K, Funke C et al (2006) The formation of extracellular matrix during chondrogenic differentiation of mesenchymal stem cells correlates with increased levels of xylosyltransferase I. Stem Cells 24:2252–2261
Reiser J, Zhang X-Y, Hemenway CS. et al. (2005). Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases.
Schaap-Oziemlak AM, Raymakers RA, Bergevoet SM et al (2009) MicroRNA hsa-miR-135b regulates mineralization in osteogenic differentiation of human unrestricted somatic stem cells. Stem Cells Dev 19:877–885
Schindeler A, Little DG (2006) Ras − MAPK signaling in osteogenic differentiation: friend or foe? J Bone Miner Res 21:1331–1338
Serra T, Planell JA, Navarro M (2013) High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater 9:5521–5530
Shor L, Güçeri S, Wen X et al (2007) Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro. Biomaterials 28:5291–5297
Undale AH, Westendorf JJ, Yaszemski MJ et al. (2009). Mesenchymal stem cells for bone repair and metabolic bone diseases. Paper presented at Mayo Clinic Proceedings (Elsevier).
Vats A, Tolley N, Polak J et al (2002) Stem cells: sources and applications. Clin Otolaryngol Allied Sci 27:227–232
Venugopal J, Prabhakaran MP, Zhang Y et al (2010) Biomimetic hydroxyapatite-containing composite nanofibrous substrates for bone tissue engineering. Philos Trans Royal Soc London A: Math, Phys Eng Sci 368:2065–2081
Wang Y, Liu L, Guo S (2010) Characterization of biodegradable and cytocompatible nano-hydroxyapatite/polycaprolactone porous scaffolds in degradation in vitro. Polym Degrad Stab 95:207–213
Wutticharoenmongkol P, Pavasant P, Supaphol P (2007) Osteoblastic phenotype expression of MC3T3-E1 cultured on electrospun polycaprolactone fiber mats filled with hydroxyapatite nanoparticles. Biomacromolecules 8:2602–2610
Xin X, Hussain M, Mao JJ (2007) Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials 28:316–325
Zuk PA, Zhu M, Ashjian P et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295
Acknowledgments
The present work is part of an MSc thesis supported financially (Grant No. 5/104/577) by The Umbilical Cord Stem Cell Research Center (UCSRC), Tabriz University of Medical Sciences, Tabriz, Iran. Also, we thank Tarbiat Modares University for providing technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Hoseinzadeh, S., Atashi, A., Soleimani, M. et al. MiR-221-inhibited adipose tissue-derived mesenchymal stem cells bioengineered in a nano-hydroxy apatite scaffold. In Vitro Cell.Dev.Biol.-Animal 52, 479–487 (2016). https://doi.org/10.1007/s11626-015-9992-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-015-9992-x