Abstract
Curcumin, a naturally occurring phenolic compound, has a diversity of antitumor activities. It has been previously demonstrated that curcumin can inhibit the invasion and metastasis of tumors through activation of the tumor suppressor DnaJ-like heat shock protein 40 (HLJ1). However, the specific roles and mechanisms of curcumin in regulating the malignant behaviors of non-small cell lung cancer (NSCLC) cells still remain unclear. In this study, we found that curcumin could inhibit the proliferation and invasion of NSCLC cells and induce G0/G1 phase arrest. Metastasis-associated protein 1 (MTA1) overexpression has been detected in a wide variety of aggressive tumors and plays an important role on cell invasion and metastasis. Our results showed that curcumin could effectively inhibit the MTA1 expression of NSCLC cells. Further research on the subsequent mechanism showed that curcumin inhibited the proliferation and invasion of NSCLC cells through MTA1-mediated inactivation of Wnt/β-catenin pathway. Wnt/β-catenin signaling was reported to play a critical cooperative role on promoting lung tumorigenesis. Thus, these investigations provided novel insights into the mechanisms of curcumin on inhibition of NSCLC cell growth and invasion and showed potential therapeutic strategies for NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-small cell lung carcinoma (NSCLC) is any type of epithelial lung cancer other than small cell lung carcinoma (SCLC), which accounts for 75~80% of all primary lung cancer and has been a major public health problem worldwide (Brognard et al. 2001; Ren et al. 2004). Despite major advances made in combination with intensive chemotherapy and aggressive surgical techniques, the prognoses of NSCLC patients are still dismal due to invasion and metastasis (Hoffman et al. 2000; Steeg 2006). As a class, NSCLCs are relatively insensitive to chemotherapy. Thus, it is important to develop more efficient chemotherapies to be used in advanced (metastatic) NSCLC and prevent NSCLC progression and improve patient survival rates.
Curcumin is a naturally occurring phenolic compound shown to have a wide variety of antitumor activities in many different cancers, such as gastric cancer, osteosarcoma, and lung adenocarcinoma (Chen et al. 2008; Prakobwong et al. 2011; Li et al. 2012). Its anticancer effects involved in cell proliferation, invasion, metastasis, apoptosis, and resistance to chemotherapy. Cai et al. (2009) found that curcumin inhibited gastric cancer cell growth by down-regulating the mRNA and the protein expression of cyclin D1 and suppressing transition of the cells from G1 to S phase (Prakobwong et al. 2011). Li et al. reported that curcumin caused osteosarcoma cell proliferation inhibition through inactivation of Notch-1 signaling (Li et al. 2012). In lung cancers, many studies found that curcumin induced apoptosis of non-small cell lung cancer cells and lung adenocarcinoma cells (Chen et al. 2010; Pongrakhananon et al. 2010; Wu et al. 2010; Lee et al. 2011).
However, few studies focused on the function and mechanism of curcumin on the cell proliferation and invasion of NSCLC cells. Li et al. showed that curcumin inhibited NSCLC A549 cell proliferation through regulation of Bcl-2/Bax and cytochrome C (Li et al. 2013a). Chen et al. reported that curcumin was able to transcriptionally regulate HLJ1 expression through the JNK/JunD pathway and inhibited lung cancer cell invasion and metastasis by modulating E-cadherin expression, and it could also suppress the metalloproteinase (MMP) expression (Chen et al. 2008).
However, the specific roles and mechanisms of curcumin in regulating the cell proliferation and invasion of non-small cell lung cancer (NSCLC) cells still remain unclear. In this study, we identified the anti-proliferation and anti-invasion effects of curcumin on NSCLC. Our results demonstrated that curcumin exhibited inhibitory effects on cell proliferation and invasion via suppression of cell cycle and MTA1 expression. Overexpression of metastasis-associated protein 1 (MTA1) has been detected in a wide variety of human aggressive tumors and plays an important role in the malignant biological behaviors such as invasion, metastasis, and angiogenesis (Zhu et al. 2012). Recent study by Li et al. showed that MTA1 protein played an important role in regulating the migration and invasion of NSCLC, and down-regulation of MTA1 protein leads to the inhibition of migration, invasion, and angiogenesis of non-small cell lung cancer cell line (Li et al. 2013b). Here, we conclude that inactivation of MTA1 by curcumin can be a potential targeted approach for the prevention of tumor progression of NSCLC.
Materials and Methods
Cell culture and experimental reagents.
The human NSCLC cell lines 95D and A549 cells were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM supplemented with 10% fetal bovine serum (Gibco, Gaithersburg, MD) at 37°C in 5% CO2. Primary antibodies for MTA1 were obtained from Cell Signaling Technology (Beverly, MA). The small interfering RNA (siRNA) targeting MTA1 gene and negative control siRNA were chemically synthesized by GenePharma Co., Ltd. (Shanghai, China). FuGENE HD was purchased from Roche. Curcumin, MTT, and all other chemicals were obtained from Sigma Chemical Company (St. Louis, MO).
MTT assay for cell viability.
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was used to determine relative cell growth. Human NSCLC cell lines 95D and A549 cells were plated at 5 × 103 cells per well in 96-well plates with six replicate wells at the indicated concentrations. After incubation for indicated time, the cell proliferation assay was performed as the manufacturer’s instructions. The absorbance was measured at 450 nm using an enzyme-linked immunosorbent assay plate reader. Each data point represents the mean of a minimum of 6 wells. The viability of untreated cells was assumed to be 100%.
Cell cycle analysis.
After treatment, NSCLC cell lines 95D and A549 cells were trypsinized and subsequently fixed with ice-cold 70% ethanol for at least 1 h. After extensive washing, the cells were suspended in Hank’s balanced salt solution (HBSS) containing 50 μg/ml propidium iodide (PI, Sigma-Aldrich) and 50 μg/ml RNase A (Boehringer Mannheim, Indianapolis, IN) and incubated for 1 h at room temperature for subsequent FACScan analysis (Becton-Dickinson, San Jose, CA). Cell cycle analysis was performed by the ModFit LT software.
Cell transfection efficiency analysis.
Transfected cells were digested with trypsin (0.25%), which was neutralized with 10% FCS, and the cells were pelleted and resuspended. After centrifugation at 500×g for 5 min, the supernatant was discarded. Then, cells were washed twice with phosphate-buffered saline (PBS). After adjusting the cell density of 1 × 106/ml, cell transfection efficiency was measured by flow cytometry. Cells transfected with empty vector (no green fluorescence) were used as negative control.
Real-time quantification by reverse transcription polymerase chain reaction.
The ABI 7300 HT Sequence Detection system (Applied Biosystems, Foster City, CA) was used for real-time reverse transcription polymerase chain reaction (RT-PCR) assays. All the primers of MTA1 (sense primer 5′-CAGCTACGAGCAGCACAACG-3′, antisense primer 5′-TGTCCGTGGTTTGCCAGA-3′) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) endogenous controls (sense primer 5′-GGTGGTCTCCTCTGACTTCAACA-3′, antisense primer 5′-GTTGCTGTAGCCAAATTCGTTGT-3′) for mRNA assays were purchased from Sangon Biotech (Shanghai, China). The cycling conditions were as follows: initial denaturation at 95°C for 30 s, followed by 40 cycles at 95°C for 5 s, 60°C for 30 s, and 72°C for 15 s. Relative gene expression was calculated via a 2−ΔΔCt method.
Western blot analysis.
To determine the levels of protein expression, soluble proteins were isolated by lysis buffer (137 mM NaCl, 15 mM EGTA, 0.1 mM sodium orthovanadate, 15 mM MgCl2, 0.1% Triton X-100, 25 mM MOPS, 100 μM phenylmethylsulfonyl fluoride and 20 μM leupeptin, adjusted to pH 7.2). One-dimensional sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was performed with a corresponding gel concentration using the discontinuous Laemmli buffer system (Bio-Rad Laboratories, Richmond, CA). The electrophoresed proteins were transferred to a polyvinylidene difluoride membrane and subjected to immunoblot analysis with antibodies to purified MTA1, β-catenin, cyclin D1, and MMP7. The reaction was detected by enhanced chemiluminescence (Amersham Life Science, Arlington Heights, IL). The membranes were reprobed with a GAPDH antibody (1:2000 dilution, Santa Cruz Biotechnology, Dallas, TX) after washing as a gel loading control.
In vitro invasion assays.
Transwell membranes coated with Matrigel (BD Biosciences, San Jose, CA) were used to assay the invasion of NSCLC cell lines 95D and A549 cells in vitro. Transfected 95D and A549 cells were plated at 5 × 103 cells per well in the upper chamber in serum-free medium. Then, 20% FBS was added to the medium in the lower chamber. After 24 h, non-invading cells were removed from the top well with a cotton swab while invading cells were also removed from the bottom cells. Either of them were stained with 0.1% crystal violet for 30 min at 37°C and then washed with PBS. Stained 95D and A549 cells that migrated and cells that did not migrate were assessed by absorbance at 570-nm wavelength using a microplate reader separately, and the percentage of cells that migrated compared to those that did not migrate was calculated. Three independent experiments were performed and used to calculate fold migration relative to a blank control.
Statistical analysis.
All tests were performed using SPSS Graduate Pack 11.0 statistical software (SPSS, Chicago, IL). Descriptive statistics, including the mean and SE, in addition to one-way ANOVAs were used to determine significant differences. P < 0.05 was considered statistically significant.
Results
Curcumin inhibits the proliferation of NSCLC cell lines 95D and A549 cells.
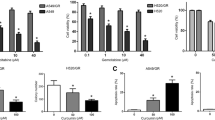
Previous research has shown that the effects of curcumin could be cytotoxic or cytostatic. In initial experiments, the cytotoxic effect of curcumin on NSCLC cell lines 95D and A549 cells was analyzed by using MTT assays. As shown in Fig. 1A , the cell viability of 95D and A549 cells decreased in a concentration-dependent manner after treatment with curcumin for 24 h. Data from MTT cell proliferation assay also revealed that the cell viability was about 53 ± 3.1 and 43 ± 7.1, respectively, when 95D and A549 cells were treated with 30 μM curcumin. Thus, we selected 30 μM curcumin to treat 95D and A549 cells for 24, 48, and 72 h. The results showed that 30 μM curcumin exhibited obvious anti-proliferation which resulted in large number of cell death in a time-dependent manner (Fig. 1 B ). Considering the cell proliferation ability change always accompanying cell cycle change, we analyzed the effects of curcumin on 95D and A549 cell cycle distribution by using a flow cytometric assay. Cell cycle distribution of 95D and A549 cells was treated with 0, 15, and 30 μM of curcumin for 72 h. As shown in Fig. 1 C , curcumin treatment resulted in an increased number of cells in G0/G1 phase, indicating a curcumin-induced G0/G1 phase arrest. These data suggest that the decreased proliferation in curcumin-treated 95D and A549 cells is at least partially a result of the cell cycle arrest in G0/G1 phase by curcumin.
Effects of curcumin on the cell viability of NSCLC cell lines 95D and A549 cells. (A) 95D and A549 cells were treated with indicated concentration of curcumin for 24 h. After that, cell viability of 95D and A549 cells was measured by MTT assay. (B) 95D and A549 cells were treated with 30 μM concentration for 24, 48, and 72 h. Then, cell viability of 95D and A549 cells was measured by MTT assay. C 95D and A549 were treated with 0, 15, and 30 μM of curcumin for 72 h. After that, the cell cycle distribution of 95D cells was analyzed by a flow cytometric assay. *P < 0.05; **P < 0.01.
Curcumin inhibits cell invasion of NSCLC cell line 95D and A549 cells.
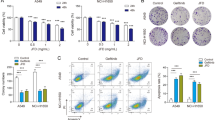
To measure the effects of curcumin on 95D and A549 cell invasiveness, a better indication of NSCLC migratory and invasive properties in vivo, we employed a Transwell invasion assay system. In order to avoid the impact of cell proliferation inhibition on cell invasion, we calculated the percentage of cells that migrated compared to those that did not migrate. In this test, only invasive cells have the ability to digest the Matrigel and migrate through the pores in the membrane. Then, 95D and A549 cells were preincubated with different concentrations of curcumin for 24 h and equal amount of the cells was seeded into the upper part of transwell chamber. After 24-h cultivation, the invaded cells were fixed and stained with Hoechst. As shown in Fig. 2 , the treatment of 95D and A549 cells for 24 h with 0, 15, and 30 μM of curcumin resulted in a concentration-dependent inhibition of cell invasion. Thus, our results strongly indicate that curcumin plays an important role in decreasing the invasive potential of NSCLC 95D and A549 cells.
Curcumin reduced MTA1 expression in NSCLC cell lines 95D and A549 cells.
The association between MTA1 protein and migration potential of 95D cells was explored and verified by Li et al. (Li et al. 2013b). To further understand whether the molecular mechanism of curcumin-induced invasion inhibition was involved in MTA1 expression decease, the expression of MTA1 mRNA in 95D and A549 cells treated with 0, 15, and 30 μM curcumin for 48 h was assessed by real-time RT-PCR analysis. As shown in Fig. 3 A , mRNA levels after curcumin treatment suggested that curcumin induced the transcriptional inactivation of MTA1 in 95D and A549 cells. Moreover, Western blot results also demonstrated that MTA1 protein levels were deceased after curcumin treatment in 95D and A549 cells (Fig. 3 B ). These results suggest that curcumin regulated the transcription and translation of the MTA1 gene.
Effects of curcumin on the expression of MTA1 in NSCLC cell lines 95D and A549 cells. 95D cells and A549 cells were treated with 0, 15, and 30 μM curcumin for 48 h. (A) The expression of MTA1 mRNA in 95D and A549 cells was assessed using real-time RT-PCR analysis. (B) The protein of MTA1 in 95D and A549 cells was assessed by using Western blot analysis. **P < 0.01.
Down-regulation of MTA1 protein induced proliferation and invasion inhibition in NSCLC cell lines 95D and A549 cells.
To further confirm the role of MTA1 on the proliferation and invasion of 95D and A549 cells, we used the MTA1 siRNA to down-regulate the MTA1 expression. As shown in Fig. 4A , Western blot showed that MTA1 siRNA significantly reduced the expression of MTA1 protein compared with MTA1 siRNAnc treatment. As shown in Fig. 4 B , this down-regulation of MTA1 significantly induced cell proliferation inhibition on 95D and A549 cells. Moreover, the association between MTA1 protein and invasion potential of 95D and A549 cells was further analyzed by Transwell assay. Our results demonstrated that the number of 95D and A549 cells migrating through the pores in the membrane by MTA1 siRNA was significantly less than that of 95D and A549 cells treated with MTA1-siRNAnc (Fig. 4 C ). These findings demonstrated that MTA1 was involved in cell proliferation and invasion of NSCLC cell lines 95D and A549 cells.
Effects of MTA1 siRNA on the proliferation and invasion of 95D and A549 cells. A 95D and A549 cells were transfected with MTA1 siRNA. After that, the expression of MTA1 was analyzed by Western blot. B MTA1 siRNA inhibited the cell proliferation of 95D and A549 cells. C MTA1 siRNA inhibited the cell invasion of 95D and A549 cells. **P < 0.01.
Up-regulation of MTA1 reverses the antitumor effects of curcumin on NSCLC cell lines 95D and A549 cells.
We further examined whether up-regulation of MTA1 counteracts the antitumor effects of curcumin on NSCLC cell lines 95D and A549 cells. As shown in Fig. 5A , after 48-h transfection, MTA1 overexpressing vectors (short for MTA1 vector) (transfection efficiency, approximately 83.7% for 95D cells and 87.1% for A549 cells; Fig. 5B ) effectively abolished the reduction of MTA1 repressed by curcumin. Cells were subsequently treated with 30 μM curcumin for 6 h. After that, cells were washed with drug-free medium and then treated with MTA1 overexpressing vectors for 48 h. The resulting up-regulation of MTA1 levels rescued the proliferation and invasion inhibition induced by curcumin (Fig. 5C, D ). These findings suggest that MTA1 repression may contribute to the anti-proliferation and anti-invasion activity induced by curcumin.
Up-regulation of MTA1 reverses the antitumor effects of curcumin on NSCLC cell lines 95D and A549 cells. (A) 95D and A549 cells were transfected with MTA1 overexpressing vectors or 30 μM curcumin, and the expression of MTA1 was analyzed by Western blot. (B) The transfection efficiency of 95D and A549 cells were observed under a fluorescence microscope. (C) The cell viability of 95D and A549 cells upon transfection with curcumin and MTA1 overexpressing vectors was measured by the MTT assay. (D) The cell invasion of 95D and A549 cells upon transfection with curcumin and MTA1 overexpressing vectors was measured by Transwell assay. **P < 0.01.
MTA1 downstream pathway was further confirmed after curcumin administration.
Wnt/β-catenin signaling is a known oncogenic pathway that plays a well-defined role in colon and skin cancers. Recent data showed Wnt/β-catenin signaling could accelerate mouse lung tumorigenesis by imposing an embryonic distal progenitor phenotype on lung epithelium (Pacheco-Pinedo et al. 2011). Chen et al. found that Wnt/β-catenin signaling, which induces MMP-7 expression in lung cancer cells, involved in lung cancer invasion (Chen et al. 2014), while functional MTA1-Wnt1 signaling resulted in nuclear translocation of β-catenin in breast tumor progression and metastasis (Yan et al. 2012). However, whether MTA1 could mediate Wnt/β-catenin signaling in lung cancer was still unclear. Thus, we hypothesized that MTAl and its downstream targets of Wnt (β-catenin, Cyclin D1, and matrix metalloproteinase (MMP)-7) occurred corresponding changes after curcumin treatment. To confirm this hypothesis, MTA1 downstream pathway proteins were further detected. 95D and A549 cells were treated with 30 μM curcumin for 48 h, and then, the downstream Wnt/β-catenin pathway of MTAl including β-catenin, Cyclin D1, and MMP-7 proteins was detected by Western blot. The results showed that β-catenin, Cyclin D1, and MMP-7 were significantly down-regulated in 95D and A549 cells after curcumin treatment (Fig. 6A ). Further, 95D and A549 cells were co-treated with curcumin and MTA1 overexpressing vectors, and our results showed that exogenous expression of MTA1 could rescue alteration of β-catenin, cyclin D1, and MMP7 levels induced by curcumin (Fig. 6B ). Moreover, the cells were transfected with MTA1 siRNA, and the data showed that MTA1 knockdown had effects on β-catenin, cyclin D1, and MMP7 protein levels similar to curcumin treatment in NSCLC (Fig. 6C ). These findings suggest that curcumin represses 95D and A549 cells through the inactivation of MTAl-Wnt/β-catenin pathway.
MTA1 downstream pathway proteins in 95D and A549 cells after curcumin treatment. (A) After curcumin treatment for 48 h, Wnt/β-catenin signaling including β-catenin, Cyclin D1, and MMP-7 proteins were detected by Western blot. (B) After curcumin combined MTA1 overexpressing vectors treatment, Wnt/β-catenin signaling including β-catenin, Cyclin D1, and MMP-7 proteins were detected by Western blot. (C) After MTA1 siRNA treatment for 48 h, Wnt/β-catenin signaling including β-catenin, Cyclin D1, and MMP-7 proteins were detected by Western blot.
Discussion
The cell proliferation and invasion are two of the major causes of therapeutic failure in patients with NSCLC. Inhibiting the proliferation and invasion of NSCLC could help to suppress the cancer progresses. However, the effective and safe drug and its detailed molecular mechanisms on the proliferation and invasion of NSCLC are still unclear. Thus, it is urgent to find a drug to focus on and abolish the cell proliferation and invasion of NSCLC.
Traditional medicines are generally free of the deleterious side effects and usually inexpensive. Curcumin, a component of turmeric, is one such agent that is safe, affordable, and efficacious. Curcumin derived from the Indian spice turmeric and possesses several active components. It is believed to have anti-inflammatory, antioxidant, and perhaps even anticancer properties. In fact, it could target ten causative factors involved in cancer development. Curcumin alters cellular signaling to enhance healthy control over cellular replication, which tightly regulates the cellular reproductive cycle, helping to stop uncontrolled proliferation of new tissue in tumors of human lung carcinoma A-549 cells, acute promyelocytic leukemia HL-60 cells, melanoma A375 cell line, and so on (Ravindran et al. 2009). It is also reported to induce apoptosis in rapidly reproducing lung adenocarcinoma cells without affecting normal tissue (Zhang et al. 2010). In addition, curcumin regulates tumor suppressor pathways and triggers endoplasmic reticulum stress and mitochondrial dysfunction-mediated death in hepatocellular carcinoma cells (Cheng et al. 2010). Recently, curcumin interferes with tumor invasiveness and blocks molecules of kinds of cancer cells including breast carcinoma cells, prostate cancer cells, colorectal cancer cells, and lung cancer A549cells that would otherwise open pathways to penetration of tissue (Lin et al. 2009; Mudduluru et al. 2011; Kim et al. 2012; Cheng et al. 2013). However, there is little knowledge of the bioactivity of curcumin on NSCLC.
In the present study, our results, for the first time, demonstrated that curcumin could efficiently inhibit the proliferation of NSCLC cell lines 95D and A549 cells and induce a significant G0/G1 cell cycle arrest in 95D and A549 cells by flow cytometry analysis. Furthermore, we found that curcumin could also inhibit the cell invasion of 95D and A549 cells. To further elucidate the molecular mechanisms of cell invasion inhibition by curcumin, we investigated the expression of MTA1 in the cells treated with curcumin, which plays a key role in the process of cell invasion and metastasis (Li et al. 2013b).
MTA1, a component of the nucleosome remodeling and histone deacetylation (NuRD) complex, is widely overexpressed in a variety of human cell lines (breast, ovarian, lung, gastric, and colorectal cancer but not melanoma or sarcoma) and cancerous tissues (breast, esophageal, colorectal, gastric, and pancreatic cancer) and plays a crucial role in cell survival, proliferation, migration, and invasion (Cong et al. 2011). Nicolson et al. has reported that experimental inhibition of MTA1 protein expression using antisense phosphorothioate oligonucleotides resulted in inhibition of growth and invasion of human MDA-MB-231 breast cancer cells (Nicolson et al. 2003). Jiang et al. showed that down-regulation of MTA1 by RNAi approach led to re-expression of estrogen receptor (ER) alpha in ER-negative breast cancer cell lines MDA-MB-231 and reduced protein levels of MMP-9 and CyclinD1, as well as decreased tumor cell invasion and proliferation (Jiang et al. 2011). Recently, Li et al. found that average expression of the MTA1 gene in NSCLC primary carcinoma tissue and lymph nodes with metastasis was remarkably higher than that in normal tissue and lung innocence tissue, and the overexpression of the MTA1 gene correlates with invasion and metastasis of NSCLC (Li et al. 2008). And inhibition of MTA1 protein prevents the migration, invasion, and angiogenesis of NSCLC (Li et al. 2013b). In this study, we used MTA1 siRNA to knock down the expression of MTA1 in NSCLC cell lines 95D and A549 cells and our results demonstrated that down-regulation of MTA1 protein led to cell proliferation and invasion inhibition of 95D and A549 cells. Further research by us showed that overexpression of MTA1 could rescue the inhibition effects of curcumin on cell proliferation and invasion of 95D and A549 cells. Therefore, inhibition of MTA1 protein may have important therapeutic applications for treating and preventing cell proliferation and invasion in NSCLC, and curcumin inhibiting the proliferation and invasion of NSCLC was at least in part through down-regulating the expression of MTA1 protein. Wnt/β-catenin alterations are prominent in human malignancies including lung cancers (Stewart. 2014). Wnt signaling was reported to be aberrantly activated by kinds of mechanisms, and its main function was to inhibit the proteolysis of β-catenin, and then, free β-catenin could enter the nucleus to activate the target genes of Wnt (Gao et al. 2014). Previous studies showed that nuclear β-catenin expression leads to increase the transcription of target genes, including c-myc, cyclinD1, and MMP7, which affected the proliferation and invasion of tumor cells (Lustig and Behrens 2003; Gao et al. 2014). And Cyclin D1 was reported to mainly regulate G1 phase progression to control tumor proliferation (Nigg 1995). Grigoryan et al. reported that the Wnt signaling pathway was highly active in lung cancer cells, which leads to cell metastasis and proliferation (Grigoryan et al. 2008). To confirm whether MTA1 could mediate Wnt/β-catenin signaling in lung cancer, the expression of MTA1 was knockdown by siRNA and detected the expression of Wnt/β-catenin signaling. Our results showed that the downstream Wnt/β-catenin pathway of β-catenin and the subsequent Cyclin D1 and MMP-7 were significantly decreased after MTA1 knockdown, and up-regulation of MTA1 expression could rescue curcumin-induced β-catenin, Cyclin D1, and MMP-7 decrease. These results demonstrated that MTA1 and its downstream Wnt/β-catenin pathway played an important role in the proliferation and invasion of NSCLC.
Taken together, our study identifies MTA1-Wnt/β-catenin as new molecular targets of curcumin that may have important clinical applications for NSCLC chemoprevention and therapy and provide an innovative therapeutic strategy for the treatment of NSCLC.
References
Brognard J, Clark AS, Ni Y, Dennis PA (2001) Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res 61:3986–3997
Cai XZ, Wang J, Li XD, Wang GL, Liu FN, Cheng MS, Li F (2009) Curcumin suppresses proliferation and invasion in human gastric cancer cells by downregulation of PAK1 activity and cyclin D1 expression. Cancer Biol Ther 8(14):1360–1368
Chen HW, Lee JY, Huang JY, Wang CC, Chen WJ, Su SF, Huang CW, Ho CC, Chen JJ, Tsai MF, Yu SL, Yang PC (2008) Curcumin inhibits lung cancer cell invasion and metastasis through the tumor suppressor HLJ1. Cancer Res 68:7428–7438
Chen QY, Lu GH, Wu YQ, Zheng Y, Xu K, Wu LJ, Jiang ZY, Feng R, Zhou JY (2010) Curcumin induces mitochondria pathway mediated cell apoptosis in A549 lung adenocarcinoma cells. Oncol Rep 23(5):1285–1292
Chen X, Meng J, Yue W, Yu J, Yang J, Yao Z, Zhang L (2014) Fibulin-3 suppresses Wnt/β-catenin signaling and lung cancer invasion. Carcinogenesis. 2014 Feb 22
Cheng CY, Lin YH, Su CC (2010) Curcumin inhibits the proliferation of human hepatocellular carcinoma J5 cells by inducing endoplasmic reticulum stress and mitochondrial dysfunction. Int J Mol Med 26(5):673–678
Cheng TS, Chen WC, Lin YY, Tsai CH, Liao CI, Shyu HY, Ko CJ, Tzeng SF, Huang CY, Yang PC, Hsiao PW, Lee MS (2013) Curcumin-targeting pericellular serine protease matriptase role in suppression of prostate cancer cell invasion, tumor growth, and metastasis. Cancer Prev Res 6:495–505
Cong L, Pakala SB, Ohshiro K, Li D, Kumar R (2011) SUMOylation and SUMO-interacting Motif (SIM) of Metastasis Tumor Antigen 1 (MTA1) synergistically regulate its transcriptional repressor function. J Biol Chem 286:43793–43808
Gao Y, Song C, Hui L, Li CY, Wang J, Tian Y, Han X, Chen Y, Tian DL, Qiu X, Wang E (2014) Overexpression of RNF146 in non-small cell lung cancer enhances proliferation and invasion of tumors through the Wnt/β-catenin signaling pathway. PLoS ONE 9(1):e85377
Grigoryan T, Wend P, Klaus A, Birchmeier W (2008) Deciphering the function of canonical Wnt signals in development and disease: conditional loss and gain of function mutations of β-catenin in mice. Genes Dev 22:2308–2341
Hoffman PC, Mauer AM, Vokes EE (2000) Lung cancer. Lancet 355:479–485
Jiang Q, Zhang H, Zhang P (2011) ShRNA-mediated gene silencing of MTA1 influenced on protein expression of ER alpha, MMP-9, CyclinD1 and invasiveness, proliferation in breast cancer cell lines MDA-MB-231 and MCF-7 in vitro. J Exp Clin Cancer Res 30:60
Kim JM, Noh EM, Kwon KB, Kim JS, You YO, Hwang JK, Hwang BM, Kim BS, Lee SH, Lee SJ, Jung SH, Youn HJ, Lee YR (2012) Curcumin suppresses the TPA-induced invasion through inhibition of PKCα-dependent MMP-expression in MCF-7 human breast cancer cells. Phytomedicine 19(12):1085–1092
Lee JY, Lee YM, Chang GC, Yu SL, Hsieh WY, Chen JJ, Chen HW, Yang PC (2011) Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: the versatile adjuvant for gefitinib therapy. PLoS ONE 6(8):e23756
Li D, Qian J, Hong Z (2008) Expression and clinical significance of MTA1 in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 11(6):775–779
Li S, Tian H, Yue W, Li L, Gao C, Si L, Li W, Hu W, Qi L, Lu M (2013a) Down-regulation of MTA1 protein leads to the inhibition of migration, invasion, and angiogenesis of non-small-cell lung cancer cell line. Acta Biochim Biophys Sin 45:115–122
Li Y, Zhang S, Geng JX, Hu XY (2013b) Curcumin inhibits human non-small cell lung cancer A549 cell proliferation through regulation of Bcl-2/Bax and cytochrome C. Asian Pac J Cancer Prev 14(8):4599–4602
Li Y, Zhang J, Ma D, Zhang L, Si M, Yin H, Li J (2012) Curcumin inhibits proliferation and invasion of osteosarcoma cells through inactivation of Notch-1 signaling. FEBS J 279(12):2247–2259
Lin SS, Lai KC, Hsu SC, Yang JS, Kuo CL, Lin JP, Ma YS, Wu CC, Chung JG (2009) Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF). Cancer Lett 285(2):127–133
Lustig B, Behrens J (2003) The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol 129(4):199–221
Mudduluru G, George-William JN, Muppala S, Asangani IA, Kumarswamy R, Nelson LD, Allgayer H (2011) Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci Rep 31(3):185–197
Nicolson GL, Nawa A, Toh Y, Taniguchi S, Nishimori K, Moustafa A (2003) Tumor metastasis-associated human MTA1 gene and its MTA1 protein product: role in epithelial cancer cell invasion, proliferation and nuclear regulation. Clin Exp Metastasis 20(1):19–24
Nigg EA (1995) Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays 17:471–480
Pacheco-Pinedo EC, Durham AC, Stewart KM, Goss AM, Lu MM, Demayo FJ, Morrisey EE (2011) Wnt/β-catenin signaling accelerates mouse lung tumorigenesis by imposing an embryonic distal progenitor phenotype on lung epithelium. J Clin Invest 121(5):1935–1945
Pongrakhananon V, Nimmannit U, Luanpitpong S, Rojanasakul Y, Chanvorachote P (2010) Curcumin sensitizes non-small cell lung cancer cell anoikis through reactive oxygen species-mediated Bcl-2 downregulation. Apoptosis 15(5):574–585
Prakobwong S, Gupta SC, Kim JH, Sung B, Pinlaor P, Hiraku Y, Wongkham S, Sripa B, Pinlaor S, Aggarwal BB (2011) Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis 32:1372–1380
Ravindran J, Prasad S, Aggarwal BB (2009) Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J 11(3):495–510
Ren H, Tang X, Lee JJ, Feng L, Everett AD, Hong WK, Khuri FR, Mao L (2004) Expression of hepatoma-derived growth factor is a strong prognostic predictor for patients with early-stage non-small-cell lung cancer. J Clin Oncol 22:3230–3237
Steeg PS (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12:895–904
Stewart DJ (2014) Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst 106(1):djt356
Wu SH, Hang LW, Yang JS, Chen HY, Lin HY, Chiang JH, Lu CC, Yang JL, Lai TY, Ko YC, Chung JG (2010) Curcumin induces apoptosis in human non-small cell lung cancer NCI-H460 cells through ER stress and caspase cascade- and mitochondria-dependent pathways. Anticancer Res 30(6):2125–2133
Yan D, Avtanski D, Saxena NK, Sharma D (2012) Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires β-catenin activation via Akt/GSK3- and MTA1/Wnt1 protein-dependent pathways. J Biol Chem 287(11):8598–8612
Zhang J, Zhang T, Ti X, Shi J, Wu C, Ren X, Yin H (2010) Curcumin promotes apoptosis in A549/DDP multidrug-resistant human lung adenocarcinoma cells through an miRNA signaling pathway. Biochem Biophys Res Commun 399(1):1–6
Zhu X, Zhang X, Wang H, Song Q, Zhang G, Yang L, Geng J, Li X, Yuan Y, Chen L (2012) MTA1 gene silencing inhibits invasion and alters the microRNA expression profile of human lung cancer cells. Oncol Rep 28(1):218–224
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Yimin Lu and Changjiang Wei Contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lu, Y., Wei, C. & Xi, Z. Curcumin suppresses proliferation and invasion in non-small cell lung cancer by modulation of MTA1-mediated Wnt/β-catenin pathway. In Vitro Cell.Dev.Biol.-Animal 50, 840–850 (2014). https://doi.org/10.1007/s11626-014-9779-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-014-9779-5